Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5625
Peer-review started: May 7, 2020
First decision: September 14, 2020
Revised: September 21, 2020
Accepted: October 12, 2020
Article in press: October 12, 2020
Published online: November 26, 2020
Processing time: 202 Days and 11.4 Hours
Endometrial stromal sarcoma (ESS) is a rare malignant mesenchymal tumor. Early in the disease, the findings on magnetic resonance imaging are similar to those of leiomyoma. When the lesion involves both vascular and cardiac tissue, it might be misdiagnosed as intravenous leiomyomatosis, which is not common in the clinic.
We present the case of a 34-year-old female patient with tumor embolus, which extended from the right iliac vein and ovarian vein to the inferior vena cava (IVC), and then to the right atrium and right ventricle, and finally protruded into the pulmonary artery. The patient had undergone a hystero-myomectomy 7 years previously. Based on the findings of the imaging examinations, the diagnosis of intravenous leiomyomatosis was considered preoperatively. The patient then underwent complete resection of the endovascular and intracardiac tumor embolus. The postoperative pathology results confirmed metastatic ESS with endovascular and intracardiac involvement. The patient was discharged from hospital in good condition, and there was no sign of recurrence 5 mo after the operation.
Extending from the iliac vein and ovarian vein to the IVC, this metastatic ESS invaded both vascular and cardiac tissues. For patients with ESS involving vascular and cardiac tissues, pathological examinations are essential for the differential diagnosis, such as intravenous leiomyomatosis. In addition, due to the high recurrence rate of ESS, long-term and close follow-up evaluation is necessary.
Core Tip: Endometrial stromal sarcoma (ESS) is a rare mesenchymal malignant tumor which is infrequently complicated by endovascular and intracardiac involvement. We report a case of ESS with diffuse myometrial infiltration, as well as endovascular and intracardiac involvement. From the iliac vein and ovarian vein to the inferior vena cava, the metastatic ESS in our patient invaded vascular and cardiac tissues.
- Citation: Fan JK, Tang GC, Yang H. Endometrial stromal sarcoma extending to the pulmonary artery: A rare case report. World J Clin Cases 2020; 8(22): 5625-5631
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5625.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5625
Endometrial stromal sarcoma (ESS) is a rare malignant mesenchymal tumor, accounting for an estimated 0.2% of all malignant uterine tumors and 10% to 15% of uterine sarcomas, with an annual incidence of 1-2 per million women[1,2]. The 2014 World Health Organization (WHO) classification scheme included the latest molecular findings, and divided ESS into low-grade endometrial stromal sarcoma (LGESS), high-grade endometrial stromal sarcoma (HGESS) and undifferentiated uterine sarcoma (UUS)[2-4]. LGESS is the second most common malignant mesenchymal tumor of the uterus, and its incidence is lower than that of leiomyosarcoma[3]. There are differences in the age of onset of ESS among the subtypes. The highest incidence of LGESS, HGESS and UUS is observed in those aged 40-55 years, 28-67 years (mean 50 years) and 60 years, respectively[2]. The clinical manifestations are abnormal uterine bleeding, pelvic mass and pelvic pain, but approximately 25% of LGESS patients have no obvious symptoms[2-4]. The ESS tends to spread to the nearby lymph nodes and veins, but rarely involves cardiac tissues[5]. We present the case of a 34-year-old female patient with ESS, extending from the right iliac vein and ovarian vein, to the inferior vena cava (IVC) and then to the right atrium and right ventricle, finally protruding into the pulmonary artery. The purpose of this report is to present this rare, but meaningful and educational case, by discussing the relevant clinical and imaging features, and the diagnosis when encountering such cases in future clinical work.
A 34-year-old female patient was admitted to the hospital due to vaginal bleeding, fatigue and shortness of breath for 4 d without chest pain, hemoptysis, dyspnea or other symptoms.
Computed tomography (CT) examination revealed a filling defect in the IVC before admission, which was initially thought to be a thrombus. Anticoagulant therapy was administered immediately. No other conditions were recorded when admitted to our hospital.
Seven years ago, the patient had undergone a hystero-myomectomy (no medical records/pathological specimens were available). No other significant history of past illnesses was identified.
The patient had no previous or family history of similar illnesses.
Physical examination showed a surgical scar in the lower abdomen. A palpable hard mass was found in the lower abdomen, with poor mobility and mild tenderness. Vital signs were stable with a blood pressure of 135/80 mmHg, a heart rate 90 bpm, respiratory rate of 21 breaths/min and O2 saturation of 98%. The electrocardiogram showed sinus rhythm and incomplete right bundle branch block.
The laboratory examinations showed elevated levels of cancer antigen 125 (47.73 U/mL), FDP (20.31 μg/mL) and D-Di (6.06 μg/mL), while the results of other laboratory examinations were in the normal range.
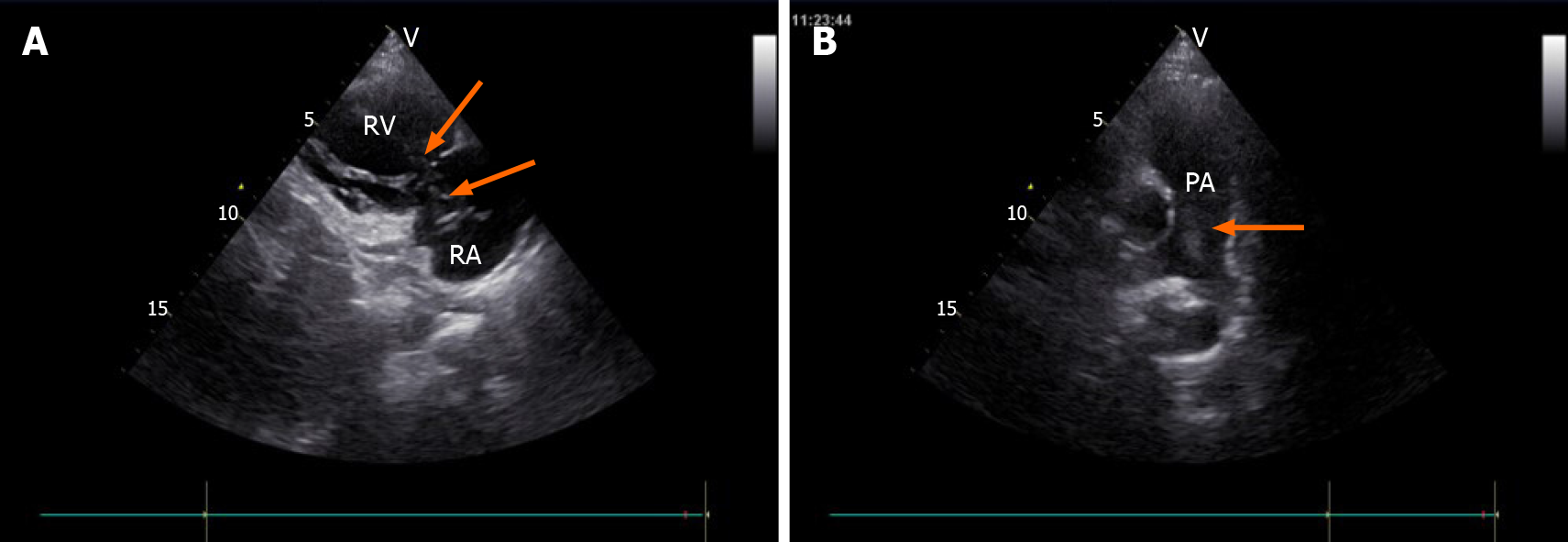
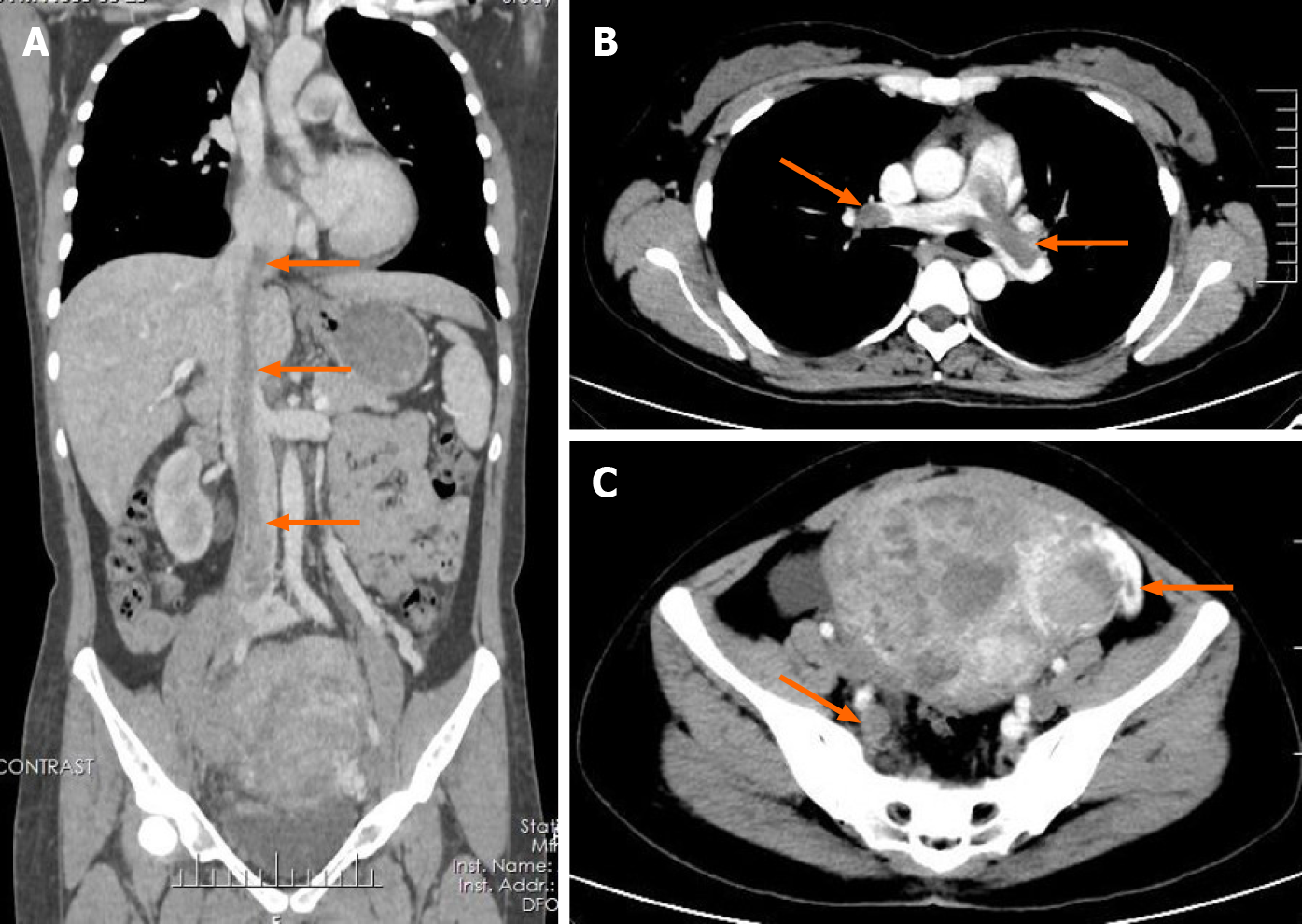
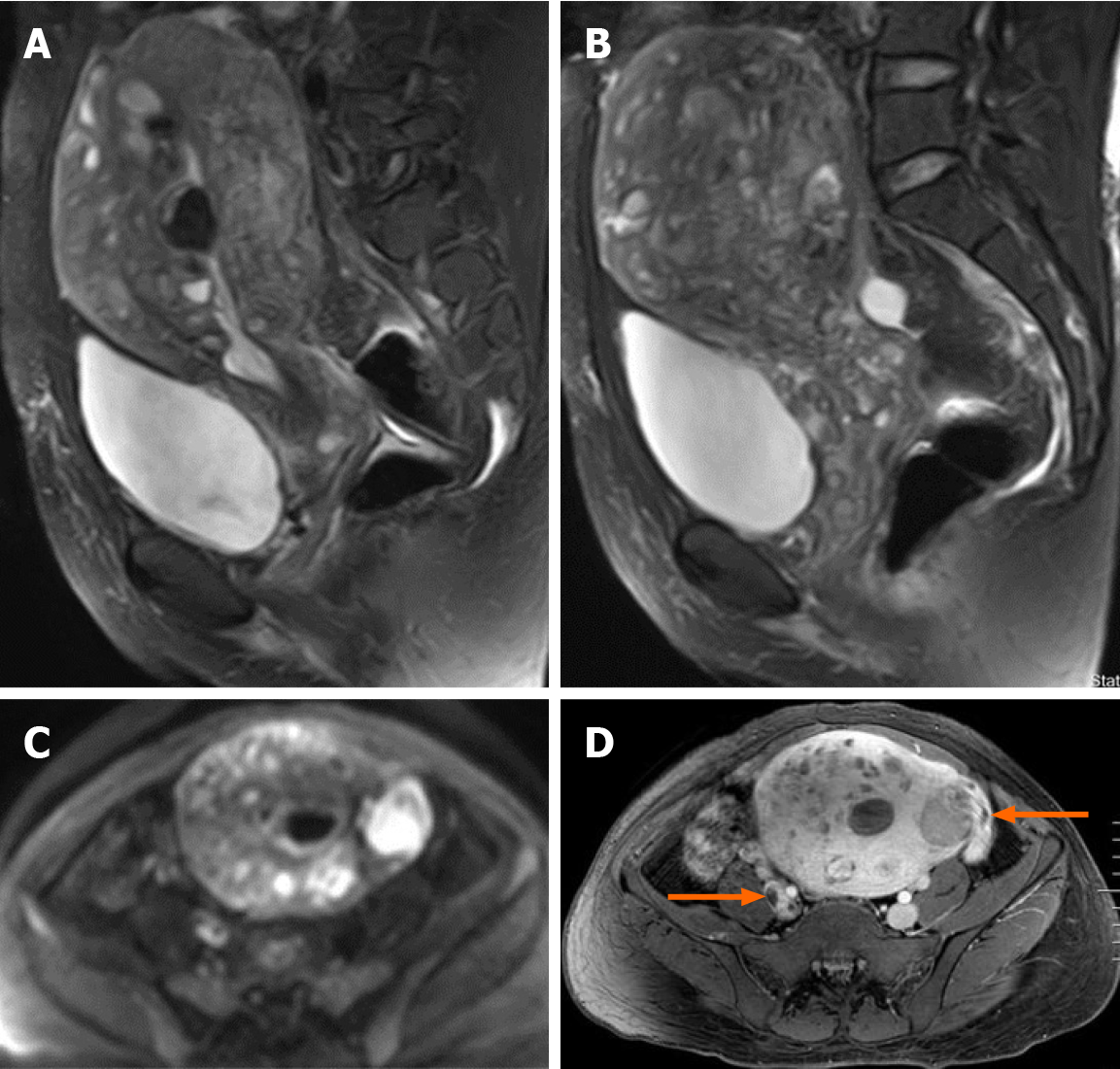
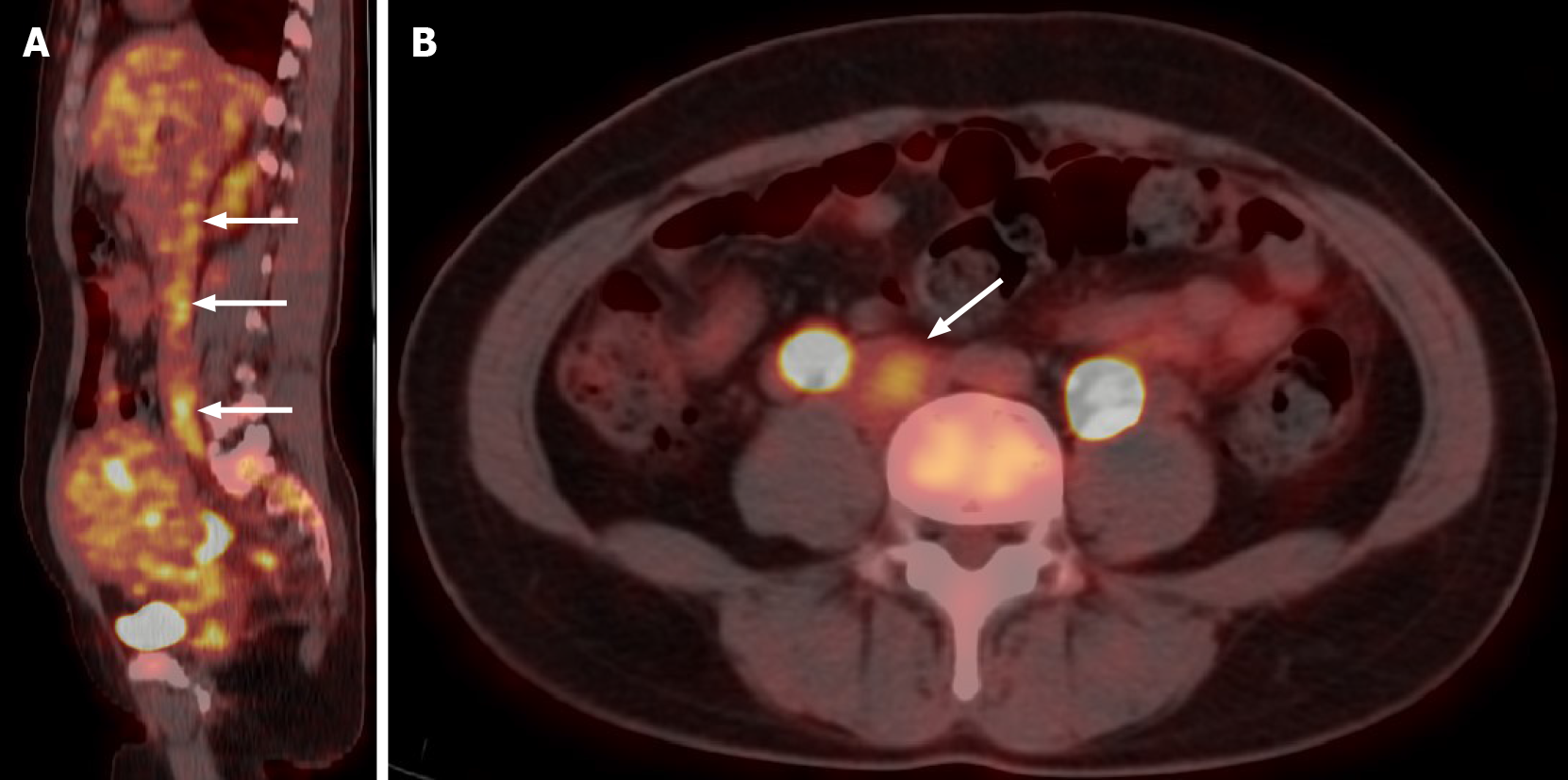
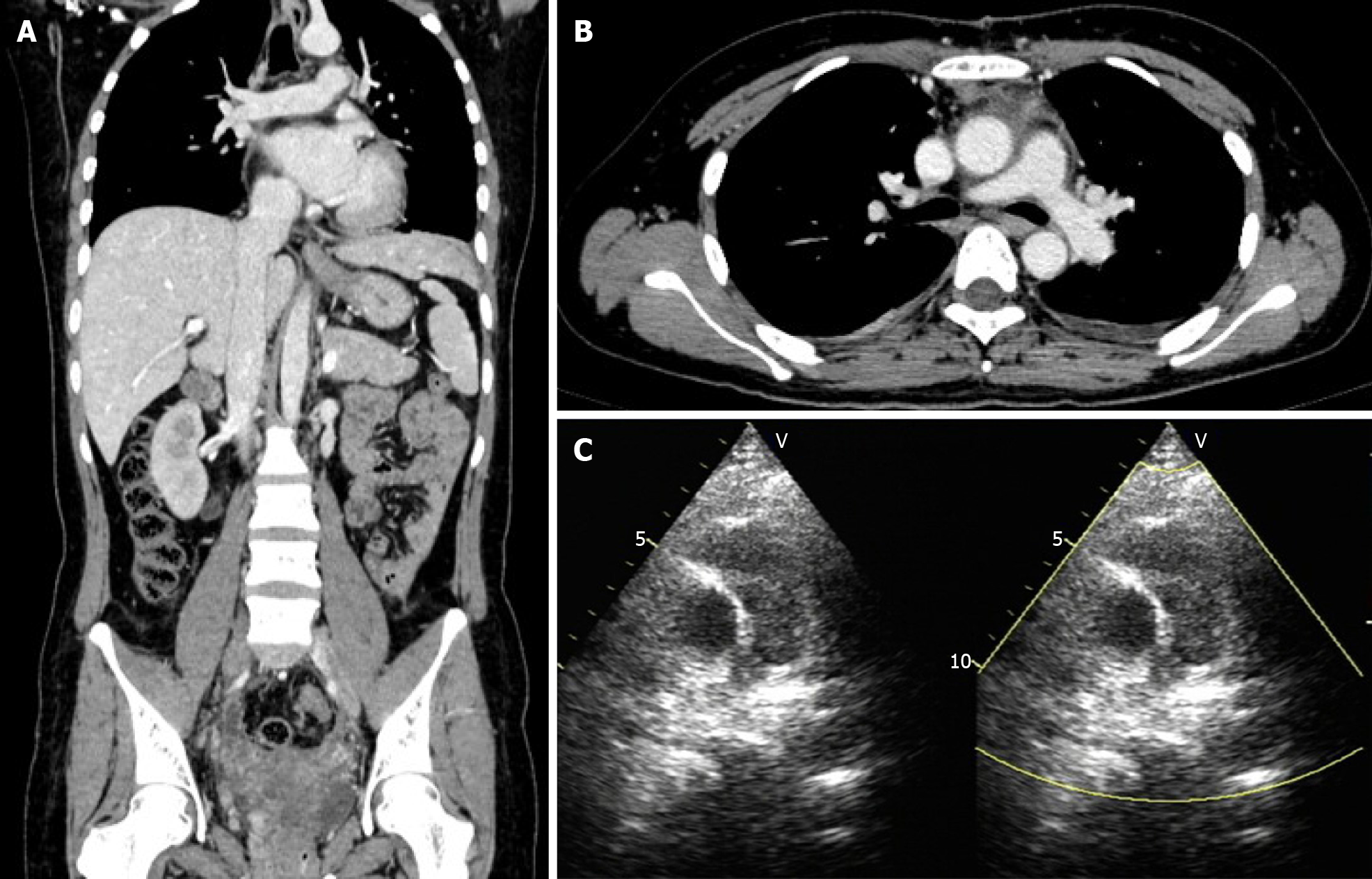
Transvaginal ultrasonography showed that the uterus was 10.0 cm × 8.5 cm × 8.9 cm in size with multiple heterogeneously echogenic lesions. Transthoracic echocardiography revealed a strip-shaped mass extending to the right atrium and right ventricle, and protruding into the pulmonary artery (Figure 1). The diameter of the pulmonary artery was widened, and the pulmonary artery pressure was 66 mmHg. An enlarged uterus and multiple mixed-density masses in the myometrium were found on enhanced CT images (Figure 2A and C). Filling defects were observed in the right iliac vein, ovarian vein, IVC, right atrium, right ventricle, pulmonary trunk and bilateral pulmonary artery branches (Figure 2). Magnetic resonance imaging (MRI) showed extensive myometrial thickening which was heterogeneously hyperintense on T2-weighted images (Figure 3A and B). Diffuse infiltration of the cervix and vaginal vault was noted without rectal or pelvic wall involvement. Increased signal intensity of the lesions was observed on diffusion weighted imaging (DWI) (Figure 3C). Following an intravenous injection of contrast medium, contrast enhancement of the lesions was heterogeneous and less intense than in the myometrium. The vessels around the uterus and cervix increased, and filling defects in the right iliac vein and left ovarian vein were noted (Figure 3D). 18F-FDG positron emission tomography/computed tomography (PET/CT) demonstrated slightly high 18F-fluorodeoxyglucose uptake of the tumor in the IVC (Figure 4), and the maximum standard uptake value was approximately 2.8.
High-grade ESS.
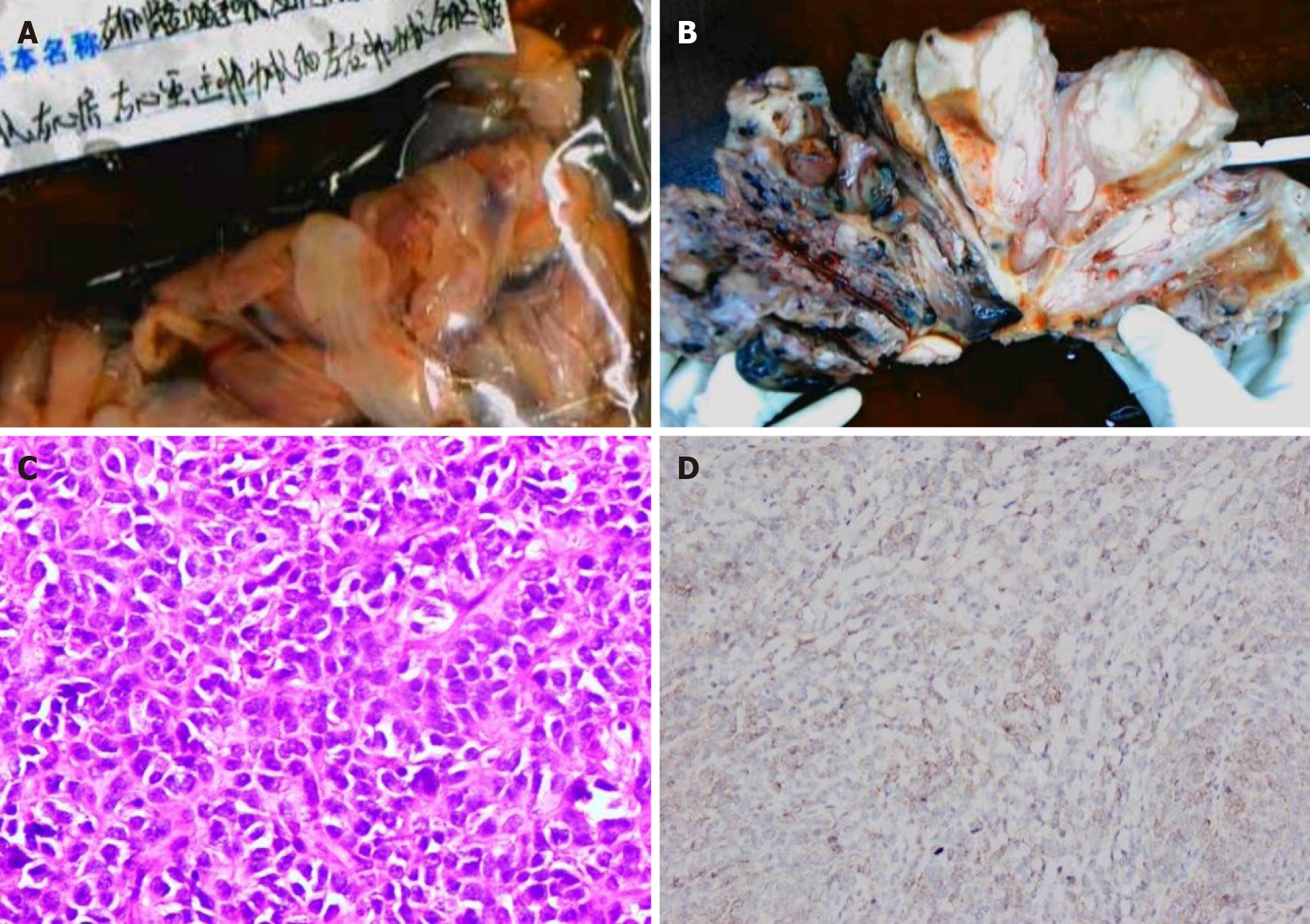
On hospitalization day seven, a combined operation was performed by cardiac surgeons, vascular surgeons and gynecologists (Figure 5A). The endovascular and cardiac tumors were completely removed with no adhesion or infiltration between the tumors and the vessel, and no obvious thrombus on the tumor surface. The gynecologist then performed a subtotal hysterectomy. The size of the uterine tumor was approximately 12 cm × 11cm × 10 cm, and the tumor infiltrated nearly the whole layer of the myometrium. Cut sections showed multiple grey-white nodules and necrotic cystic areas in the myometrium, and some protruded into the uterine cavity as polypoidal masses (Figure 5B). The right ovary was removed, as a cauliflower-shaped lesion approximately 1.0 cm × 0.5 cm in size was found on the surface of the ovary.
The patient recovered well and was discharged 20 d after the operation. Transthoracic echocardiography after surgery revealed improved pulmonary hypertension (Figure 6C). The patient refused to be referred to the oncology department for further chemoradiotherapy. Five months after the operation, the patient was in good condition. Follow-up enhanced CT showed complete removal of the lesion (Figure 6A and B), and there was no sign of recurrence.
Staging is an important factor in determining the prognosis of ESS patients[2-4]. The biological behavior and prognosis of HGESS fall between those of LGESS and UUS[2]. According to the size, extent of invasion and distant metastasis of the tumor, the International Federation of Gynecology and Obstetrics (FIGO) staging is as follows: A tumor limited to the uterus is I [< 5 cm in diameter (I A) and ≥ 5 cm (I B)]; a tumor extending beyond the uterus but limited to the pelvic cavity is II [adnexal involvement (II A) and other pelvic cavity invasion (II B)]; a tumor outside the pelvic cavity is Ш; a tumor invading the bladder and rectum is IV A, and distant metastases is IV B[2,4]. The 5-year overall survival rate of patients with stage I and II LGESS exceeds 90%, but decreases to 50% for stage Ш and IV[2,4,6]. This patient was considered to have stage IVB, which has a poor prognosis. However, there was no distant metastasis to parenchymal organs or lymph nodes, at least according to the medical imaging findings. In this case, we were more concerned about the endovascular mass, due to the possibility of pulmonary embolism leading to sudden heart failure.
For patients with ESS accompanied by endovascular or intracardiac metastasis, preoperative imaging examinations are important. The imaging examinations can evaluate the extent of the tumor thrombus and show the relationship between the tumor thrombus and vessel wall clearly. The MRI findings of ESS commonly show a polypoid endometrial mass, a heterogeneously low signal on TI-weighted images and a hyperintense signal on T2-weighted images, and moderate contrast enhancement[7]. Worm-like extension bands caused by lymphatic and vascular invasion appear as hypointense bands on T2-weighted images, which represent preserved normal myometrium[7,8]. Distinctively, our patient presented features of a tumor with diffuse myometrial infiltration, and normal myometrium was not clearly demonstrated. Usually, the tumor shows marginal irregularity, which can be attributed to tumor invasion along the vessels and lymphatics[7]. However, MRI is neither specific nor sensitive to the diagnosis of these lesions[4]. Even histological examinations often miss early-stage ESS, with a failure rate as high as 40%[9].
The appearance of early-stage ESS on MRI, although similar to that of leiomyoma, generally has an infiltrative margin. The results of DWI and apparent diffusion coefficient (ADC) values may have the potential to differentiate ESS from benign leiomyomas[10]. ESS shows a high signal on DWI, and the ADC value of ESS is significantly lower than that of benign leiomyoma[10]. When accompanied by endovascular or intracardiac involvement, ESS may be misdiagnosed as intravenous leiomyomatosis, which has a similar biological behavior to ESS and can display vascular invasion. The manifestations of these two lesions on CT and MRI are similar. Thus, pathological immunohistochemical examinations are essential for the differential diagnosis.
Compared with LGESS, patients with HGESS have earlier and more frequent recurrences (usually no more than 1 year), and are more likely to die of the disease[2,4]. The common sites of tumor recurrence are the local pelvic cavity, distant abdomen and lungs, or both[2-4].
Tumor stage is the most important factor affecting the prognosis of ESS patients. Metastatic ESS extending from the iliac vein and ovarian vein to the IVC and invading vascular and cardiac tissues was seen in this patient. The results of DWI and ADC values are helpful in differentiating early-stage ESS from benign leiomyoma. For patients with endovascular and intracardiac involvement, ESS should be differentiated from intravenous leiomyomatosis, and pathological examinations are essential. Due to the high recurrence rate of ESS, it is necessary to carry out long-term and close follow-up evaluations.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Teragawa H S-Editor: Zhang H L-Editor: Webster JR P-Editor: Liu JH
| 1. | Rauh-Hain JA, del Carmen MG. Endometrial stromal sarcoma: a systematic review. Obstet Gynecol. 2013;122:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynaecol Obstet. 2018;143 Suppl 2:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 3. | Ali RH, Rouzbahman M. Endometrial stromal tumours revisited: an update based on the 2014 WHO classification. J Clin Pathol. 2015;68:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Horng HC, Wen KC, Wang PH, Chen YJ, Yen MS, Ng HT; Taiwan Association of Gynecology Systematic Review Group. Uterine sarcoma Part II-Uterine endometrial stromal sarcoma: The TAG systematic review. Taiwan J Obstet Gynecol. 2016;55:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Gabal S, Ashour Z, Hamada G, Aziz SA, Khairy H, Badawy H, Hamada EM, Saied K. Low-grade endometrial stromal sarcoma with intravenous extension to the heart. Medscape J Med. 2009;11:23. [PubMed] |
| 6. | Chan JK, Kawar NM, Shin JY, Osann K, Chen LM, Powell CB, Kapp DS. Endometrial stromal sarcoma: a population-based analysis. Br J Cancer. 2008;99:1210-1215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Santos P, Cunha TM. Uterine sarcomas: clinical presentation and MRI features. Diagn Interv Radiol. 2015;21:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Shah SH, Jagannathan JP, Krajewski K, O'Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012;199:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Scher D, Nghiem W, Aziz S, Rahbar R, Banks W, Venbrux A, Sarin S. Endometrial Stromal Sarcoma Metastatic from the Uterus to the Inferior Vena Cava and Right Atrium. Tex Heart Inst J. 2015;42:558-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Tamai K, Koyama T, Saga T, Morisawa N, Fujimoto K, Mikami Y, Togashi K. The utility of diffusion-weighted MR imaging for differentiating uterine sarcomas from benign leiomyomas. Eur Radiol. 2008;18:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |