Published online Dec 6, 2020. doi: 10.12998/wjcc.v8.i23.5918
Peer-review started: June 29, 2020
First decision: August 8, 2020
Revised: August 29, 2020
Accepted: October 13, 2020
Article in press: October 13, 2020
Published online: December 6, 2020
Processing time: 157 Days and 22.9 Hours
Implant vagus nerve stimulation is an adjunctive treatment for intractable epilepsy when patients are not suitable for resective surgery.
To identify the safety and efficacy of vagus nerve stimulation in children with intractable epilepsy and analyze the effects on different epilepsy syndromes.
Eligible children with intractable epilepsy were admitted to the study. We collected data from preoperative assessments as the baseline. During the follow-up time, we recorded the process of seizures (frequency, duration, and seizure type), the changes of drugs or parameters, the complications, etc. The mean reduction rate of seizures, response rate, and McHugh scale were chosen as the outcomes.
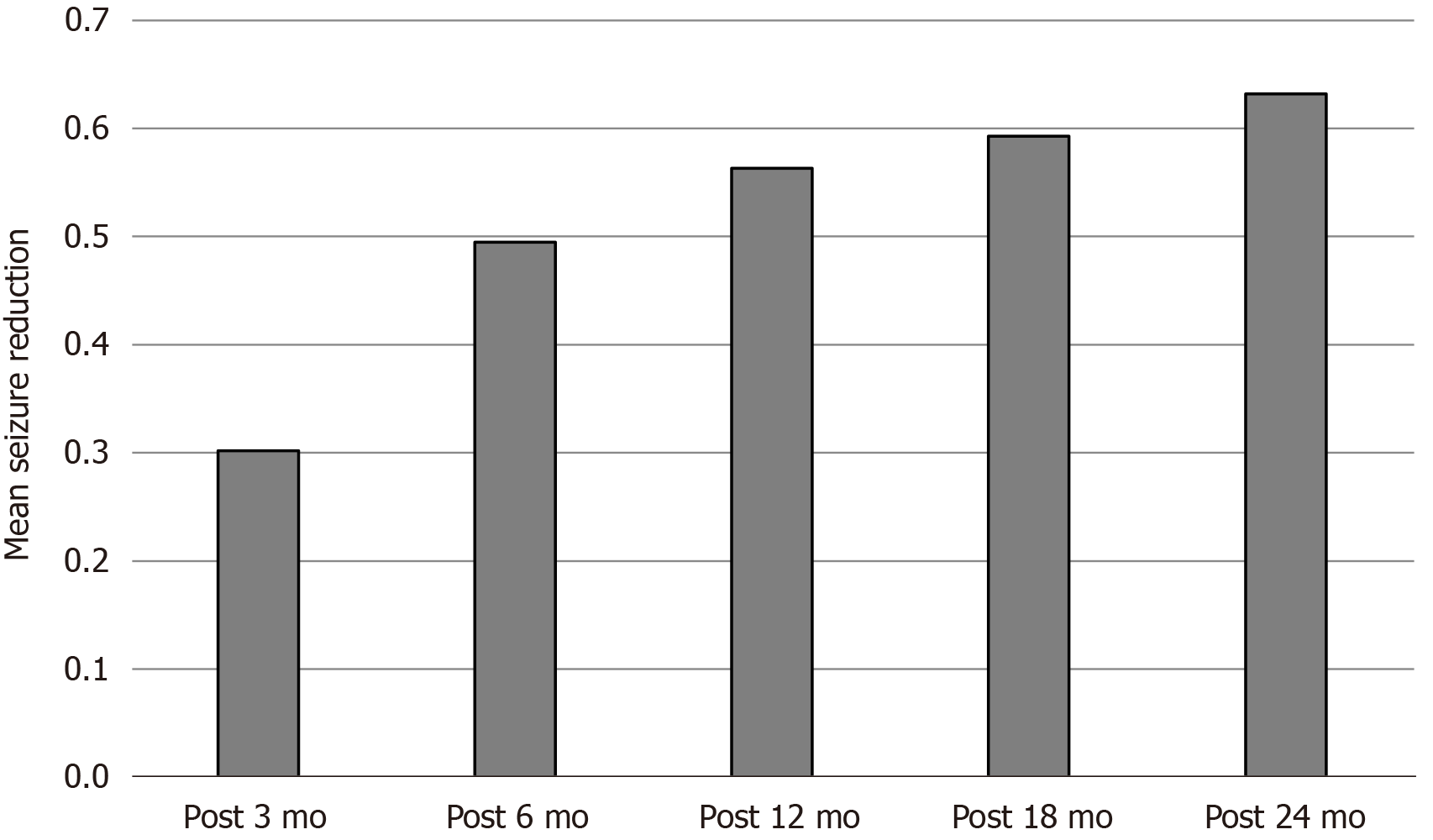
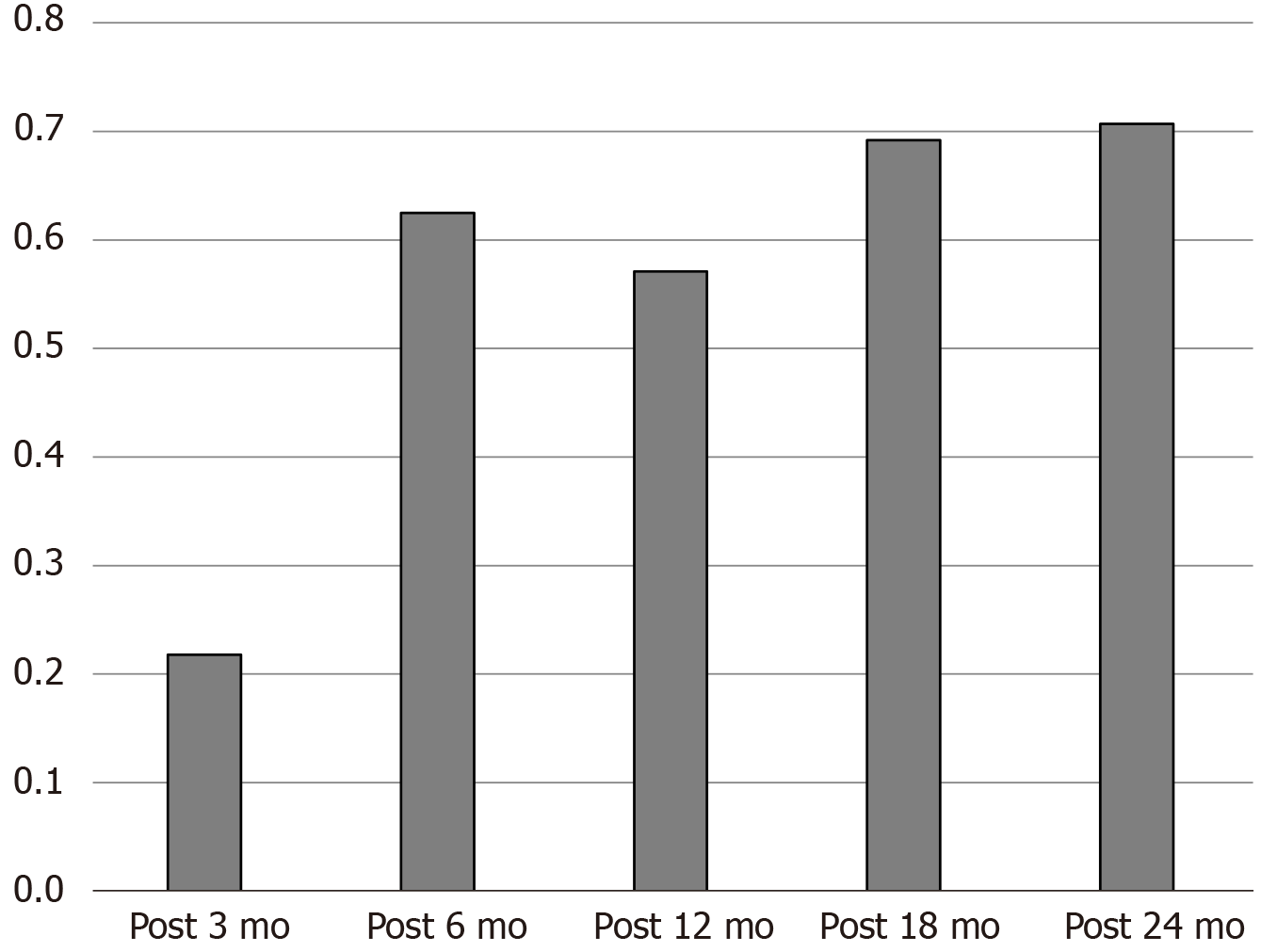
A total of 213 patients were implanted with Tsinghua Pins vagus nerve stimulators, and the average age was 6.6 years. In the follow-up time of postoperative 3 mo, 6 mo, 12 mo, 18 mo, and 24 mo, the average reduction rate was 30.2%, 49.5%, 56.3%, 59.4%, and 63.2%, while the response rate was 21.8%, 62.5%, 57.1%, 69.2%, and 70.7%. In addition, implanted vagus nerve stimulation had different effects on epilepsy syndromes. The reduction rate of West syndrome increased from 36.4% (postoperative 6 m) to 74.3% (postoperative 24 m). The reduction rate of Lennox-Gastaut syndrome improved from 25.4% to 73.1% in 24 mo. The chi-square test of the five efficacy grades showed P < 0.05. The comparison between the 3-mo follow-up and the 6-mo follow-up showed P < 0.05, and the comparison between the 6-mo follow-up and the 24-mo follow-up showed P > 0.05.
Vagus nerve stimulation is safe and effective in children with intractable epilepsy, and the seizure reduction occurred in a time-dependent manner. Moreover, patients with West syndrome may get the most benefits.
Core Tip: We provided a preliminary analysis of the safety and efficacy of implanted vagus nerve stimulation in the treatment of refractory epilepsy in children.
- Citation: Fang T, Xie ZH, Liu TH, Deng J, Chen S, Chen F, Zheng LL. Preliminary analysis of the effect of vagus nerve stimulation in the treatment of children with intractable epilepsy. World J Clin Cases 2020; 8(23): 5918-5925
- URL: https://www.wjgnet.com/2307-8960/full/v8/i23/5918.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i23.5918
Epilepsy is a chronic neurological disease characterized by excessive discharge of brain neurons caused by various etiologies. Children with epilepsy account for 1% to 2% of the children, which is the most common chronic neuropathic disease in children. The incidence rate of epilepsy in China is about 4% to 7%, while the annual rate is 30/100000, of which more than 50% are younger than 15-years-old. There are 20% to 30% of patients with epilepsy who cannot effectively control seizures after receiving standardized antiepileptic drugs, which progresses to drug-refractory epilepsy[1-3]. For these patients, surgical resection of epileptic foci, neuromodulation, and ketogenic diet are effective treatments[4,5]. Among them, implant vagus nerve stimulation (IVNS) is widely used in clinical practice and is a safe and effective neuromodulation treatment[6-9]. At present, more than 80000 epilepsy patients worldwide have received IVNS treatment, of which about 20000 children who were younger than 12-years-old.
IVNS is a pulse signal generated by the generator that increases the impulse of the vagus nerve on one side of the neck, inhibits the abnormality synchronized discharge of the brain neuron network and terminates or attenuates the seizure. IVNS has a certain effect on most types of seizures without considering the pathogenesis, abnormal electroencephalography (EEG), or abnormal imaging. Compared with adults, children’s vagus nerve conduction is slower and often requires higher current intensity. The width of pulse required for signaling to the cortex is longer because children’s vagus nerve does not contain myelin. A study showed that IVNS can reduce the frequency of seizures, and the overall effect on children is better than adults. The seizure reduction rate in children was more than 90% is higher than in adults[7]. In 2013, the American Academy of Neurology published a guideline for IVNS treatment of epilepsy and recommended indications for children with epilepsy, Lennox-Gastaut (LG) syndrome, and adult epilepsy with depression[10]. This study will provide a preliminary analysis of the safety and efficacy of IVNS in the treatment of refractory epilepsy in children.
This study was a single-center retrospective study of children with refractory epilepsy who underwent IVNS in our hospital. The safety and efficacy of IVNS in the treatment of refractory epilepsy in children was analyzed.
Subjects were selected to meet the following criteria: (1) Age less than 18 years; (2) Taking two or more regular combined antiepileptic drugs, seizure > 4 times/mo, duration > 2 years; and (3) Multifocal or diffusive discharge seizures, surgery cannot remove the lesion or patients with epilepsy who are ineligible for reoperation after surgery. Four families refused resection surgery and required IVNS surgery.
Baseline was collected from all subjects prior to IVNS and was performed by a physician from our epilepsy center following the standard procedures. The recorded information included: (1) General: name, gender, age, past medical history, family history; (2) Seizures: onset time, aura, type, duration, frequency; (3) Video EEG; (4) Medication: type, dose, medication time, adverse reactions; and (5) Brain magnetic resonance imaging/computed tomography examination, Wechsler intelligence scale.
This study used the vagus nerve stimulator produced by Cyberonics (Houston, TX, United States). The pulse generator model is Type 102; the wire type is Type 302.
The implantation is a standard clinical procedure. The surgery was performed on the left vagus nerve. The patient’s supine head was slightly to the right, and the neck and shoulders were appropriately raised to fully expose the neck. The left anterior midline was parallel to the striate transverse incision about 3 cm. The skin, subcutaneous, and cervical fascia were incised, and the carotid artery pulsation was touched. The carotid sheath was opened by blunt dissection, and the internal carotid artery, vagus nerve, and internal jugular vein were identified. Three times under the surgical magnifying glass, the vagus nerve trunk is about 3 cm. The left subclavian parallel skin pattern was slightly curved 4 cm separated into the surface of the pectoralis major fascia, and the skin capsule was made to open the neck and chest tunnel. The fixed electrode was carefully wrapped, the stimulating electrode was placed around the vagus nerve, and both were fixed on the carotid sheath and neck muscle respectively. The wire was maintained with a certain degree of slack through two U-shaped sputum to prevent the wire from falling off during neck movement. On the pulse generator, we tested that the wire impedance was not greater than 1, and the minimum stimulation parameter was turned on during surgery. We checked for no bleeding, correct equipment, and that the slits are in turn.
The pulse generator was turned on during the operation. The initial output current was set to 0.25 mA, signal frequency 30 Hz, pulse width 250 ms, signal start time 30 s, and signal off time 5 min. The patient feels that before or during the attack, and magnetism can be used to enhance the stimulation. The magnet output current is 0.5 mA, the start-up time is 60 s, and the pulse width is 500 ms.
The instrument parameters, drug types, and drug dose adjustments were completed by the specialists of the epilepsy center of the hospital, and the patients were followed up once every 3 mo. Patients and their families recorded the epilepsy diary in detail. The specialists recorded the seizure, medication, and malnutrition at 1 mo postoperatively, 3 mo postoperatively, 6 mo postoperatively, 9 mo postoperatively, and 12 mo postoperatively. Reaction, etc., was recorded in the epilepsy diary. The follow-up time of this group of children was more than 3 mo.
The rate of seizure reduction = (baseline frequency - postoperative frequency) / baseline frequency. The mean frequency of episodes (times/mo) 3 mo before treatment was the baseline frequency, and the mean frequency of episodes (times/mo) during follow-up was the postoperative frequency. The effective rate was the percentage of patients with a reduction rate of ≥ 50% of the total number of people. The McHugh efficacy grades were as follows: Grade I, seizure frequency reduction ≥ 80%; grade II, seizure frequency reduced by 50% to 79%; grade III, seizure frequency decreased by < 50%; grade IV, benefit only when using magnets; grade V, no improvement.
Adverse events, treatment measures and outcomes were recorded during the study. All data were analyzed using SPSS 19.0 statistical software. The 2 test was used to compare the rates between different groups. P < 0.05 was considered statistically significant.
A total of 213 children (34 cases of G111 type and 179 cases of G112 type) with refractory epilepsy who were treated with Tsinghua Pinchi VNS in our hospital from January 2017 to June 2019 were included in this study. The ages of the patients ranged from 7 mo to 15-years-old, with 75% of the children aged younger than 3-years-old. The average type of medication before surgery was 2.8. Most children had mental retardation. The average attack reduction rate in the follow-up time of postoperative 3 mo, 6 mo, 12 mo, 18 mo, and 24 mo was 30.2%, 49.5%, 56.3%, 59.4%, and 63.2% (Figure 1). The response rate was 21.8%, 62.5%, 57.1%, 69.2%, and 70.7% (Figure 2).
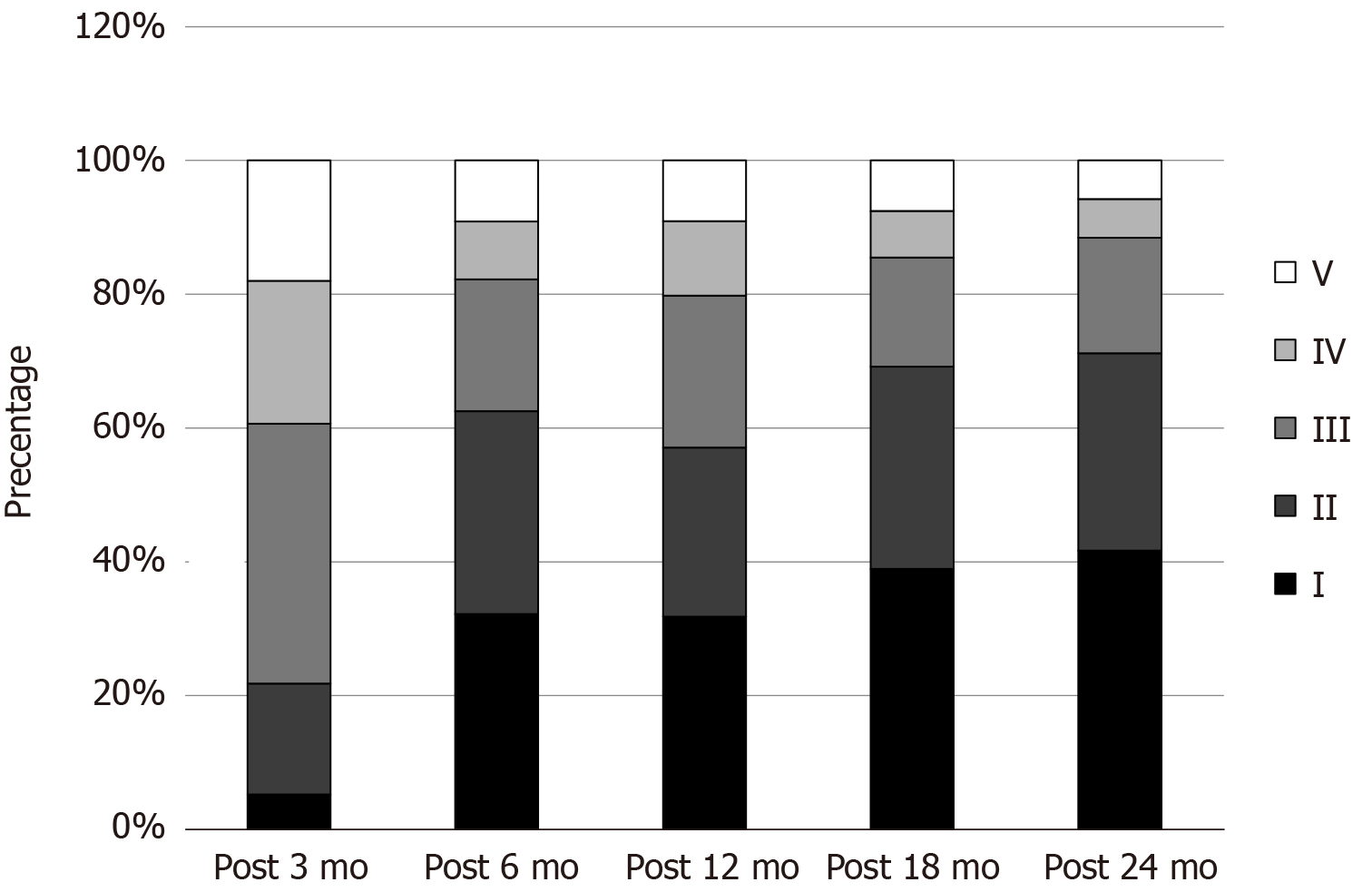
According to the McHugh classification, the chi-square test of the five efficacy grades showed P < 0.05. The comparison between the 3-mo follow-up and the 6-mo follow-up was significant (P < 0.05), and the comparison between the 6-mo follow-up and the 24-mo follow-up was significant (P > 0.05). It showed that the curative effect gradually improved with time. The curative effect was significantly better at 6 mo after surgery than at 3 mo after surgery. Although there was an improvement in 24 mo after surgery compared with 6 mo after surgery, there was no statistical difference (Figure 3). A larger sample size may still be needed.
There were a few surgical complications. One of the 213 patients had a subcutaneous hematoma after implantation of the electrode generator after operation. The hematoma was absorbed after compression dressing, which did not cause other adverse reactions. One patient had the stimulator removed due to an infection. Some patients had a hoarse voice and symptoms of dysphagia after starting up, but the symptoms disappeared after 1 wk.
The essence of epilepsy is in the abnormal activity of the intracranial neural network caused by the excessively synchronized discharge of neurons. The specific mechanism of IVNS currently remains unclear. Antiepileptic effects are generally believed to be exerted by causing synchronization or desynchronization of brain electrical activity, long-term changes in neurotransmitter concentration, and redistribution of cerebral blood flow. In addition, the brain network topology of epileptic seizures deviates from normal anatomy, and vagus nerve stimulation can promote brain network structure reconstruction and inhibit seizures[11].
In recent years, the treatment of IVNS in children with refractory epilepsy has gradually increased[12]. Morris reported that 55% of children with refractory epilepsy had a reduction rate of more than 50% after IVNS[13]. A retrospective cohort study by Fernandez et al[14] showed that the number of epileptic seizures decreased in children 1 yr after IVNS, but the overall number of episodes was not significantly reduced. Majkowska-Zwolińska et al[15] and other studies suggested that IVNS can significantly reduce the sudden death due to epilepsy.
In this study, the average rate of seizure reduction was 66.6% 12 mo after surgery, and the effective rate was 77.8%, indicating that IVNS was effective in treating refractory epilepsy and could reduce seizures by more than half in 70% of patients.
IVNS reduces the frequency of seizures and gradually increases the therapeutic effect by prolonging the treatment time. After 2 years of follow-up of 347 children with refractory epilepsy, Orosz et al[16] found that patients with a seizure reduction rate greater than 50% accounted for 32.5%, 37.6%, and 43.8% at 6 mo, 12 mo, and 24 mo, respectively. However, Galbarriatu et al[12] believed that from 3 mo after surgery to 15 years after surgery, the average rate of reduction in patients was maintained at 30% to 32%, and the rate of reduction of seizures was greater than 50%, which accounted for 34.48%. They found that there was no significant increase in the therapeutic effect as the treatment time for IVNS was prolonged. In this study, the average rate of reduction in seizure episodes gradually increased from 8.8% 1 mo postoperatively to 56.6% 12 mo postoperatively, and the effective rate increased from 20% to 78%, suggesting that the efficacy of refractory epilepsy with IVNS treatment in children has a cumulative effect as the treatment time is prolonged.
The McHugh classification is an important criterion for evaluating postoperative outcomes in patients with epilepsy. Meng et al[17] performed a McHugh classification of 94 patients with refractory epilepsy after 6 years of IVNS. Grade I patients accounted for 35.5%, and 11 patients accounted for 28.7%, of whom 8.5% were completely seizure free. In this study, the proportion of McHugh grade I patients increased from 6.7% 1 mo postoperatively to 44.4% 12 mo postoperatively. The proportion of McHugh grade II patients gradually increased from 13.3% to 33.3%. The proportion of grade V patients gradually decreased from 46.7% to 11.1%, which is consistent with literature reports.
The treatment and prognosis of refractory epilepsy in children are influenced by the type of seizure and epilepsy syndrome. Kossoff et al[18] found that the onset of dystonia had the best response to IVNS followed by tonic attack. Cersósimo et al[19] found that IVNS resulted in better seizure control in patients with LG syndrome (30 patients), and the average rate of seizure reduction was greater than 55%.
In this study, the mean rate of seizure reduction in patients with LG syndrome was 55.1% 6 mo postoperatively. The mean rate of seizure reduction in patients with West syndrome increased significantly with treatment duration at 12 mo postoperatively; it reached 83.3%. The study also found that IVNS had a good effect on occipital lobe epilepsy, and the rate of seizure reduction was 56.0% 3 mo after surgery. There was no significant change in the frequency of seizures in patients with Otahara syndrome, but the onset time was obviously shortened.
In our study, one of 32 patients had a subcutaneous hematoma at the location of the implanted electrode generator postoperatively, and the hematoma was absorbed after compression dressing without causing other adverse reactions. No postoperative surgical superficial or deep incision infection occurred in any of the patients. Some patients showed short-term hoarseness and dysphagia after the machine was turned on. After 1 wk, the symptoms disappeared, and there was no long-term discomfort. At present, the majority of studies[20] reported that the IVNS infection rate is approximately 0% to 8%, and the infection rate of children is significantly higher than that of adult patients. The rate of vagus nerve injury is approximately 4%. The proportion of short-term neurological impairment in children with epilepsy craniotomy is 14%.
Compared with our case, the proportion of children of a younger age was more than 80%, and the proportion of infection and nerve damage was far lower than that reported in the literature. The reason may lie in the advantages of multidisciplinary cooperation of functional neurosurgery combined with head and neck surgeons to carry out the operation. Also, the use of diluted iodophor saline in the operation reduces the occurrence of surgical complications.
The results of this study are consistent with literature reports. The limitation is that the sample size is small, and the follow-up time is short, which can only explain the initial efficacy. There is a certain bias, and randomized, double-blind studies are needed to further confirm the efficacy of IVNS.
This article is the largest single-center vagal stimulator reported to date for the treatment of childhood epilepsy. This article analyzed the clinical efficacy of this group of cases in detail, summarized the occurrence of complications, and provided detailed information. This article has practical value in promoting the application of vagus audit stimulator in children.
IVNS is effective and safe for the treatment of refractory epilepsy in children. The curative effect has a cumulative effect. Children with epilepsy syndrome, such as infantile spasms, have a good response to IVNS treatment, which is safe.
Epilepsy is a chronic neurological disease characterized by excessive discharge of brain neurons caused by various etiologies. Implant vagus nerve stimulation (IVNS) is a pulse signal generated by the generator that increases the impulse of the vagus nerve on one side of the neck and inhibits the abnormality synchronized discharge of the brain neuron network and terminates or attenuates the seizure.
This study will provide a preliminary analysis of the safety and efficacy of IVNS in the treatment of refractory epilepsy in children.
IVNS is an adjunctive treatment for intractable epilepsy where patients are not suitable for resective surgery. This research was designed to identify the safety and efficacy of vagus nerve stimulation in child intractable epilepsy and analyze the effects on different epilepsy syndromes.
Eligible children with intractable epilepsy were admitted to the research. We collected data from preoperative assessments as the baseline. During the follow-up time, we recorded the process of seizures (frequency, duration, and seizure type), the changes of drugs or parameters, the complications, etc. We chose the mean reduction rate of seizures, response rate, and McHugh scale as the indictors.
IVNS is effective and safe for the treatment of refractory epilepsy in children. The curative effect has a cumulative effect. Children with epilepsy syndrome, such as infantile spasms, have a good response to IVNS treatment, which is safe.
Vagus nerve stimulation is safe and effective in child intractable epilepsy, and the seizure reduction occurs in a time-dependent manner. Moreover, patients with West syndrome may get the most benefits.
IVNS is effective and safe for the treatment of refractory epilepsy in children, and the curative effect has a cumulative effect. More extensive cases should be selected for case-control studies.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chaturvedi P, Forrest E S-Editor: Huang P L-Editor: Filipodia P-Editor: Li JH
| 1. | Panebianco M, Bresnahan R, Ramaratnam S, Marson AG. Lamotrigine add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst Rev. 2020;3:CD001909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Liu C, Wen XW, Ge Y, Chen N, Hu WH, Zhang T, Zhang JG, Meng FG. Responsive neurostimulation for the treatment of medically intractable epilepsy. Brain Res Bull. 2013;97:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Moro-De Faes G, Serrano-Moyano B, Cantarin-Extremera V, Moreno-Vinues B, Garcia-Fernandez M, Perez-Jimenez MA, Rivero-Martin MB, Garcia-Ezquiaga J, Duat-Rodriguez A, Ruiz-Falco Rojas ML. [Ten years' experience with vagus nerve stimulation in a paediatric population]. Rev Neurol. 2018;67:382-386. [PubMed] |
| 4. | Kun Y, Zejun D, Jian Z, Feng Z, Changqing L, Xueling Q. Surgical histopathologic findings of 232 Chinese children cases with drug-resistant seizures. Brain Behav. 2020;10:e01565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Uchida D, Yamamoto T, Yamazoe T, Iijima K, Fujimoto A, Enoki H, Tanaka T. [Experience and Solution of Complications of Vagus Nerve Stimulation Therapy in 139 Implant Patients with Medically Refractory Epilepsy]. No Shinkei Geka. 2017;45:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | San-Juan D, Dávila-Rodríguez DO, Jiménez CR, González MS, Carranza SM, Hernández Mendoza JR, Anschel DJ. Neuromodulation techniques for status epilepticus: A review. Brain Stimul. 2019;12:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Pintea B, Hampel K, Boström J, Surges R, Vatter H, Lendvai IS, Kinfe TM. Extended Long-Term Effects of Cervical Vagal Nerve Stimulation on Headache Intensity/Frequency and Affective/Cognitive Headache Perception in Drug Resistant Complex-Partial Seizure Patients. Neuromodulation. 2017;20:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Flesler S, Reyes G, Fortini S, Ramos B, Cersosimo R, Bartuluchi M, Caraballo R. [Vagus nerve stimulation: treatment of 158 pediatric patients with a long-term follow-up]. Rev Neurol. 2017;64:496-501. [PubMed] |
| 9. | Benbadis SR, Geller E, Ryvlin P, Schachter S, Wheless J, Doyle W, Vale FL. Putting it all together: Options for intractable epilepsy: An updated algorithm on the use of epilepsy surgery and neurostimulation. Epilepsy Behav. 2018;88S:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Dibué-Adjei M, Kamp MA, Vogelsang J, Wiltfang J, Wolff-Menzler C. [Vagus Nerve Stimulation for Affective Disorders]. Fortschr Neurol Psychiatr. 2020;88:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Fraschini M, Demuru M, Puligheddu M, Floridia S, Polizzi L, Maleci A, Bortolato M, Hillebrand A, Marrosu F. The re-organization of functional brain networks in pharmaco-resistant epileptic patients who respond to VNS. Neurosci Lett. 2014;580:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Galbarriatu L, Pomposo I, Aurrecoechea J, Marinas A, Agúndez M, Gómez JC, Acera MA, Martínez MJ, Valle E, Maestro I, Mateos B, Cabrera A, Fernández J, Iturri F, Garamendi I. Vagus nerve stimulation therapy for treatment-resistant epilepsy: a 15-year experience at a single institution. Clin Neurol Neurosurg. 2015;137:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Morris GL 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the guideline development subcommittee of the american academy of neurology. Epilepsy Curr. 2013;13:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Fernandez L, Gedela S, Tamber M, Sogawa Y. Vagus nerve stimulation in children less than 3 years with medically intractable epilepsy. Epilepsy Res. 2015;112:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Majkowska-Zwolińska B, Zwoliński P, Roszkowski M, Drabik K. Long-term results of vagus nerve stimulation in children and adolescents with drug-resistant epilepsy. Childs Nerv Syst. 2012;28:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Orosz I, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, Griens R, Vallée L, Boon P, Rittey C, Jayewardene AK, Bunker M, Arzimanoglou A, Lagae L. Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia. 2014;55:1576-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Meng FG, Jia FM, Ren XH, Ge Y, Wang KL, Ma YS, Ge M, Zhang K, Hu WH, Zhang X, Hu W, Zhang JG. Vagus Nerve Stimulation for Pediatric and Adult Patients with Pharmaco-resistant Epilepsy. Chin Med J (Engl). 2015;128:2599-2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Helen Cross J, Dahlin MG, Donner EJ, Klepper J, Jehle RS, Kim HD, Christiana Liu YM, Nation J, Nordli DR Jr, Pfeifer HH, Rho JM, Stafstrom CE, Thiele EA, Turner Z, Wirrell EC, Wheless JW, Veggiotti P, Vining EP; Charlie Foundation; Practice Committee of the Child Neurology Society; Practice Committee of the Child Neurology Society; International Ketogenic Diet Study Group. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 343] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Cersósimo RO, Bartuluchi M, Fortini S, Soraru A, Pomata H, Caraballo RH. Vagus nerve stimulation: effectiveness and tolerability in 64 paediatric patients with refractory epilepsies. Epileptic Disord. 2011;13:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Rychlicki F, Zamponi N, Cesaroni E, Corpaci L, Trignani R, Ducati A, Scerrati M. Complications of vagal nerve stimulation for epilepsy in children. Neurosurg Rev. 2006;29:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |