Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5781
Peer-review started: August 13, 2020
First decision: August 21, 2020
Revised: August 28, 2020
Accepted: September 11, 2020
Article in press: September 11, 2020
Published online: November 26, 2020
Processing time: 103 Days and 15.2 Hours
Enteropathy-associated T-cell lymphoma (EATL) is a rare invasive lymphoma derived from gastrointestinal epithelial T lymphocytes. EATL involving the whole gastrointestinal tract accompanied with early colon cancer is extremely rare.
We present the case of a 67-year-old man with diarrhea for more than 5 mo whose colonoscopy in another hospital showed multiple colonic polyps, which indicated moderate to severe dysplasia and focal early cancer. Therefore, he was referred to our hospital for further endoscopic treatment. Colonoscopy after admission showed that the mucosa of the terminal ileum and the entire colon were slightly swollen and finely granular. Endoscopic mucosal resection was performed for colonic polyps located in the liver flexure of the colon and descending colon, respectively. Histopathological findings revealed diffuse infiltration of medium-sized lymphoid cells in the colonic mucosa and visible lymphoepithelial lesions. The histopathology of the polyp in the descending colon indicated moderately differentiated adenocarcinoma limited to the mucosa with negative resection margins. Additionally, immunohistochemical analysis showed positive staining for CD7 and CD8. Therefore, we arrived at a diagnosis of EATL with early colon cancer. Subsequently, the patient was transferred to the hematology department for chemotherapy. The patient’s diarrhea was not significantly relieved after receiving chemotherapy, and he ultimately died of severe myelosuppression.
EATL should be considered in unexplained chronic diarrhea. EATL progresses rapidly with a poor prognosis, especially when accompanied with early colon cancer.
Core Tip: Enteropathy-associated T-cell lymphoma (EATL) is a rare aggressive lymphoma of the gastrointestinal tract. We present the case of a male patient with chronic diarrhea for more than 5 mo, and colonoscopy showed multiple polyps with the pathology of early colonic cancer. Endoscopic mucosal resection was performed. Postoperative histopathological and immunohistochemistry results suggested EATL with moderately differentiated adenocarcinoma confined to the mucosa. This is a rare case of EATL involving the whole colon, duodenum, and small intestine, complicated with early colon cancer. Nonspecific clinical symptoms and typical endoscopy appearance pose difficulty in making an accurate diagnosis. More attention should be paid to the swollen colonic mucosa with a granular appearance in unexplained chronic diarrhea to avoid misdiagnosis.
- Citation: Zhang MY, Min CC, Fu WW, Liu H, Yin XY, Zhang CP, Tian ZB, Li XY. Early colon cancer with enteropathy-associated T-cell lymphoma involving the whole gastrointestinal tract: A case report. World J Clin Cases 2020; 8(22): 5781-5789
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5781.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5781
Enteropathy-associated T-cell lymphoma (EATL) is a rare aggressive lymphoma derived from gastrointestinal epithelial T lymphocytes[1]. This lymphoma has a very low incidence and comprises less than 1% of all malignant lymphomas. It presents with a high malignancy and poor prognosis and progresses rapidly. EATL mainly occurs in the small intestine, especially the proximal jejunum, and is rarely found in the stomach and colorectum. The case of EATL occurring in the entire colon with early colon cancer is extremely rare. Here, we report a case of EATL discovered after endoscopic treatment of early colon cancer that involved the whole colon with suspected duodenum and small bowel involvement. We also performed a literature review of EATL.
A 67-year-old Chinese man was referred to our hospital with diarrhea for more than 5 mo and for management of early colon cancer discovered by colonoscopy in another hospital.
The patient visited a hospital for the first time due to diarrhea of yellow watery stool 8-9 times per day on December 20, 2018. Colonoscopy suggested colon polyps with a pathology of tubular adenoma. No significant improvement was observed in the symptoms after antidiarrheal treatment. The patient underwent colonoscopy in another hospital again, which revealed multiple polyps in the liver curvature of the colon, cecum, and descending colon on April 23, 2019. The biopsy pathology of the polyp (liver curvature) in our hospital’s consultation indicated villous adenoma, and some glands showed moderate-to-severe dysplasia with focal early carcinogenesis, roughly showing in situ adenocarcinoma changes. Therefore, the patient was admitted to our hospital for evaluation and treatment of early colon cancer.
The patient underwent right lung nodule resection 1 year prior and lumbar disc herniation surgery 28 years ago. He had no history of other diseases or allergic drugs. He had a smoking history of 30 years and had quit smoking for 8 years. His younger brother died of lung cancer. The patient had no familial history of genetic diseases.
The patient’s personal and family history was unremarkable.
On physical examination upon admission, the patient’s height and weight were 160 cm and 55 kg, respectively, and he had a blood pressure of 96/58 mmHg and pulse rate of 80 beats/min. There were no other pathognomonic signs during physical examination, except for enhanced bowel sounds at 8 beats/min.
After admission, the patient underwent thorough evaluations including routine blood tests, routine urine tests, routine fecal tests, occult blood tests, blood biochemistry, infection indices, and serum tumor markers. He had hypoproteinemia and hypokalemia with serum albumin and potassium levels of 29.61 g/L and 3.37 mmol/L, respectively. The C-reactive protein level was 7.79 mg/L. His erythrocyte sedimentation rate and white blood cell count were normal. He had a slightly elevated carcinoembryonic antigen level of 5.11 ng/mL. The levels of IgG antibodies to Epstein-Barr virus capsid and nuclear antigen were more than 50.00 and 7.42 AU/mL, respectively. The antinuclear antibody was positive, with a titer of 1:3200. Immunoglobulin E significantly increased with a value of 265.30 IU/mL. The fecal occult blood test results were negative, and stool flora analysis was normal. No other significant abnormal laboratory results were recorded in this patient.
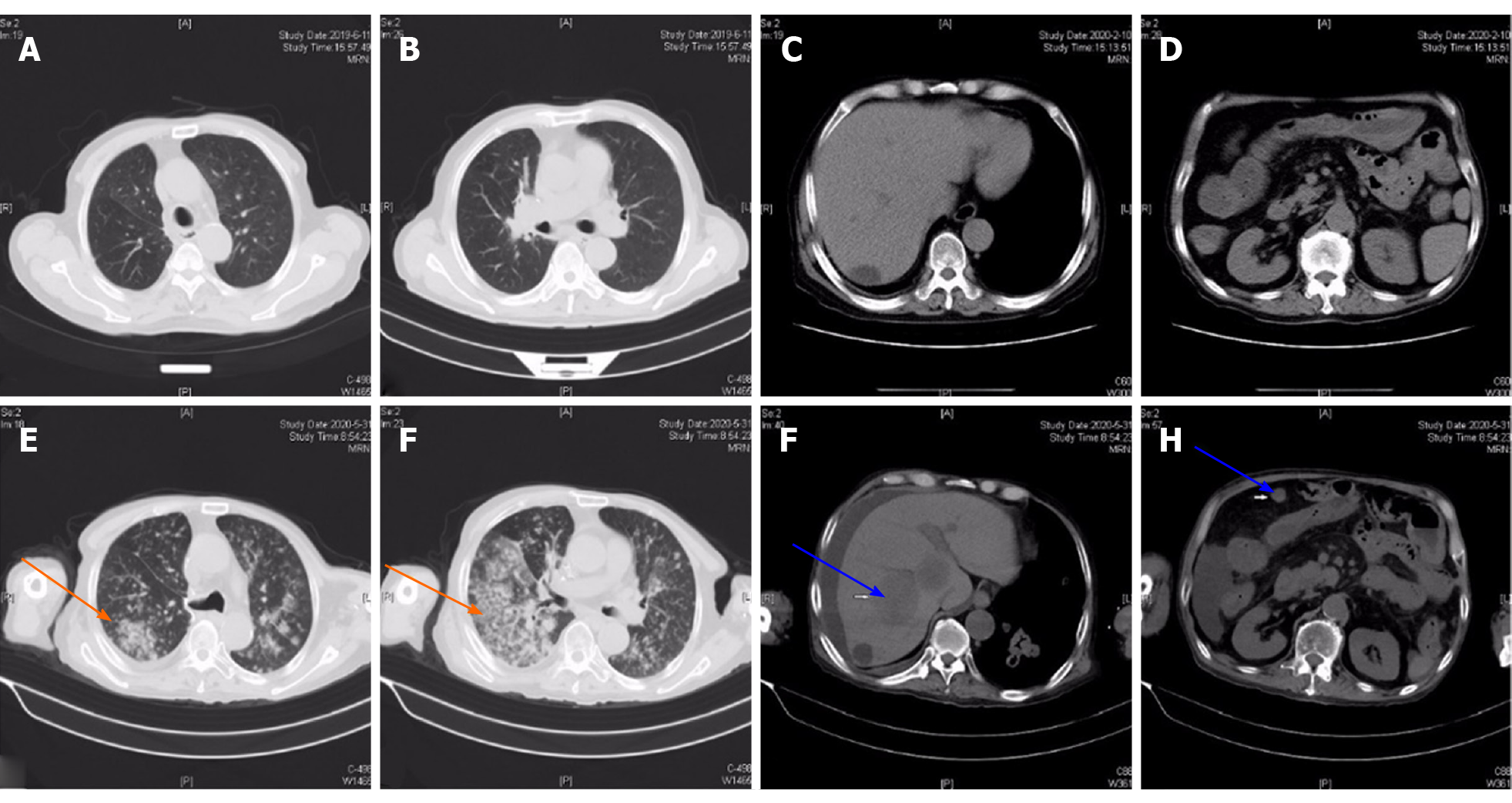
Chest computed tomography (CT) showed a clear lung field (Figure 1A and B). We observed a liver cyst and part of the small intestine that was slightly dilated on abdominal CT (Figure 1C and D). Chest CT on April 14, 2020 revealed new nodules in the lower lobe of both lungs. However, multiple masses in both lungs had progressed more than before excluding tumorous lesions on chest CT on May 31, 2020 (Figure 1E and F). Abdominal CT revealed multiple nodules in the right lobe of the liver and multiple soft tissue nodules in the abdominal cavity with a greater possibility of tumor and ascites, and the colorectal wall was slightly thickened (Figure 1G and H).
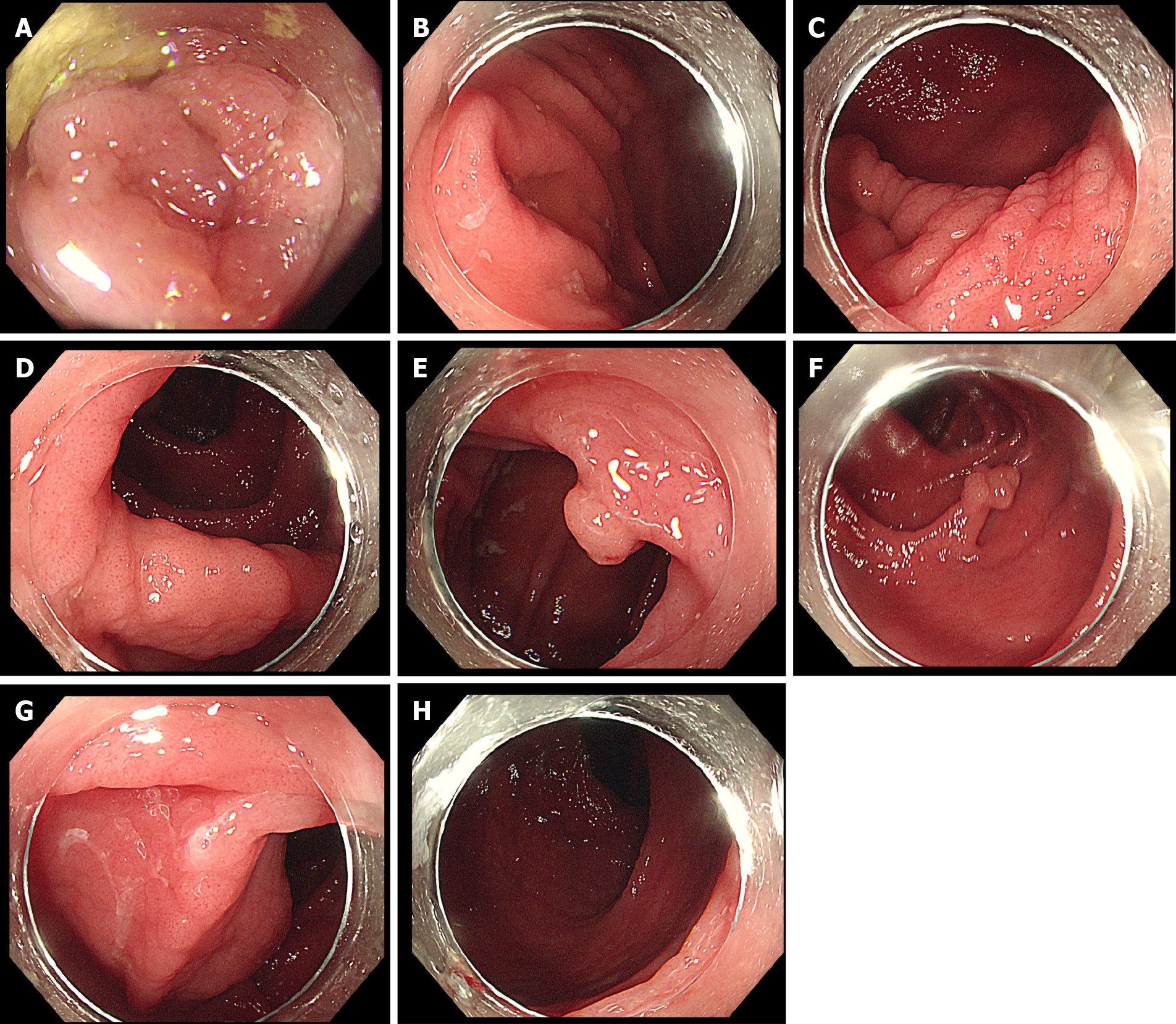
Colonoscopy in our hospital revealed a hemispherical polyp with a diameter of approximately 5 mm in the liver curve and a long-pedicle polyp in the descending colon, with a lobular pattern of 10 mm in diameter at the tip. At the same time, it was found that the terminal ileum, ileocecal valve, and entire colonic mucosa were slightly swollen, with pit patterns of types I-II, which were finely grained (Figure 2 ).
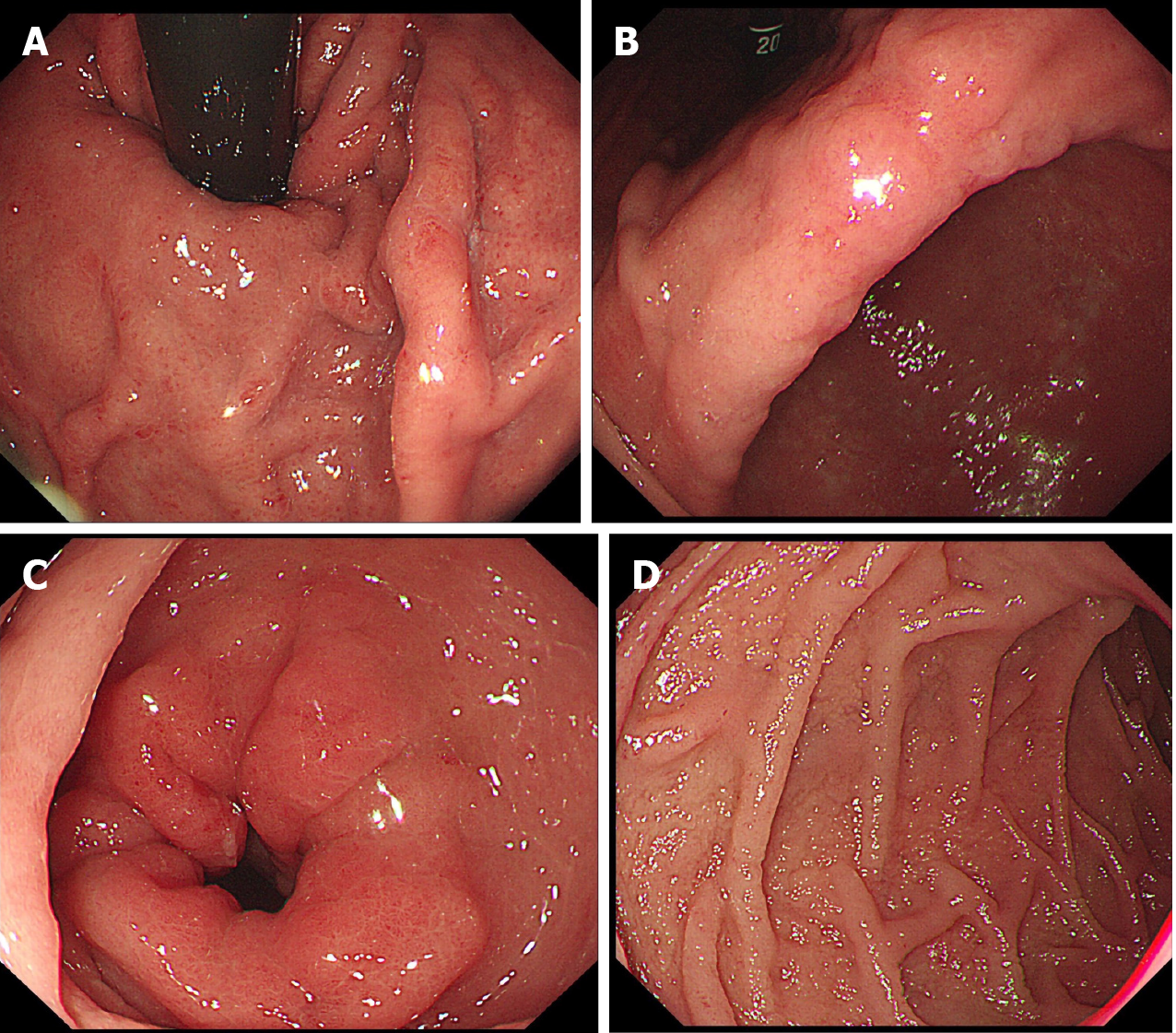
In addition, gastroscopy showed swelling of the whole gastric mucosa and flaky erosions in the gastric angle and antrum with fine granular mucosa. The mucosa of the descending duodenum was also swollen in the form of fine granules (Figure 3 ).
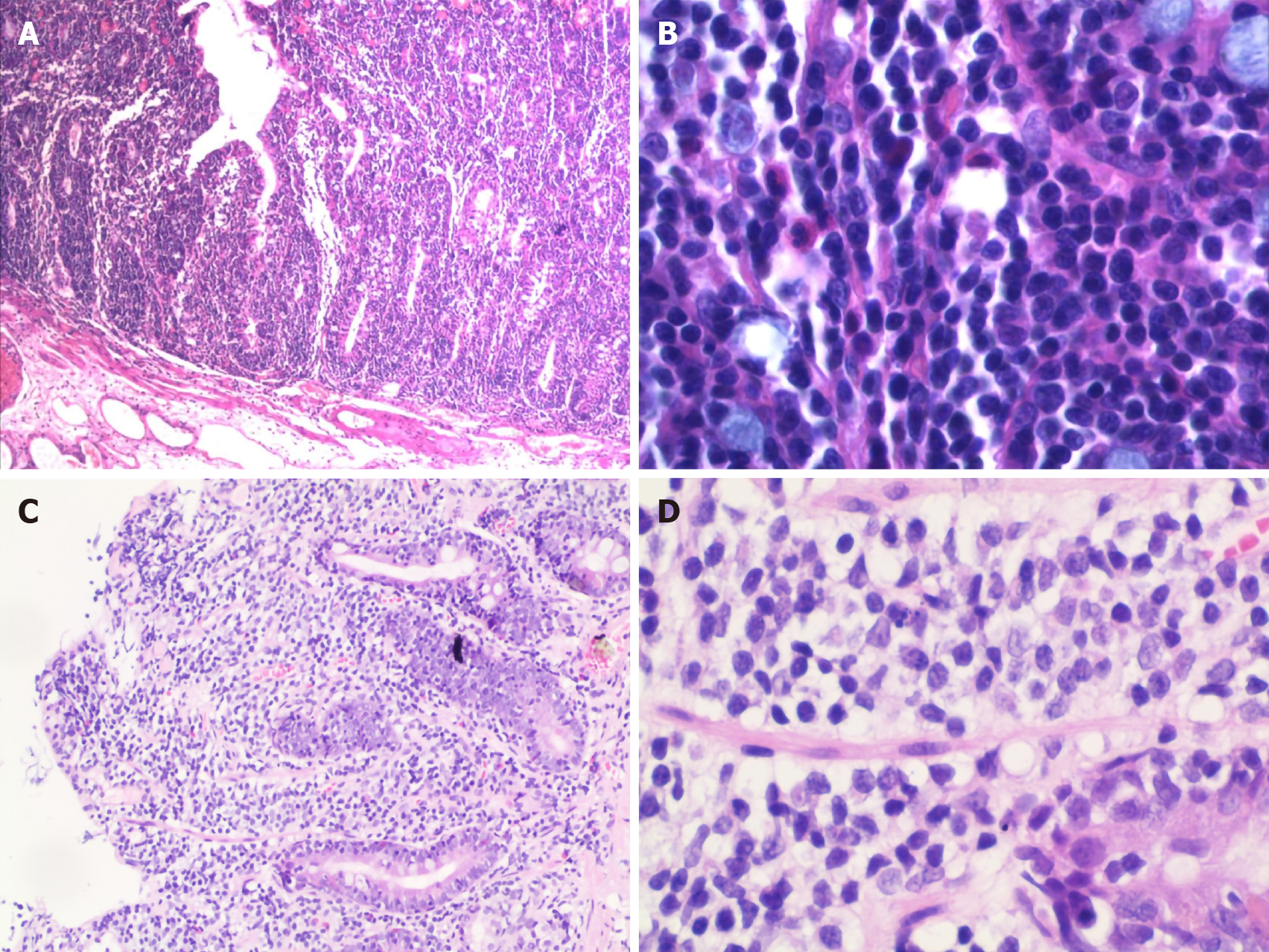
After admission, we performed endoscopic mucosal resection to remove the two polyps located in the liver curve of the colon and descending colon, respectively. Simultaneously, the entire colonic mucosa was swollen and finely granular. Routine hematoxylin and eosin staining revealed tubular adenoma with diffuse proliferation of atypical lymphocytes in the mucosal layer in the polyp of the liver curve.
The polyp of the descending colon was diagnosed as villous adenoma with focal carcinoma–adenocarcinoma (moderately differentiated) limited to the mucosal layer. However, the high-magnification view showed that diffusely proliferated atypical lymphocytes infiltrated mainly in the mucosal epithelium and lamina propria, partially invading the submucosa. The lymphocytes had a relatively simple morphology and medium-sized cell bodies containing few cytoplasm and large oval or irregular nuclei with some visible nucleoli (Figure 4). Additionally, immuno-histochemical staining was positive for CD3, CD7, CD8, CD43, and Bcl-2 but negative for CD20, CD4, and CD56, and the Ki-67 index was approximately 80%. In situ hybridization, Epstein-Barr virus-encoded RNA, and IGH, IGK, and IGL rearrangement were negative.
Based on the clinical history, endoscopic performance, and histopathological investigations, the patient was diagnosed with EATL and early colon cancer.
The patient was transferred to the hematology department on June 24, 2019 for further treatment. He received 12 chemotherapy cycles including five cycles of CHOP chemotherapy regimen (cyclophosphamide 1 g on day 1; vindesine 4 mg on day 1; doxorubicin liposome 60 mg on day 1; and dexamethasone 20 mg on days 1-5), two cycles of GeMoxED chemotherapy regimen (gemcitabine 1.8 g on day 1; oxaliplatin 0.2 g on day 1; etoposide 100 mg on days 1-4; and dexamethasone 40 mg on days 1-4), and subsequent five chemotherapy cycles in which different chemotherapy drugs were administered according to different clinical conditions. PET-CT on November 1, 2019 showed increased segmental metabolism in the small intestine, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum lumen, with an SUVmax of approximately 6.6.
During the chemotherapy period, the patient’s diarrhea was significantly relieved. He developed severe bone marrow suppression and perianal abscess and died on June 2, 2020.
EATL is a rare peripheral non-Hodgkin T-cell lymphoma originating from T lymphocytes in the intestinal epithelium. It has a lower incidence and accounts for only 5.4% of all lymphomas. EATL is most common in Europe (9.1%), followed by North America (5.8%) and Asia (1.9%). EATL is divided into two types according to the World Health Organization: Classic (type 1) and single-phase (type 2)[1]. EATL1 is characterized by diverse cell morphology and negativity for CD56 and 1Q or 5Q chromosome gain, which is closely related to celiac disease and HLA-DQ2 haploidy [2]. EATL2, a monomorphic epitheliotropic intestinal T-cell lymphoma, has a medium-sized cell body with a simple morphology[3]. This type of EATL has a unique immunophenotype: Positivity for CD3, CD8, CD56, CD103, TCRβ, MYC oncogene locus gain, and rare gains of 1Q and 5Q chromosomes[4]. This patient was diagnosed with EATL1.
The clinical manifestations of EATL lack specificity, which is also one of the reasons for misdiagnosis. Abdominal pain, fatigue, and anorexia are the most common symptoms of EATL[5,6]. There was no obvious difference in clinical manifestations between EATL1 and EATL2[7]. Few patients have hepatosplenomegaly and itching, and some present with acute abdominal diseases such as bowel perforation and bowel obstruction[8,9]. However, diarrhea was the main manifestation in this patient. Colonoscopy was performed twice before the final diagnosis, only indicating colon polyps and inflammation. Diarrhea was not significantly relieved after taking antidiarrheal drugs and probiotics. After resection of the colon polyps, the patient was pathologically confirmed to have EATL. Therefore, the patient’s diarrhea was caused by EATL. EATL complicated with colon polyp carcinogenesis is extremely rare. In our case, the long-pedicle polyp in the descending colon was pathologically villous adenoma with malignant component, which was diagnosed as early colon cancer. However, whether mucosal heterotypic lymphocyte infiltration in patients with EATL is more likely to cause polyps to undergo malignant transformation has not been reported in the literature.
It is difficult to diagnose EATL using endoscopy[10]. EATL most frequently occurs in the small intestine (mainly jejunum) and occasionally other gastrointestinal sites, such as the colon or stomach[8]. Abdominal CT in this case revealed that part of the small intestine in the abdominal cavity was slightly dilated, indicating the possible involvement of the small intestine. However, due to rapid disease progression and poor general condition, endoscopy or capsule endoscopy was not performed during diagnosis and treatment. EATL usually manifests as multiple raised ulcers or nodules under endoscopy, and a few manifested as large masses that often infiltrate the mesenteric and mesenteric lymph nodes. It is also reported that diffuse mucosal thickening, edema, and ulcers can be observed on endoscopy. There are numerous coarse or fine particles on the diffusely thickened mucosa, the so-called mosaic sign[11-13]. In this patient, the obvious lesions occurred in the colon, which manifested as obvious congestion and swelling of the terminal ileum and entire colonic mucosa, with slightly thickened surface capillaries with no ulcers and nodules on colonoscopy. Gastroscopy showed that the duodenal mucosa was swollen and finely granular. Diffuse atypical lymphocytes were infiltrated in the lamina propria in the pathology. In addition, abdominal CT showed slight dilation and effusion of part of the small intestine in the abdominal cavity. PET-CT revealed increased strip metabolism in the upper and middle esophagus and increased segmental strip metabolism in the small intestine. In summary, the results of all auxiliary examinations indicated that EATL widely involved the gastrointestinal tract. In addition to the definite lesions of the total colon, the duodenum and small intestine were also likely to be involved.
The diagnosis of EATL mainly depends on the pathology and immuno-histochemistry results[14]. At the same time, it needs to be differentiated from the following diseases: (1) Peripheral intestinal T-cell lymphoma generally does not show symptoms of bowel disease; (2) In intestinal B-cell lymphoma, especially diffuse large B-cell lymphoma, positive B lymphocyte markers such as CD20 can contribute to distinguishing it from EATL; (3) Extranodal NK/T-cell lymphoma is distinctly characterized by positivity for NK (CD2) and EBV markers[15,16]. Most NK(T) cell lymphomas are derived from NK cells and lack TCR rearrangement, while EATL has TCR rearrangement[17,18]; and (4) Patients with inflammatory bowel disease and Behcet’s disease often have ulcers on colonoscopy and specific histopathological manifestations.
Currently, there are no standardized chemotherapy regimens for the treatment of EATL. The National Comprehensive Cancer Network recommends first-line treatment options for EATL. Currently, there are no standardized chemotherapy regimens for the treatment of EATL. The following options may be considered, including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone); CHOP or CHOP-14 with or without etoposide; CHOP followed by IVE (ifosfamide, vincristine, etoposide); Dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin); HyperCVAD alternating with high-dose methotrexate and cytarabine, according to NCCN recommended first-line treatment plans for Non-Hodgkin’s Lymphomas[19].
It is reported that patients may benefit from adding brentuximabvedotin (anti-CD30) to routine chemotherapy[20]. This patient received CHOP and GeMoxED chemotherapy therapy for 1 year in the hematology department after the diagnosis of EATL. However, his diarrhea symptoms were not obviously relieved during the chemotherapy period. He was not reexamined by colonoscopy, but abdominal CT showed that the colorectal intestinal wall was slightly thickened with no obvious ulcers. Unfortunately, the patient developed secondary infection and serious bone marrow suppression due to chemotherapy. The wide range of involvement resulted in poor treatment outcomes, and the patient died of secondary infection and multiple organ failure.
We present a rare case of EATL involving the whole colon, duodenum, and small intestine, complicated with early colon cancer. Nonspecific clinical symptoms pose difficulty in making an accurate diagnosis. The disease was delayed for 5 mo because two colonoscopies in other hospitals only revealed colonic polyps. Endoscopic resection of polyps was subsequently performed to confirm the final diagnosis. During chemotherapy, his diarrhea did not improve significantly, and multiple metastatic tumor lesions in the lungs, liver, and abdominal cavity appeared in a short time. Ultimately, the patient died of multiple organ failure and secondary infection resulting from severe bone marrow suppression. He did not undergo another colonoscopy to evaluate changes in the colon mucosa. However, we speculate that this patient was characterized by mucosal infiltration based on the abdominal CT 1 mo before his death, presenting a diffusely thickened colorectal wall without ulcers. In the future, lymphoma should be taken into consideration when encountering repeated diarrhea and swollen colonic mucosa with a granular appearance, particularly when symptomatic treatment does not yield satisfactory results. It is necessary to strengthen the follow-up of such patients and accumulate experience to avoid misdiagnosis.
We thank all the authors for helping with the writing and publication of this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demir Y S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117:5019-5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1442] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 2. | Ondrejka S, Jagadeesh D. Enteropathy-Associated T-Cell Lymphoma. Curr Hematol Malig Rep. 2016;11:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Deleeuw RJ, Zettl A, Klinker E, Haralambieva E, Trottier M, Chari R, Ge Y, Gascoyne RD, Chott A, Müller-Hermelink HK, Lam WL. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 4. | Chander U, Leeman-Neill RJ, Bhagat G. Pathogenesis of Enteropathy-Associated T Cell Lymphoma. Curr Hematol Malig Rep. 2018;13:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Sun ZH, Zhou HM, Song GX, Zhou ZX, Bai L. Intestinal T-cell lymphomas: a retrospective analysis of 68 cases in China. World J Gastroenterol. 2014;20:296-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | van de Water JM, Cillessen SA, Visser OJ, Verbeek WH, Meijer CJ, Mulder CJ. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol. 2010;24:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Chandesris MO, Malamut G, Verkarre V, Meresse B, Macintyre E, Delarue R, Rubio MT, Suarez F, Deau-Fischer B, Cerf-Bensussan N, Brousse N, Cellier C, Hermine O. Enteropathy-associated T-cell lymphoma: a review on clinical presentation, diagnosis, therapeutic strategies and perspectives. Gastroenterol Clin Biol. 2010;34:590-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL, Müller-Hermelink K, Rüdiger T, Coiffier B, Gascoyne RD, Berger F, Tobinai K, Au WY, Liang R, Montserrat E, Hochberg EP, Pileri S, Federico M, Nathwani B, Armitage JO, Weisenburger DD. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 9. | Tse E, Gill H, Loong F, Kim SJ, Ng SB, Tang T, Ko YH, Chng WJ, Lim ST, Kim WS, Kwong YL. Type II enteropathy-associated T-cell lymphoma: a multicenter analysis from the Asia Lymphoma Study Group. Am J Hematol. 2012;87:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Hong YS, Woo YS, Park G, Lee K, Kang SH, Lee HW, Kim ER, Hong SN, Chang DK, Kim YH, Rhee PL, Kim JJ. Endoscopic Findings of Enteropathy-Associated T-Cell Lymphoma Type II: A Case Series. Gut Liver. 2016;10:147-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Yanai S, Matsumoto T, Nakamura S, Fujisawa K, Ueki T, Hirahashi M, Yao T, Iida M. Endoscopic findings of enteropathy-type T-cell lymphoma. Endoscopy. 2007;39 Suppl 1:E339-E340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Joyce AM, Burns DL, Marcello PW, Tronic B, Scholz FJ. Capsule endoscopy findings in celiac disease associated enteropathy-type intestinal T-cell lymphoma. Endoscopy. 2005;37:594-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Sato Y, Ono M, Sagawa T, Takimoto R, Hirakawa M, Ohnuma H, Sato T, Iyama S, Murase K, Miyanishi K, Kobune M, Kato J. Endoscopic findings of enteropathy-type T-cell lymphoma by double-balloon enteroscopy and capsule endoscopy. Dig Endosc. 2010;22:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Tomita S, Kikuti YY, Carreras J, Kojima M, Ando K, Takasaki H, Sakai R, Takata K, Yoshino T, Bea S, Campo E, Nakamura N. Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Mod Pathol. 2015;28:1286-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Suzuki R. NK/T-cell lymphomas: pathobiology, prognosis and treatment paradigm. Curr Oncol Rep. 2012;14:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ishida F, Kwong YL. Diagnosis and management of natural killer-cell malignancies. Expert Rev Hematol. 2010;3:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Kabul S, Uğraş N, Yerci Ö, Öztürk E. Perforation of the small intestine caused by enteropathy-associated T cell lymphoma. Turk J Surg. 2018;34:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Tomita S, Kikuti YY, Carreras J, Nakamura N. Monomorphic epitheliotropic intestinal T-cell lymphoma with T-cell receptor (TCR) of silent phenotype shows rearrangement of TCRβ or TCRγ gene. Pathol Int. 2019;69:117-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, Byrd JC, Czuczman MS, Fayad LE, Glenn MJ, Gockerman JP, Gordon LI, Harris NL, Hoppe RT, Horwitz SM, Kelsey CR, Kim YH, LaCasce AS, Nademanee A, Porcu P, Press O, Pro B, Reddy N, Sokol L, Swinnen LJ, Tsien C, Vose JM, Wierda WG, Yahalom J, Zafar N. Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2011;9:484-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Nijeboer P, Malamut G, Mulder CJ, Cerf-Bensussan N, Sibon D, Bouma G, Cellier C, Hermine O, Visser O. Enteropathy-associated T-cell lymphoma: improving treatment strategies. Dig Dis. 2015;33:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |