Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5773
Peer-review started: September 7, 2020
First decision: September 23, 2020
Revised: September 30, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: November 26, 2020
Processing time: 79 Days and 12.8 Hours
Stroke is one of the leading causes of death and disability worldwide. In patients suffering from strokes and other acute brain injuries, the prevalence of pituitary dysfunction is high, and growth hormone deficiency is commonly found. Previous studies have demonstrated that administration of recombinant human growth hormone provides adult growth hormone deficiency (AGHD) patients with beneficial effects such as improving body compositions and quality of life. Nevertheless, other physiological benefits of growth hormone substitution are still controversial and inconclusive.
A female with a history of hypertension suffered intracranial hemorrhage, intraventricular hemorrhage, and hydrocephalus at 56 years of age. Her mobility, fluency of speech, and mentality were impaired ever since the event occurred. After five years, the 61-year-old patient was further diagnosed with AGHD and received six-month growth hormone replacement therapy (GHRT). After six months of GHRT, the patient’s body composition was improved. A substantial improvement in Mini-Mental State Examination score was also observed, accompanying with ameliorations in mobility, fluency of speech, and mentality.
In addition to improvements in body composition, GHRT for AGHD may provide further beneficial effects in patients with cognitive or motor impairments due to intracerebral hemorrhage.
Core Tip: We present a case report of a female patient suffered nontraumatic intracerebral hemorrhage and was diagnosed with adult growth hormone deficiency five years later. Unexpected improvements in cognitive function, fluency of speech, and mobility were observed after six months of growth hormone replacement therapy, suggesting that growth hormone replacement therapy may provide further beneficial effects in adult growth hormone deficiency patients with cognitive or motor impairments due to intracerebral hemorrhage.
- Citation: Liu JT, Su PH. Amelioration of cognitive impairment following growth hormone replacement therapy: A case report and review of literature. World J Clin Cases 2020; 8(22): 5773-5780
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5773.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5773
Stroke remained one of the leading causes of death throughout the world and was among the top ten diseases contributing to years lived with disability in 2010[1]. The long-term disability caused by stroke results in immense health and economic burdens. As a subtype of stroke, nontraumatic intracerebral hemorrhage (ICH) accounts for around 10% of all strokes, with hypertension being the most significant risk factor[2]. The long-term neurologic sequelae of stroke can result in significant impacts on the patients’ cognitive function, motor performance, and quality of life.
Growth hormone (GH) plays a crucial role in regulating physiological and metabolic status in adults. Development of neuropsychiatric-cognitive, cardiovascular, neuromuscular, metabolic, and skeletal problems can be observed in adult patients with growth hormone deficiency[3]. While it is known that pituitary tumors, craniopharyngioma, and idiopathic GHD are major causes of adult growth hormone deficiency (AGHD)[4,5], increased number of studies have shown that stroke and other acute brain injuries[6], including traumatic brain injury (TBI) and subarachnoid hemorrhage, are among the etiologies of AGHD[3,7].
GH replacement therapy (GHRT) has been in clinical use for three decades[8] and has been reported to normalize AGHD-related signs and symptoms, including depression, anxiety, fatigue, lack of strength, and altered body composition[3]. Benefits such as improved voluntary physical activity and quality of life[9,10], increased lean body mass, reduced fat mass[11,12], and improved lipid profile[13] have been demonstrated in patients receiving GHRT. Nevertheless, other than improvements in body composition, the beneficial effects of GHRT in AGHD patients are mainly inconclusive. Moreover, studies regarding GHRT for patients suffering from ICH are scarce. Herein, we report a female patient who was diagnosed with AGHD five years after the event of ICH occurred. It is encouraging that her cognitive function, fluency of speech, and mobility drastically improved after six months of GHRT. The results of body composition, serum insulin-like growth factor-1 (IGF-1) level, and lipid profiles were also presented.
In late 2017, a 61-year-old female patient visited the clinic for regular follow-up. The patient’s consciousness was clear, but with dullness and impaired cognitive function.
In 2013, the patient was brought to the emergency department after losing control of her bike because of sudden-onset right-sided limb weakness. The physical examination revealed a glasgow coma scale (GCS) of nine (E3M5V1). Brain computed tomography showed left putaminal intracranial hemorrhage, intraventricular hemorrhage, and hydrocephalus. Following surgical interventions, the patient (GCS of 14 [E4M6V4]) was discharged and transferred to another hospital for rehabilitation. In the following four years, the patient continuously received intensive rehabilitation therapy. Although her deep tendon reflex increased during follow-up, the patient’s right-sided hemiparesis remained and continuously affected her right dominant side.
The patient had a history of hypertension and hyperglyceridemia.
The patient’s physical examination revealed no remarkable findings.
The glucagon stimulation test showed a significantly reduced GH (< 3 μg/L).
The patient was diagnosed with GHD, as examined with the glucagon stimulation test.
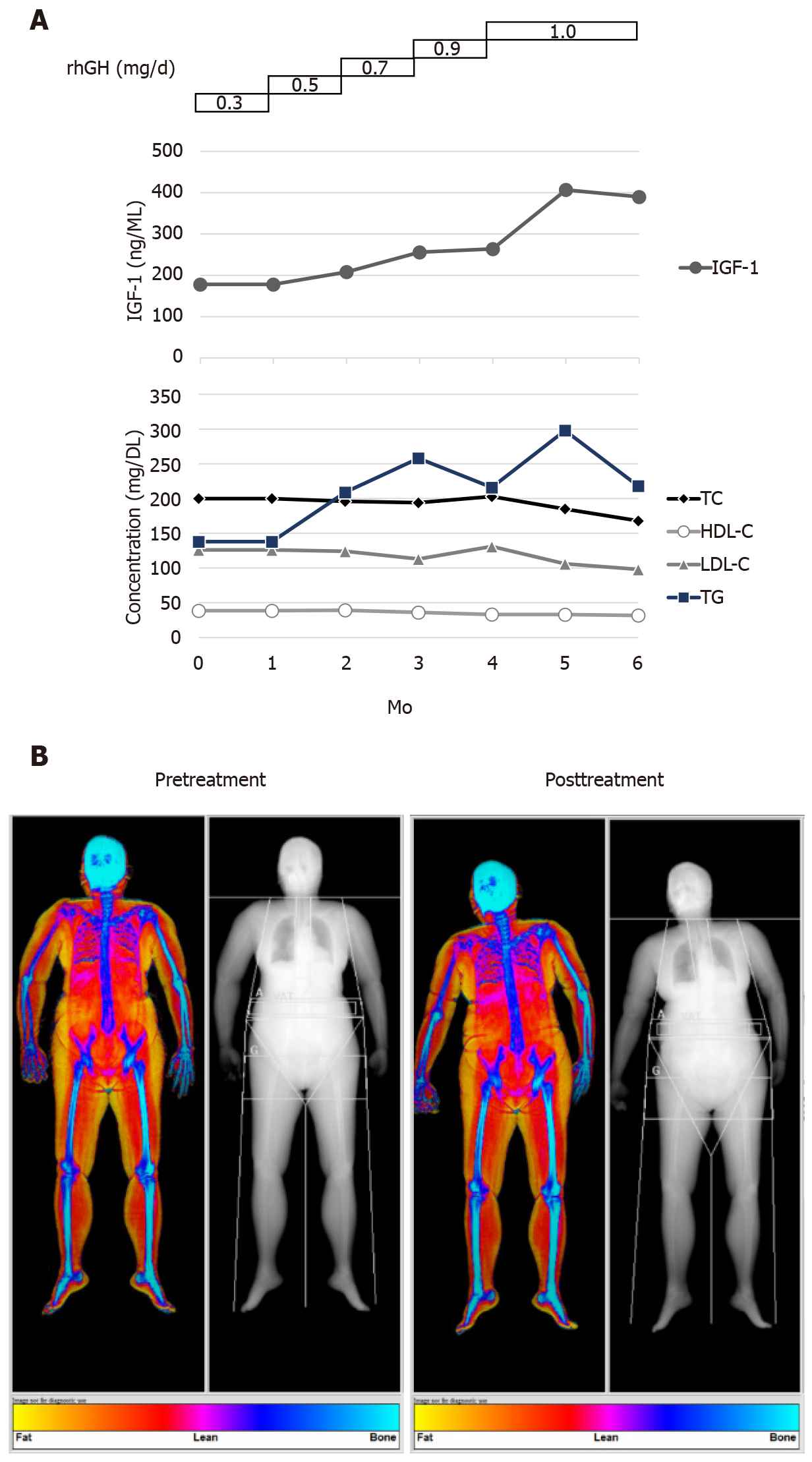
In early 2018, the patient started a 6-mo GHRT. Subcutaneous administration of recombinant human growth hormones (rhGH) was initiated at a daily dose of 0.3 mg through an electronic injection device. During the 6-mo treatment period, the dose was increased stepwise to 1.0 mg/d (Figure 1A). According to the data retrieved from the electronic device, the patient received a full 100% of the recommended dosage. Her serum IGF-1 level gradually increased in response to rhGH administration (Figure 1A). No undesirable adverse effects were observed during the treatment period.
In line with the previous studies, the results of dual-energy X-ray absorptiometry showed that GHRT substantially improved her body composition, as lean body mass increased from 37.5 to 40.5 kg and fat mass reduced from 28.6 to 24.8 kg (Table 1 and Figure 1B). The percentage of body fat also decreased from 43.3% to 38.0% (Table 1). The lipid profile during the 6-mo treatment period is displayed in Figure 1A.
| Pretreatment | Posttreatment | Change from pretreatment | |
| Lean body mass (kg) | 37.5 | 40.5 | +3.0 |
| Fat mass (kg) | 28.6 | 24.8 | -3.8 |
| Fat (%) | 43.3 | 38.0 | -5.3 |
| Weight (kg) | 68.0 | 67.0 | -1.0 |
| BMI (kg/m2) | 29.0 | 28.6 | -0.4 |
| Fat mass/Height2 | 12.2 | 10.6 | -1.6 |
| Lean/Height2 | 15.2 | 16.5 | +1.3 |
| Est. VAT mass (g) | 796 | 630 | -136 |
| Est. VAT volume (cm3) | 861 | 681 | -180 |
| Est. VAT area (cm2) | 165 | 131 | -34 |
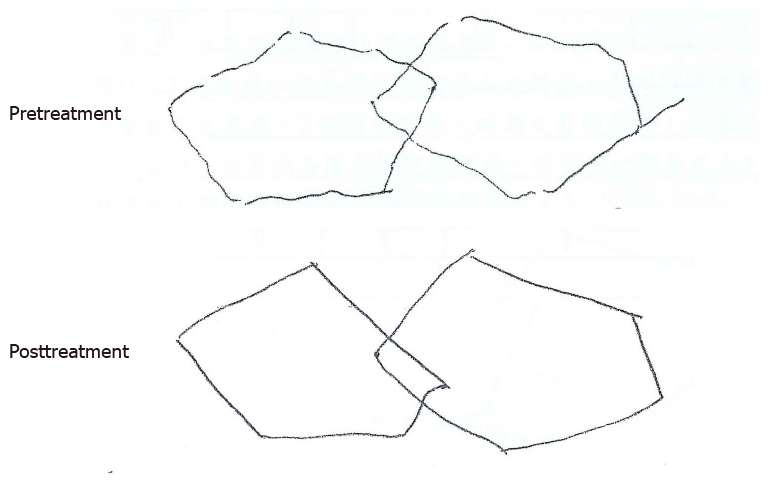
Before starting GHRT, the patient could not stand up, walk, or turn her body around without assistance due to right-sided limb weakness. She scored 14 on the GCS and 16 on the Mini-Mental State Examination (MMSE), accompanied by symptoms of disorientation, incoherent speech, and difficulty in making full sentences. Nevertheless, after six months of treatment, the patient was able to stand up and walk without any help. She could also turn her body around in approximately 6 s. At the end of treatment, the patient had a GCS of 15 and an MMSE of 22; the latter suggested a substantial improvement in cognitive function. Although the pentagon copying tasks were both scored zero before and after 6-mo GHRT, the right-hand tremor significantly improved posttreatment (Figure 2). Her speech was much more fluent, with good and positive responses to physicians’ and her family’s talk.
In the present report, a female patient who suffered ICH was diagnosed with AGHD five years later. Improvements in cognitive function, fluency of speech, and mobility were observed after six months of rhGH treatment. To the best of our knowledge, there is no case report presenting the effects of GHRT on physiological functions in patients with AGHD caused by ICH.
The beneficial effects of GHRT on body compositions are well established in randomized controlled trials[14]. A mean increase of 2-5.5 kg in lean body mass and a mean reduction of 4-6 kg in fat mass has been shown in previous literature[14]. With respect to serum lipid profile, Florakis et al[15] found that significant reductions in total and low-density lipoprotein-cholesterol (LDL-C) levels were observed at 6 mo of GH treatment, whereas an increment in high-density lipoprotein-cholesterol (HDL-C) level became evident at 18 mo and no significant changes were observed for triglycerides (TG) at all time points. Another study conducted by Feldt-Rasmussen et al[13] also reported that serum total cholesterol (TC) and LDL-C reduced significantly upon 12-mo GH treatment, and no significant alterations in HDL-C or TG were seen. In line with previous studies, the patient in this report showed that treatment of GH resulted in an increase in lean body mass, a reduction in fat mass, and slight declines in TC and LDL-C. Nevertheless, a gradual decrease in HDL-C and fluctuations in TG were noted during the 6-month treatment period. Because the patient had a medical history of hyperglyceridemia, it is not clear whether the HDL-C and TG levels were also affected by the patient’s comorbidity.
The literature concerning the prevalence of pituitary dysfunction or GHD following stroke is scarce. According to a prospective study conducted by Bondanelli et al[16], 30.3% and 35.4% of the patients were found to have GHD at 1-3 mo and 12-15 mo after an ischemic stroke, respectively. A recent study reported that 7 out of 13 patients fulfilling the criteria of GHD when tested within a week post-stroke[17]. Apart from the studies related to stroke, accumulating studies have also suggested that both TBI and subarachnoid hemorrhage are conditions at high risk of acquired GHD[18]. Impaired pituitary function was found in about one-fifth of patients after TBI, and in 5%-8% GHD was identified[19,20]. Although the association between GHD and nontraumatic ICH is still unclear, it would be expected that patients with nontraumatic ICH were also at risk of developing GHD. Owing to the lack of routine examination of pituitary hormones for patients who experienced cerebrovascular events, GHD may be unrecognized, and the patients remained unaware of their conditions unless further clinical manifestations appear months or years later. Thus, the incidence or prevalence of GHD due to cerebrovascular events could be largely underestimated.
Cognitive impairment is among the complaints reported by patients with GHD, particularly memory difficulties[21,22]. In addition, GHD was also associated with attention and verbal memory disorders after TBI[23]. The impact of GHRT on cognitive functions has been assessed with various performance tests but with inconsistent results. While some studies indicated that administration of GH help recovery of cognitive functions, as examined by means like verbal memory tests or neuroimaging[24-26], significant changes were not found in several other studies[27,28]. In our case, the patient’s cognitive impairment was evaluated with MMSE before and after a 6-mo GH treatment. A difference of 6 points was observed between the two evaluations. The results are consistent with Devesa et al[29]’ report that cognitive impairments were mitigated following GHRT in patients with GHD caused by TBI, as reflected by improvements in MMSE scores[29]. These findings suggest that GHRT may provide beneficial effects for AGHD patients with cognitive impairments.
Motor improvements have been previously revealed in patients receiving growth hormone treatment. In a series of TBI cases, the administration of growth hormone was initiated 2.5 mo to 11 years after TBI. Motor improvements were found in months after commencing the treatment as assessed with Functional ambulation categories and Tinetti balance and gait tests in both GHD and non-GHD patients[29]. In our case, the patient received GHRT five years after ICH and was able to stand up and walk without assistance following the treatment. In addition to the neurotrophic effects of GH[30], the improved body composition and enhanced muscle strength may also contribute to the patient’s motivation to leave her bed.
During the entire 6-month treatment period, IGF-1 Levels were checked every month to monitor the patient’s response to dose changes over time. The IGF-1 concentration increased from 178 ng/mL before the treatment to 390 ng/mL at the end of treatment. In healthy female Chinese adults aged 60-64 years, the 2.5 and 97.5 percentile values for serum IGF-1 are 46.5 and 210.4, respectively[31]. Because no unfavorable side effects were shown and the improvements in mobility and neurological symptoms emerged, the dosage of rhGH at 1 mg/d (a dosage within the recommended range of locally approved prescribing information) was maintained with close monitoring for the last two months, and the treatment was planned to be stopped afterward. The case showed a substantial improvement in MMSE while having an elevated IGF-1 Level. Low serum IGF-1 Levels were reported to be independently associated with unfavorable functional outcome and death in patients with ischemic stroke[32]. Nevertheless, the association between IGF-1 and cognitive performance is still controversial. In elderly subjects, it was previously shown that circulating IGF-I may be positively associated with certain cognitive functions[33,34], despite that an inverse relationship between IGF-1 Level and cognitive performance was also reported[35]. Thus, whether IGF-1 was positively related to cognitive function requires future studies for further elucidation.
Although it is not known whether the ameliorations in cognition or motor performance could be directly attributable to GH treatment for the present case, the improvements following GHRT is prominent and encouraging, suggesting cognitive and motor impairments of ICH patients may be partially reversed by growth hormone treatment. Current evidence regarding the beneficial effects of GHRT on cognitive and motor function remains limited. Without severe loss of neurological function, the effects may be too subtle to be detected in the general AGHD patients. Therefore, to further confirm whether GH treatment can reverse cognitive deterioration and neurological sequelae cerebrovascular events, randomized controlled studies in the cohorts of ICH or TBI are needed.
Medical writing assistance was funded by Merck Ltd., the funder did not have any roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mrzljak A S-Editor: Zhang L L-Editor: A P-Editor: Wang LYT
| 1. | Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, Flaxman D, Foreman, Gabriel S, Gakidou E, Kassebaum N, Khatibzadeh S, Lim S, Lipshultz SE, London S, Lopez, MacIntyre MF, Mokdad AH, Moran A, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Pope C, Roberts T, Salomon J, Schwebel DC, Shahraz S, Sleet DA, Murray, Abraham J, Ali MK, Atkinson C, Bartels DH, Bhalla K, Birbeck G, Burstein R, Chen H, Criqui MH, Dahodwala, Jarlais, Ding EL, Dorsey ER, Ebel BE, Ezzati M, Fahami, Flaxman S, Flaxman AD, Gonzalez-Medina D, Grant B, Hagan H, Hoffman H, Kassebaum N, Khatibzadeh S, Leasher JL, Lin J, Lipshultz SE, Lozano R, Lu Y, Mallinger L, McDermott MM, Micha R, Miller TR, Mokdad AA, Mokdad AH, Mozaffarian D, Naghavi M, Narayan KM, Omer SB, Pelizzari PM, Phillips D, Ranganathan D, Rivara FP, Roberts T, Sampson U, Sanman E, Sapkota A, Schwebel DC, Sharaz S, Shivakoti R, Singh GM, Singh D, Tavakkoli M, Towbin JA, Wilkinson JD, Zabetian A, Murray, Abraham J, Ali MK, Alvardo M, Atkinson C, Baddour LM, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dellavalle RP, Jarlais, Dicker D, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Foreman, Forouzanfar MH, Freedman G, Freeman MK, Gakidou E, Gillum RF, Gonzalez-Medina D, Gosselin R, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayarman S, Johns N, Kassebaum N, Khatibzadeh S, Lan Q, Leasher JL, Lim S, Lipshultz SE, London S, Lopez, Lozano R, Lu Y, Mallinger L, Meltzer M, Mensah GA, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran A, Naghavi M, Narayan KM, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Raju M, Razavi H, Ritz B, Roberts T, Sacco RL, Salomon J, Sampson U, Schwebel DC, Shahraz S, Shibuya K, Silberberg D, Singh JA, Steenland K, Taylor JA, Thurston GD, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wulf S, Murray; U. S. Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1843] [Article Influence: 153.6] [Reference Citation Analysis (1)] |
| 2. | Aguilar MI, Brott TG. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Gupta V. Adult growth hormone deficiency. Indian J Endocrinol Metab. 2011;15 Suppl 3:S197-S202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Stochholm K, Gravholt CH, Laursen T, Jørgensen JO, Laurberg P, Andersen M, Kristensen LØ, Feldt-Rasmussen U, Christiansen JS, Frydenberg M, Green A. Incidence of GH deficiency - a nationwide study. Eur J Endocrinol. 2006;155:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Abs R, Bengtsson BA, Hernberg-Stâhl E, Monson JP, Tauber JP, Wilton P, Wüster C. GH replacement in 1034 growth hormone deficient hypopituitary adults: demographic and clinical characteristics, dosing and safety. Clin Endocrinol (Oxf). 1999;50:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 165] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Booij HA, Gaykema WDC, Kuijpers KAJ, Pouwels MJM, den Hertog HM. Pituitary dysfunction and association with fatigue in stroke and other acute brain injury. Endocr Connect. 2018;7:R223-R237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Kargi AY, Merriam GR. Diagnosis and treatment of growth hormone deficiency in adults. Nat Rev Endocrinol. 2013;9:335-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Allen DB, Backeljauw P, Bidlingmaier M, Biller BM, Boguszewski M, Burman P, Butler G, Chihara K, Christiansen J, Cianfarani S, Clayton P, Clemmons D, Cohen P, Darendeliler F, Deal C, Dunger D, Erfurth EM, Fuqua JS, Grimberg A, Haymond M, Higham C, Ho K, Hoffman AR, Hokken-Koelega A, Johannsson G, Juul A, Kopchick J, Lee P, Pollak M, Radovick S, Robison L, Rosenfeld R, Ross RJ, Savendahl L, Saenger P, Sorensen HT, Stochholm K, Strasburger C, Swerdlow A, Thorner M. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol. 2016;174:P1-P9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Spielhagen C, Schwahn C, Möller K, Friedrich N, Kohlmann T, Moock J, Kołtowska-Häggström M, Nauck M, Buchfelder M, Wallaschofski H. The benefit of long-term growth hormone (GH) replacement therapy in hypopituitary adults with GH deficiency: results of the German KIMS database. Growth Horm IGF Res. 2011;21:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Deepak D, Daousi C, Boyland E, Pinkney JH, Wilding JP, MacFarlane IA. Growth hormone and changes in energy balance in growth hormone deficient adults. Eur J Clin Invest. 2008;38:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Fernholm R, Bramnert M, Hägg E, Hilding A, Baylink DJ, Mohan S, Thorén M. Growth hormone replacement therapy improves body composition and increases bone metabolism in elderly patients with pituitary disease. J Clin Endocrinol Metab. 2000;85:4104-4112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Christ ER, Carroll PV, Russell-Jones DL, Sönksen PH. The consequences of growth hormone deficiency in adulthood, and the effects of growth hormone replacement. Schweiz Med Wochenschr. 1997;127:1440-1449. [PubMed] |
| 13. | Feldt-Rasmussen U, Wilton P, Jonsson P; KIMS Study Group; KIMS International Board. Aspects of growth hormone deficiency and replacement in elderly hypopituitary adults. Growth Horm IGF Res. 2004;14 Suppl A:S51-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Carroll PV, Christ ER, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, Hintz R, Ho K, Laron Z, Sizonenko P, Sönksen PH, Tanaka T, Thorne M. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab. 1998;83:382-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 409] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 15. | Florakis D, Hung V, Kaltsas G, Coyte D, Jenkins PJ, Chew SL, Grossman AB, Besser GM, Monson JP. Sustained reduction in circulating cholesterol in adult hypopituitary patients given low dose titrated growth hormone replacement therapy: a two year study. Clin Endocrinol (Oxf). 2000;53:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Bondanelli M, Ambrosio MR, Carli A, Bergonzoni A, Bertocchi A, Zatelli MC, Ceruti S, Valle D, Basaglia N, degli Uberti EC. Predictors of pituitary dysfunction in patients surviving ischemic stroke. J Clin Endocrinol Metab. 2010;95:4660-4668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Lillicrap T, Garcia-Esperon C, Walker FR, Ong LK, Nilsson M, Spratt N, Levi CR, Parsons M, Isgaard J, Bivard A. Growth Hormone Deficiency Is Frequent After Recent Stroke. Front Neurol. 2018;9:713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Aimaretti G, Ambrosio MR, Di Somma C, Fusco A, Cannavò S, Gasperi M, Scaroni C, De Marinis L, Benvenga S, degli Uberti EC, Lombardi G, Mantero F, Martino E, Giordano G, Ghigo E. Traumatic brain injury and subarachnoid haemorrhage are conditions at high risk for hypopituitarism: screening study at 3 months after the brain injury. Clin Endocrinol (Oxf). 2004;61:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 234] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Herrmann BL, Rehder J, Kahlke S, Wiedemayer H, Doerfler A, Ischebeck W, Laumer R, Forsting M, Stolke D, Mann K. Hypopituitarism following severe traumatic brain injury. Exp Clin Endocrinol Diabetes. 2006;114:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Berg C, Oeffner A, Schumm-Draeger PM, Badorrek F, Brabant G, Gerbert B, Bornstein S, Zimmermann A, Weber M, Broecker-Preuss M, Mann K, Herrmann BL. Prevalence of anterior pituitary dysfunction in patients following traumatic brain injury in a German multi-centre screening program. Exp Clin Endocrinol Diabetes. 2010;118:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Bengtsson BA, Edén S, Lönn L, Kvist H, Stokland A, Lindstedt G, Bosaeus I, Tölli J, Sjöström L, Isaksson OG. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Deijen JB, de Boer H, Blok GJ, van der Veen EA. Cognitive impairments and mood disturbances in growth hormone deficient men. Psychoneuroendocrinology. 1996;21:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Kozlowski Moreau O, Yollin E, Merlen E, Daveluy W, Rousseaux M. Lasting pituitary hormone deficiency after traumatic brain injury. J Neurotrauma. 2012;29:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Arwert LI, Veltman DJ, Deijen JB, van Dam PS, Drent ML. Effects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: a functional MRI study. Neuroendocrinology. 2006;83:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Moreau OK, Cortet-Rudelli C, Yollin E, Merlen E, Daveluy W, Rousseaux M. Growth hormone replacement therapy in patients with traumatic brain injury. J Neurotrauma. 2013;30:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 26. | Maric NP, Doknic M, Pavlovic D, Pekic S, Stojanovic M, Jasovic-Gasic M, Popovic V. Psychiatric and neuropsychological changes in growth hormone-deficient patients after traumatic brain injury in response to growth hormone therapy. J Endocrinol Invest. 2010;33:770-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Oertel H, Schneider HJ, Stalla GK, Holsboer F, Zihl J. The effect of growth hormone substitution on cognitive performance in adult patients with hypopituitarism. Psychoneuroendocrinology. 2004;29:839-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Mossberg KA, Durham WJ, Zgaljardic DJ, Gilkison CR, Danesi CP, Sheffield-Moore M, Masel BE, Urban RJ. Functional Changes after Recombinant Human Growth Hormone Replacement in Patients with Chronic Traumatic Brain Injury and Abnormal Growth Hormone Secretion. J Neurotrauma. 2017;34:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Devesa J, Reimunde P, Devesa P, Barberá M, Arce V. Growth hormone (GH) and brain trauma. Horm Behav. 2013;63:331-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Martínez-Moreno CG, Calderón-Vallejo D, Harvey S, Arámburo C, Quintanar JL. Growth Hormone (GH) and Gonadotropin-Releasing Hormone (GnRH) in the Central Nervous System: A Potential Neurological Combinatory Therapy? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Zhu H, Xu Y, Gong F, Shan G, Yang H, Xu K, Zhang D, Cheng X, Zhang Z, Chen S, Wang L, Pan H. Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS One. 2017;12:e0185561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Tang JH, Ma LL, Yu TX, Zheng J, Zhang HJ, Liang H, Shao P. Insulin-like growth factor-1 as a prognostic marker in patients with acute ischemic stroke. PLoS One. 2014;9:e99186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Aleman A, Verhaar HJ, De Haan EH, De Vries WR, Samson MM, Drent ML, Van der Veen EA, Koppeschaar HP. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999;84:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Wennberg AMV, Hagen CE, Machulda MM, Hollman JH, Roberts RO, Knopman DS, Petersen RC, Mielke MM. The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol Aging. 2018;66:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | van Bunderen CC, Deijen JB, Drent ML. Effect of low-normal and high-normal IGF-1 levels on memory and wellbeing during growth hormone replacement therapy: a randomized clinical trial in adult growth hormone deficiency. Health Qual Life Outcomes. 2018;16:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |