Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5729
Peer-review started: August 21, 2020
First decision: September 12, 2020
Revised: September 23, 2020
Accepted: October 1, 2020
Article in press: October 1, 2020
Published online: November 26, 2020
Processing time: 95 Days and 22.5 Hours
Unsuspected gallbladder carcinoma (UGC) refers to cholecystectomy due to benign gallbladder disease, which is pathologically confirmed as gallbladder cancer during or after surgery. Port-site metastasis (PSM) of UGC following laparoscopic cholecystectomy is rare, especially after several years.
A 55-year-old man presenting with acute cholecystitis and gallstones was treated by laparoscopic cholecystectomy in July 2008. Histological analysis revealed unexpected papillary adenocarcinoma of the gallbladder with gallstones, which indicated that the tumor had spread to the muscular space (pT1b). Radical resection of gallbladder carcinoma was performed 10 d later. In January 2018, the patient was admitted to our hospital for a mass in the upper abdominal wall after surgery for gallbladder cancer 10 years ago. Laparoscopic exploration and complete resection of the abdominal wall tumor were successfully performed. Pathological diagnosis showed metastatic or invasive, moderately differentiated adenocarcinoma in fibrous tissue with massive ossification. Immuno-histochemistry and medical history were consistent with invasion or metastasis of gallbladder carcinoma. His general condition was well at follow-up of 31 mo. No recurrence was found by ultrasound and epigastric enhanced computed tomography.
PSM of gallbladder cancer is often accompanied by peritoneal metastasis, which indicates poor prognosis. Once PSM occurs after surgery, laparoscopic exploration is recommended to rule out abdominal metastasis to avoid unnecessary surgery.
Core Tip: Port-site metastasis of unsuspected gallbladder carcinoma occurring several years after laparoscopic cholecystectomy is rare. It is often accompanied by peritoneal metastasis with poor prognosis. We report a case of a 55-year-old man with port-site metastasis after surgery for gallbladder cancer 10 years ago. Complete resection of an abdominal wall tumor was successfully performed with no recurrence after 31 mo. Although recurrence of unsuspected gallbladder carcinoma is rare, physicians should remain vigilant for this possibility. Once port-site metastasis occurs after surgery, laparoscopic exploration or positron emission tomography/computed tomography is recommended to rule out abdominal metastasis. Complete tumor resection can improve prognosis.
- Citation: Gao KJ, Yan ZL, Yu Y, Guo LQ, Hang C, Yang JB, Zhang MC. Port-site metastasis of unsuspected gallbladder carcinoma with ossification after laparoscopic cholecystectomy: A case report. World J Clin Cases 2020; 8(22): 5729-5736
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5729.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5729
Unsuspected gallbladder carcinoma (UGC) is referred to as a carcinoma found during cholecystectomy due to benign gallbladder disease, which is pathologically confirmed as gallbladder cancer during or after surgery[1]. Port-site metastasis (PSM) of UGC following laparoscopic cholecystectomy (LC) is rare with an incidence of 10.3%[2]. PSM of UGC occurring several years after surgery is even rarer with only a few cases reported. The mechanism of the PSM is unknown, and many researchers have focused on bile overflow and pneumoperitoneum. We report a case of a 55-year-old man who was diagnosed with PSM after surgery for UGC 10 years ago. Laparoscopic exploration and complete resection of the abdominal wall tumor were successfully performed. PSM with obvious ossification was found. Some studies have confirmed that ossification often indicates good prognosis. Our patient had no recurrence after follow-up of 31 mo. The good prognosis of this patient may be related to ossification. We also reviewed the literature and retrieved seven relevant papers, which included 8 cases of late-type PSM of UGC. We analyzed the clinical and pathological features of those cases and summarized the treatment strategy based on our observations.
A 55-year-old man was admitted to our hospital for a mass in the upper abdominal wall after surgery for UGC 10 years ago.
The patient underwent LC at a community hospital due to acute cholecystitis and gallstones 10 years ago. Histological analysis revealed unexpected papillary adenocarcinoma of the gallbladder with gallstones, which indicated that the tumor had spread to the muscular space (pT1b). Radical resection of gallbladder carcinoma was performed 10 d later. During the operation, abdominal wall tissue (2 cm) around the trocar hole was not removed. The patient recovered well and followed up every 3 mo in the 1st year after surgery, and then every 6 mo or 1 yr with abdominal computed tomography (CT) and tests of cancer-associated tumor markers, such as serum carcinoembryonic antigen (CEA) and serum cancer antigen 19-9 (CA19-9). During the follow-up period, the patient experienced no obvious discomfort. The patient visited our hospital 1 d after a mixed mass was found in the upper abdominal wall by ultrasound. He then was admitted to our hospital for further treatment. Enhanced magnetic resonance imaging (MRI) of the upper abdomen showed a space-occupying mass in the anterior wall of the upper abdomen.
The patient denied a history of hypertension, diabetes, and other relevant illnesses. No other surgery was performed during this period.
The patient did not have a family history of cancer.
Old surgical scars of 20 cm were seen on the abdomen, and a hard mass was felt under the xiphoid process. No other abnormalities were found in the physical examination.
Blood analysis revealed that complete blood count, blood biochemistry, and CEA, alpha fetoprotein, CA19-9, CA125, and CA153 were within normal limits, except for CA724, which was 7.98 U/mL (normal range 0-6.9 U/mL).
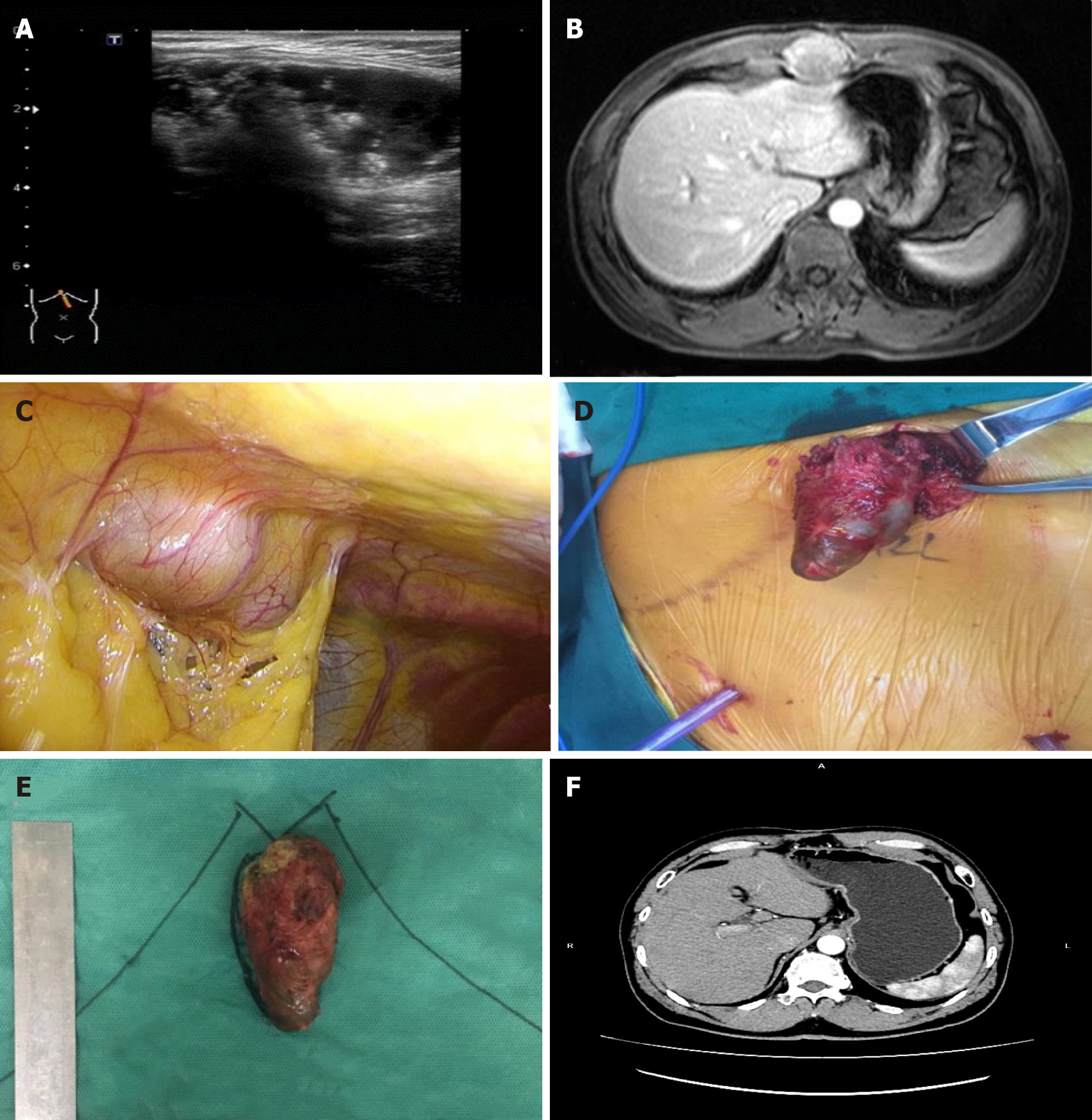
Ultrasound and enhanced MRI of the upper abdomen showed a space-occupying mass in the anterior wall of the upper abdomen (Figure 1A and 1B). No recurrence was found in other parts of the body by positron emission tomography (PET)/CT.
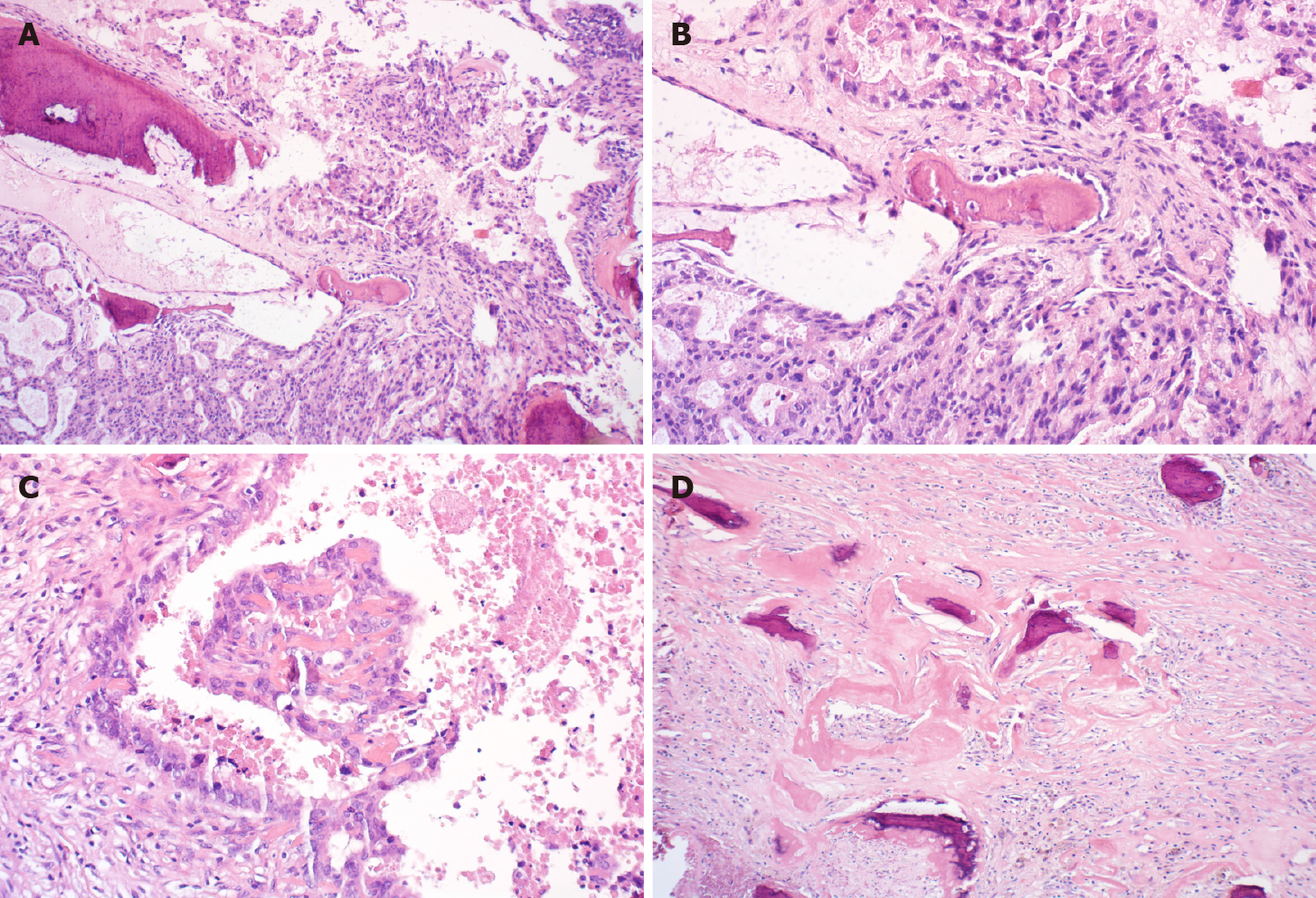
Postoperative pathology showed that a gray-red mass had partial cystic degeneration, and hard calcification of the cyst wall. Microscopic examination showed atypical ethmoid hyperplasia (Figure 2A) protruding to the cystic cavity with massive necrosis and hemorrhage and interstitial ossification around multifocal carcinoma (Figure 2B) with infiltration into the fibrous stroma (Figure 2A and 2B). There was no neurovascular invasion, and several foci of cancer cells produced a bone-like matrix (Figure 2C). Partial cyst wall thickening and fibrosis with previous hemorrhage with hemosiderin deposition and interstitial fibrous bone were observed (Figure 2D). Pathological diagnosis was metastatic or invasive, moderately differentiated adenocarcinoma in the fibrous tissue with massive ossification. After consultation with the Department of Radiotherapy and Chemotherapy in our hospital, immunohistochemical staining was recommended to determine whether the metastasis originated from the gallbladder. Immunohistochemistry showed cytokeratin (CK) 7 (+++), CK20 (++), CEA (+), villin (+++), p53 (-), Ki-67 (+) 40%, CK19 (+++), HBME1 (-), and Wilms tumor (-).
Immunohistochemistry and medical history were consistent with the invasion or metastasis of gallbladder carcinoma. The final diagnosis was PSM of gallbladder cancer.
Laparoscopic exploration and abdominal wall tumor resection were performed after excluding surgical contraindications. No ascites or metastatic nodules were found in the peritoneum and greater omentum during the operation. After the abdominal adhesion was separated, the mass located outside the peritoneum was observed with a size of approximately 5 cm × 3 cm × 3 cm (Figure 1C). An incision of about 5 cm was made above the mass in the right upper abdomen. After incision of the skin and subcutaneous tissue, the mass was found located in the muscular layer of the abdominal wall. The mass was completely removed (Figure 1D and 1E). After consultation with the Department of Radiotherapy and Chemotherapy, further chemotherapy was recommended but was refused by the patient.
The patient was followed up every 3 mo with abdominal ultrasonography and tests of cancer-associated tumor markers. No recurrence was found by abdominal enhanced CT 1 yr after the latest surgery (Figure 1F). The patient has been followed up for 31 mo and is in good condition.
In recent years, the incidence of UGC has increased annually with the widespread use of LC. About 0.25%-3.0% of patients were diagnosed with UGC after surgery, accounting for 50% of all patients with gallbladder cancer[3]. The overall prognosis of patients with gallbladder cancer is poor, and more than a third have metastasis at the time of diagnosis. In contrast, the pathological stage of UGC patients is relatively early with a pT2 of 50% and pT1 of 33.3%, and they have a relatively good prognosis[4]. Papillary adenocarcinoma of the gallbladder accounts for about 5% of all gallbladder cancers, and prognosis of these patients is significantly better than that of patients with other types of gallbladder carcinoma. This may be due to the late infiltration of the gallbladder wall and growth into the cavity. It is easy to show the symptoms of obstruction, and early diagnosis can be made[5]. PSM after UGC is rare and mostly occurs at 4-10 mo after surgery with a median time of 7 mo. It is often associated with poor prognosis such as peritoneal metastasis, and the median survival time after recurrence is only 10 mo[6]. Berger-Richardson et al[2] reported that the incidence of PSM in gallbladder cancer after LC decreased from 18.6% before 2000 to 10.3% after 2000, but it was still higher than that of other primary malignant tumors.
Cases of late-type PSM of UGC after surgery are even rarer, and only a few cases have been reported in the literature, with only 8 cases being identified over the past 3 years[7-13] as shown in Table 1. So far, the longest time to recurrence is 12 years[13]. It has been reported that late recurrence is more common in other primary tumors such as breast cancer and melanoma. This may be related to the lack of angiogenesis in micrometastasis, cell autophagy, and tumor inhibition by the immune system[14]. Ninety percent of PSM occurs in the trocar hole from where the gallbladder specimen is extracted, which is higher than that in nongallbladder extraction sites (19%)[2].
| Ref. | Age/sex | First pathology | T stage | Interval | Second pathology | IHC | Rt or Cmt | Follow-up |
| Ciulla et al[7] | 72/F | ADC | pT1 | 3 yr | ADC | Not performed | N | 2 yr, alive |
| Wettstein et al[8] | 74/F | ADC | PT1a | 40 mo | ADC | Not performed | N | 3 mo, died |
| Nakagawa et al[9] | 73/F | ADC | pT2 | 44 mo | ADC | Not performed | Unknown | Unknown |
| Sharma et al[10] | 58/F | Unknown | Unknown | 4 yr | ADC | CK19 (+), CK20 (+) | Y, Gemcitabine and Cisplatin | Unknown |
| Sultania et al[11] | 34/F | Unknown | Unknown | 5 yr | ADC | Not performed | N | 3 yr, alive |
| Sultania et al[11] | 60/F | Unknown | Unknown | 6 yr | ADC | CK7 (+), CK19 (+), CK20 (-) | Y, Gemcitabine and Oxaliplatin | 3 mo, died |
| Tsujita et al[12] | 55/M | ADC | pT2 | 139 mo | ADC | Not performed | Unknown | 20 mo, alive |
| Carboni et al[13] | 78/F | P-ADC | pT2 | 12 yr | ADC | CK7 (++), CK20 (+) | Y, Capecitabine | 6 mo, alive |
| This case | 59/M | P-ADC | pT1b | 10 yr | ADC | CK7 (+++), CK19 (+++), CK20 (++) | N | 31 mo, alive |
The possible mechanisms of PSM are as follows: (1) The outer wall of the gallbladder is contaminated by tumor cells. When the gallbladder specimen is removed, the tumor cells are directly planted on the resected specimen. Microperforation of the gallbladder wall and bile overflow caused by intraoperative clamping or improper surgical procedure are also involved. No incision protection is given during the operation, and the gallbladder specimen is not removed with an extraction bag; (2) Artificial pneumoperitoneum (CO2) affects tumor cell implantation. Under the condition of artificial pneumoperitoneum, the pressure of gas in the abdominal cavity is higher than that of atmospheric pressure and some gas leaks along the trocar, which is called chimney effect[15]. In addition, when the gas is filled into the abdominal cavity, the tumor cells may be atomized, which may cause incision metastasis of tumor cells[16]; and (3) Postoperative incision trauma, blood osmosis, low immunity, and other factors provide favorable conditions for tumor cell implantation and growth.
Pathological examination in our patient showed metastatic or invasive adenocarcinoma with massive ossification, but gallbladder carcinoma with ossification is not reported in the literature. Ossification is generally considered to be an adaptive mechanism that limits the spread of inflammation to control the spread of harmful processes and protect adjacent tissues[17]. Some studies have confirmed that ossification of breast cancer often indicates good prognosis[18]. Therefore, the good prognosis in the current patient may be related to ossification.
Although recurrence several years after UGC resection is rare, it is still necessary to be vigilant. Therefore, attention must be paid to specimen handling and incision protection during cholecystectomy to avoid bile spillover. In particular for patients with suspected gallbladder cancer, it is recommended to use extraction bags to reduce the possibility of tumor cell spread. The gallbladder specimen should be routinely dissected during the operation, and suspected cases must be examined by frozen section. It is suggested that the gallbladder should be exposed along the long axis, the adventitia or serosa of the gallbladder should be cut along the maximum diameter in the suspected lesions, and the specimens of suitable parts should be selected for frozen section examination[19].
Although the removal of abdominal wall tissue around the trocar hole after laparoscopic two-stage radical UGC has nothing to do with recurrence and prognosis[6], the prognosis is poor if the PSM occurs at trocar sites. Therefore, tissue resection should be performed on the trocar holes with clear contamination in order to reduce the possibility of implant recurrence. If PSM occurs after surgery, the patient’s condition should be comprehensively evaluated. When local recurrence was found, PET/CT was used to check whether there were other sites of metastasis.
The main treatment of PSM after gallbladder cancer is complete resection. If radical surgery is feasible, the tumor should be resected as soon as possible. As shown in Table 1, surgery was performed in all previous cases. Because PSM of gallbladder cancer is often accompanied by peritoneal metastasis, laparoscopic exploration is recommended to exclude abdominal metastasis in order to avoid unnecessary surgery. To improve prognosis, the tumor should be removed completely during the operation. In the current patient, no recurrence was found in other parts of the body by PET/CT. The abdominal wall mass was isolated outside the peritoneum and there was no peritoneal metastasis through laparoscopic exploration. Therefore, resection of the abdominal wall tumor was performed.
Limited data support combined gemcitabine, capecitabine, and radiation therapy for patients with residual cancer[20], but there is no clear guideline. As shown in Table 1, only three patients received postoperative chemotherapy, but one of them had peritoneal and pulmonary metastases at 3 mo after surgery. Our patient refused further chemotherapy treatment after operation, and there was no recurrence at follow-up of 31 mo. We recommend the follow-up strategies for these patients as follows: (1) Abdominal ultrasound and tests of cancer-associated tumor markers performed every 3 mo within 1 year after surgery; (2) An enhanced CT performed within 1 year; and (3) If there is evidence of recurrence, the patient should be followed up every 6 mo.
We report a case of PSM 10 years after resection of UGC, and postoperative pathology showed papillary adenocarcinoma complicated with massive ossification. The patient was followed up for 31 mo after surgery, and his condition is good. Although this late type of PSM is rare, we must pay attention to it. For such patients, we recommend that PET/CT should be performed before surgery to exclude metastases at other sites. Laparoscopic exploration should be performed to avoid unnecessary excessive surgery. The final diagnosis should be made based on postoperative pathology combined with medical history and immunohistochemistry to determine whether the metastasis originated from gallbladder. We hope to find more tumor patients with PSM to determine whether further treatment is needed after surgery in an attempt to provide experience in PSM of UGC.
We thank the patient for permitting us to use his data to complete this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Strainiene S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Lundgren L, Muszynska C, Ros A, Persson G, Gimm O, Valter L, Andersson B, Sandström P. Are Incidental Gallbladder Cancers Missed with a Selective Approach of Gallbladder Histology at Cholecystectomy? World J Surg. 2018;42:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Berger-Richardson D, Chesney TR, Englesakis M, Govindarajan A, Cleary SP, Swallow CJ. Trends in port-site metastasis after laparoscopic resection of incidental gallbladder cancer: A systematic review. Surgery. 2017;161:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 3. | Cavallaro A, Piccolo G, Panebianco V, Lo Menzo E, Berretta M, Zanghì A, Di Vita M, Cappellani A. Incidental gallbladder cancer during laparoscopic cholecystectomy: managing an unexpected finding. World J Gastroenterol. 2012;18:4019-4027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 4. | Søreide K, Guest RV, Harrison EM, Kendall TJ, Garden OJ, Wigmore SJ. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg. 2019;106:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 5. | Wan X, Zhang H, Chen C, Yang X, Wang A, Zhu C, Fu L, Miao R, He L, Yang H, Zhao H, Sang X. Clinicopathological features of gallbladder papillary adenocarcinoma. Medicine (Baltimore). 2014;93:e131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Fuks D, Regimbeau JM, Pessaux P, Bachellier P, Raventos A, Mantion G, Gigot JF, Chiche L, Pascal G, Azoulay D, Laurent A, Letoublon C, Boleslawski E, Rivoire M, Mabrut JY, Adham M, Le Treut YP, Delpero JR, Navarro F, Ayav A, Boudjema K, Nuzzo G, Scotte M, Farges O. Is port-site resection necessary in the surgical management of gallbladder cancer? J Visc Surg. 2013;150:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Ciulla A, Romeo G, Genova G, Tomasello G, Agnello G, Cstronovo G. Gallbladder carcinoma late metastases and incisional hernia at umbilical port site after laparoscopic cholecystectomy. G Chir. 2006;27:214-216. [PubMed] |
| 8. | Wettstein AR, Field AS, Hugh TB, Vickers CR. Implantation carcinoma developing late after laparoscopic cholecystectomy for superficial carcinoma of the gall-bladder. Aust N Z J Surg. 1999;69:406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Nakagawa S, Tada T, Furukawa H, Abe M, Hatakeyama K. Late-type recurrence at the port site of unexpected gallbladder carcinoma after a laparoscopic cholecystectomy: report of a case. Surg Today. 2000;30:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sharma P, Chatterjee P. Late Port Site Metastasis from Occult Gall Bladder Carcinoma After Laparoscopic Cholecystectomy for Cholelithiasis: The Role of (18)F-FDG PET/CT. Nucl Med Mol Imaging. 2014;48:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Sultania M, Pandey D, Sharma J, Mallick S, Mridha AR. Delayed isolated port-site metastasis of gallbladder cancer following laparoscopic cholecystectomy: report of two cases. J Gastrointest Cancer. 2014;45 Suppl 1:188-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Tsujita E, Ikeda Y, Kinjo N, Uezu I, Matsuyama J, Kawano H, Yamaguchi S, Egashira A, Minami K, Yamamoto M, Kumagai R, Taguchi K, Morita M, Toh Y, Okamura T. Late-type port-site recurrence of unexpected gallbladder carcinoma 11 years after laparoscopic cholecystectomy. Asian J Endosc Surg. 2014;7:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Carboni F, Federici O, Giofrè M, Diodoro M, Valle M. Recurrence of gallbladder carcinoma 12 years after laparoscopic cholecystectomy. Clin Res Hepatol Gastroenterol. 2019;43:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 46944] [Article Influence: 3353.1] [Reference Citation Analysis (5)] |
| 15. | Iwanaka T, Arya G, Ziegler MM. Mechanism and prevention of port-site tumor recurrence after laparoscopy in a murine model. J Pediatr Surg. 1998;33:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Wittich P, Marquet RL, Kazemier G, Bonjer HJ. Port-site metastases after CO(2) laparoscopy. Is aerosolization of tumor cells a pivotal factor? Surg Endosc. 2000;14:189-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Fuery MA, Liang L, Kaplan FS, Mohler ER 3rd. Vascular ossification: Pathology, mechanisms, and clinical implications. Bone. 2018;109:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Downs-Kelly E, Nayeemuddin KM, Albarracin C, Wu Y, Hunt KK, Gilcrease MZ. Matrix-producing carcinoma of the breast: an aggressive subtype of metaplastic carcinoma. Am J Surg Pathol. 2009;33:534-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Dincel O, Goksu M, Hatipoglu HS. Importance of routine histopathological examination of a gallbladder surgical specimen: Unexpected gallbladder cancer. J Cancer Res Ther. 2018;14:1325-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Vinuela E, Vega EA, Yamashita S, Sanhueza M, Mege R, Cavada G, Aloia TA, Chun YS, Lee JE, Vauthey JN, Conrad C. Incidental Gallbladder Cancer: Residual Cancer Discovered at Oncologic Extended Resection Determines Outcome: A Report from High- and Low-Incidence Countries. Ann Surg Oncol. 2017;24:2334-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |