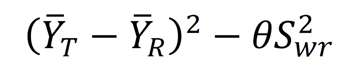Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5518
Peer-review started: June 5, 2020
First decision: July 25, 2020
Revised: August 6, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: November 26, 2020
Processing time: 173 Days and 4 Hours
The pharmacokinetics and bioequivalence of esomeprazole in healthy Chinese subjects and the effects of food on the pharmacokinetics have not been well studied.
To evaluate the pharmacokinetic characteristics of esomeprazole magnesium (Eso) enteric- coated capsule in the healthy subjects in China and the bioequivalence of the two formulations.
This study was conducted in the Phase I Clinical Trial Unit of the Affiliated Hospital of Changchun University of Chinese Medicine. A total of 64 healthy subjects were enrolled in the study. Thirty-two subjects fasted or fed, took the test or reference formulation Eso enteric-coated capsule by a four-cycle, two-sequence crossover of fasting/fed, self-controlled method. The liquid chromatography-mass spectrometry was performed to determine the drug plasma concentration at 16 different time points within 12 h after drug administration. The pharmacokinetic parameters Cmax, area under the curve (AUC)0-t, and AUC0-inf were calculated to evaluate the bioequivalence.
Pharmacokinetic parameters were evaluated after subjects took the test formulation and control formulation under fasting status. The ratio of geometric means of Cmax was 104.15%, with a confidence interval (CI) of 98.20-110.46%. The ratio of geometric means of AUC0-t was 105.26%, with a CI of 99.80-111.01%. The ratio of geometric means of AUC0-inf was 105.37%, with a CI of 99.97-111.06%. The pharmacokinetic parameters were also evaluated after subjects took the reference formulation of Eso enteric-coated capsule after eating. The upper limit of 95% CI of the geometric mean ratio of pharmacokinetic parameters of Eso enteric-coated capsules in the postprandial state Cmax was -0.1689, and the point estimate was 0.9509 (0.80-1.25). The upper limit of 95% CI of the geometric mean ratio of pharmacokinetic parameters of Eso enteric-coated capsules in the postprandial state AUC0-t was -0.1015 (≤ 0) , and the point estimate was 0.9003 (0.80-1.25). The upper limit of 95% CI of the geometric mean ratio of pharmacokinetic parameters of Eso enteric-coated capsules in the postprandial state AUC0-inf was -0.0593 (≤ 0), and the point estimate was 0.8453 (0.80-1.25). The results indicated that the two formulations were bioequivalent under both fasting and fed states.
The two types of esomeprazole tablets were bioequivalent under both fasting and fed states, and both were generally well tolerated.
Core Tip: The pharmacokinetic characteristics and bioequivalence of two types of single oral dose esomeprazole magnesium (Eso) enteric-coated capsules were assessed. The 90%CI of the ratios of geometric means of the primary pharmacokinetic parameters all fell within the acceptable limits of 80.00%-125.00%. Although meal was able to extend drug absorption, it had no impact on Cmax, AUC0-t, or AUC0-inf, of either of the two formulations under the same status. Furthermore, no significant differences in safety issues were observed between the two formulations. Therefore, the two formulations of Eso enteric-coated capsules are considered bioequivalence.
- Citation: Liu ZZ, Ren Q, Zhou YN, Yang HM. Bioequivalence of two esomeprazole magnesium enteric-coated formulations in healthy Chinese subjects. World J Clin Cases 2020; 8(22): 5518-5528
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5518.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5518
Gastroesophageal reflux disease (GERD) is the most common acid-related disease. The typical symptoms include heartburn and/or reflux[1]. GERD is the most commonly diagnosed disease in gastroenterology in the United States, affecting approximately 7% adults every day. In East Asia, the prevalence is 2.5%-7.8%[2]. Without effective treatment, patients can develop serious complications, such as esophageal stricture, ulcer, or Barrett’s esophagus[3].
The goal of GERD treatment is to reduce associated symptoms[4]. The severity and frequency of these symptoms and the degree of esophageal acid exposure are significantly related to esophagus pH[5]. Thus, suppressing gastric acid can relieve symptoms. Proton pump inhibitors (PPI) have been extensively used in the treatment of GERD and are recommended as the first-line treatment for GERD patients[6,7]. As the first option for treatment[8-12], PPIs inhibit gastric acid secretion and increase gastric pH[13]. It has been reported that esomeprazole exhibits a stronger acid inhibiting effect than omeprazole and can effectively improve the gastric pH environment in a short term[14-18].
Esomeprazole, the S-isomer of omeprazole and the first single optical isomer in the PPI family, is a common drug for giant gastric ulcers and used extensively in clinical practice. The drug inhibits gastric acid secretion[19-22] by explicitly inhibiting the H+/K+-ATPase in the gastric parietal cells, and is an alternative for PPIs[23]. Esomeprazole is a new generation of PPI with faster absorption and a stronger ability to inhibit gastric acid secretion.
The esomeprazole magnesium (Eso) enteric-coated tablets at 40 mg and 20 mg obtained marketing approval in China in 2003. The absolute bioavailability of a single dose of 40 mg was 64%, while that of one more dose every day was 89%. The corresponding values of a dose of 20 mg were 50% and 68%, respectively. The plasma protein binding rate of esomeprazole was 97%, and the plasma concentration reached a peak in about 1-2 h after oral administration[24]. Esomeprazole is entirely metabolized by the cytochrome P450 (CYP) enzymes. The metabolism is mostly via the polymorphic CYP2C19, which produces hydroxyl and dimethyl metabolites of esomeprazole. The rest is metabolized by the specific isoform CYP3A4 to produce omeprazole sulfone, a primary metabolite in plasma[25]. In addition, food intake may affect the pharmacokinetics of esomeprazole due to changes in gastric emptying, stimulation by bile flow, changes in drug metabolism, and physical or chemical drug interactions[26-28]. Therefore, the characteristics of food may exert a significant impact on the pharmacokinetics of medicines, and it is essential to determine the optimal drug administration time relative to the meal[29].
At present, the pharmacokinetics and bioequivalence of esomeprazole in healthy Chinese subjects and the effects of food on the pharmacokinetics have not been well studied. In order to better observe the bioequivalence, tolerance, and safety of esomeprazole in healthy Chinese subjects, the dose of 40 mg was chosen for this research. A single-center, open-label, single-dose, randomized, repeated, four-period, crossover bioequivalence study was conducted in healthy subjects at fasting and fed states to evaluate the pharmacokinetics and safety of esomeprazole (40 mg) in these subjects in China. The bioequivalence of the two formulations of esomeprazole was determined by area under the curve (AUC) from time 0 to the last measurable plasma concentration (AUC0-t) and the AUC from time 0 to infinity (AUC0-inf).
The design of this clinical study was based on “Technical Guidelines for Studies on Human Bioequivalence of Generic Drugs with Pharmacokinetic Endpoints”[30] issued by the China Food and Drug Administration in 2016 and “Guidance for Industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Draft Guidance”[31] issued by the Food and Drug Administration (FDA) in 2013.
The study protocol was approved by the Ethics Committee of Changchun University of Chinese Medicine Affiliated Hospital. All subjects provided written informed consent prior to participating in the study. This was a single-center, open-label, single-dose, randomized, repeated, four-period crossover bioequivalence study conducted in healthy subjects under fasting and fed states.
Two bioequivalence arms, fasting and fed states, were included in the study. Thirty-two healthy subjects were enrolled in each arm. The subjects enrolled in the study should be aged between 18-50 years, weighed ≥ 50.0 kg for males and ≥ 45.0 kg for females, with a body mass index between 18.0-28.0 kg/m2 (including boundary values). Subjects were enrolled into the study only after no significant abnormalities were found in vital signs, physical examination, laboratory tests, electrocardiogram, or imaging examination. Subjects who had participated in other clinical studies were excluded. Other exclusion criteria were: Past history of drug allergy, cardiovascular disease, hepatobiliary, renal endocrine, hematological, and gastrointestinal diseases, use of liver enzyme inhibitors or inducers within 28 d before the trial, and use of prescription drugs or herbs within two weeks before the trial; use of any other investigational products within two mo before the trial; consumption of caffeine or chocolate within 48 h of the study; and other ineligibility to participate in the study determined by the researchers.
The test formulation was Eso enteric-coated capsules, manufactured by Chia Tai Tianqing Pharmaceutical Co, Ltd, 40 mg/capsule, stored below 25°C, with an acceptable window at 15-30°C. The drugs of the same strength for subject use were all from the same lot.
The reference formulation was esomeprazole magnesium capsules (Nexium), manufactured by AstraZeneca, 40 mg/capsule, stored below 25°C, with an acceptable window 15-30°C. The drugs of the same strength for subject use were all from the same lot.
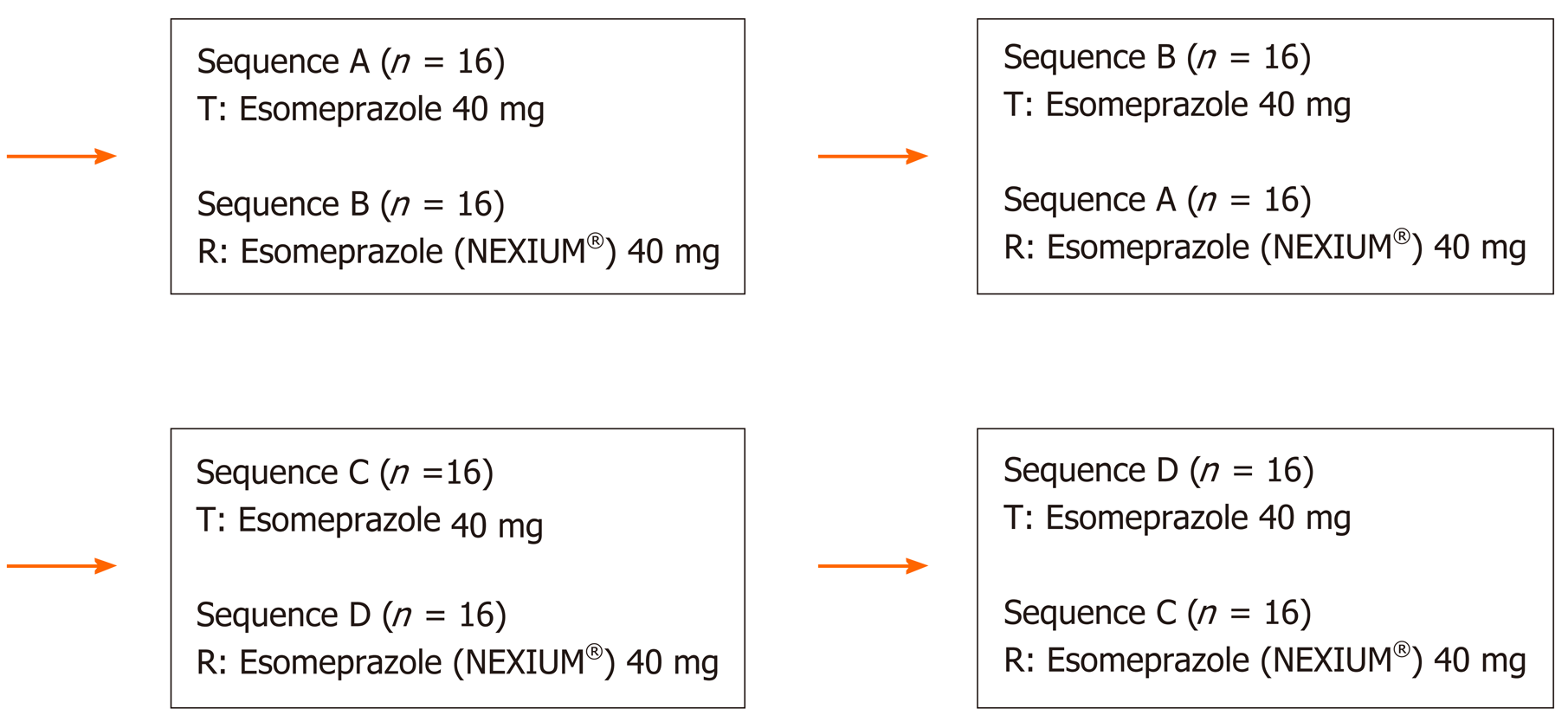
There were two independent arms, the fasting group and the fed group. After screening, in each arm, 32 eligible subjects were randomized using SAS software (version 9.4) to receive either the test formulation or reference formulation following the randomization administration chart. Subjects in the fasting group took the test or reference formulation at 40 mg orally with 240 mL warm water in the morning. Subjects in the fed group were required to have a high-fat meal at 30 min before drug administration. The eating speed was monitored to ensure that all subjects finish the meal within 30 min. The high-fat meal provided 800-1000 calories, 50% of which was from fat (approximately 150 calories of protein, 250 calories of carbohydrates, and 500-600 calories fat). The test or reference formulation was taken orally with 240 mL warm water at 30 min after meal. Subjects in both arms were required to have the standard dinner on the day before administration, fasting for at least 10 h before administration, and no water within 1 h before and 2 h after administration. Subjects were allowed to have lunch 4 h after drug administration and to have dinner 10 h after administration.
The subjects were hospitalized for a total of 8 days’ observation. The mean terminal half-life (mean ± standard deviation) of esomeprazole in plasma was 1.3 h. The washout period (dosing interval) between test cycles was set to 2 d, ten times longer than half-life. This ensured that the drug concentrations at the beginning of a cycle for all subjects are lower than the lower limit of quantification of bioanalysis to eliminate the effect of the treatment during the previous cycle on the treatment during the subsequent cycle (Figure 1).
In each cycle of fasting or fed status, pharmacokinetics analysis was conducted on samples collected at 0 h (within 60 min) before drug administration, and 15 min, 30 min, 45 min, 1 h, 1.5 h, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 5 h, 6 h, 8 h, 10 h and 12 h after drug administration. Whole blood samples were centrifuged at 2-8°C, 3500 rpm for 10 min. The plasma was obtained and stored under 70°C for pharmacokinetics analysis.
WinNonlin7.0 non-compartmental analysis was used for analyzing pharmacokinetics (PK) parameters, including Cmax, AUC0-t, AUC0-inf, Tmax, λz, t1/2, CL/F, Vz/F, and %AUCex. For samples collected within the collection window, the PK parameters were calculated using the theoretical collection time. For samples collected outside the collection window, the PK parameters were calculated using the actual collection time.
SAS (version 9.4) was used for bioequivalence analysis on the PK parameters (Cmax, AUC0-t, and AUC0-inf) after natural logarithmic conversion.
Canagliflozin plasma concentrations were determined using a validated, specific, and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS)[32]. After precipitated with a methanol solution, protein was analyzed by chromatography.
The column chromatography was performed using ACQUITY UPLC BEH C18 (1.7 µm, 2.1 mm × 50 mm). The mobile phase consisted of mobile phase A of 5% acetonitrile containing 0.1% formic acid and mobile phase B of 95% acetonitrile with 0.1% formic acid. The injection volume was 5 μL. The column temperature was 40°C. Mass spectrometry was performed using API-4000 (AB Sciex, Concord, ON, Canada). The effective quantitative range of esomeprazole was 3.00-3000 ng/mL.
Safety assessment was based on post-dosing clinical and laboratory examinations to evaluate adverse events (AEs), including all subjective symptoms reported by subjects and objective signs observed by the researchers (numbers, severity, and relationship to the study drug).
SAS (version 9.4) was used to perform bioequivalence analysis on the PK parameters (Cmax, AUC0-t, and AUC0-inf) after natural logarithmic conversion. A mixed-effect model was used. The PK parameters of the reference formulation were used to determine the within-subject standard deviation Swr.
For the primary endpoint PK parameters (Cmax, AUC0-t, and AUC0-inf): (1) Swr < 0.294, two one-sided t test with α = 0.05 was used to test the statistical hypothesis, that is, whether the 90% CI of ratios of geometric means of the pharmacokinetic parameters (Cmax, AUC0-t, and AUC0-inf) of the test and reference formulations fell within the range of 80.00% to 125.00%[33] (including the boundary value); and (2) Swr ≥ 0.294, the reference-scale average bioequivalence was used for analysis. Test and reference formulations were considered bioequivalent when the pharmacokinetic parameters of both test and reference formulations met the following criteria: (a) The 95%CI of the test and reference Formula was less than or equal to 0, and (b) The ratios of geometric means of the pharmacokinetic parameters (Cmax, AUC0-t, and AUC0-inf) of the test and reference formulations were within the range of 80.00%-125.00%[33] (including the boundary value). Non-parametric text was used to calculate Tmax (Wilcoxon-Matched Pairs method).
One hundred and eleven subjects were screened for the fasting arm. After informed consent was provided by the subjects, general information (age, height, and weight) and medical history were obtained, physical examinations (measurement of body temperature, vital signs , blood pressure, and alcohol exhalation), urine collection for routine body fluid examination, and drug screening were conducted. Blood samples were collected for biochemical examination. Thirty-two eligible subjects were enrolled following the strict inclusion/exclusion criteria, including 17 males (53.13%) and 15 females (46.88%). The demographic information of the 32 healthy subjects as the intention-to-treat population was as follows (mean ± standard deviation): Age 38.0 ± 6.68 years (range 26-49 years), weight 65.39 ± 8.288 kg (range 48.7-79.3 kg), height 164.13 ± 8.768 cm (range 144.5-178.5 cm), and body mass index 24.26 ± 2.343 kg/m2 (range 19.5-27.2 kg/m2). Using the same method, 32 subjects were included for the fed arm, including 14 males (43.75%) and 18 females (56.25%). The demographic information of the 32 healthy subjects as intention-to-treat population was as follows (mean ± standard deviation): Age 38.4 ± 7.48 years (range 42-49 years), weight 62.44 ± 10.011 kg (range 47.4-89.1 kg), height 162.28 ± 10.171 cm (range 144.5-181.0 cm), and body mass index 23.64 ± 2.370 kg/m2 (range 19.9-27.7 kg/m2).
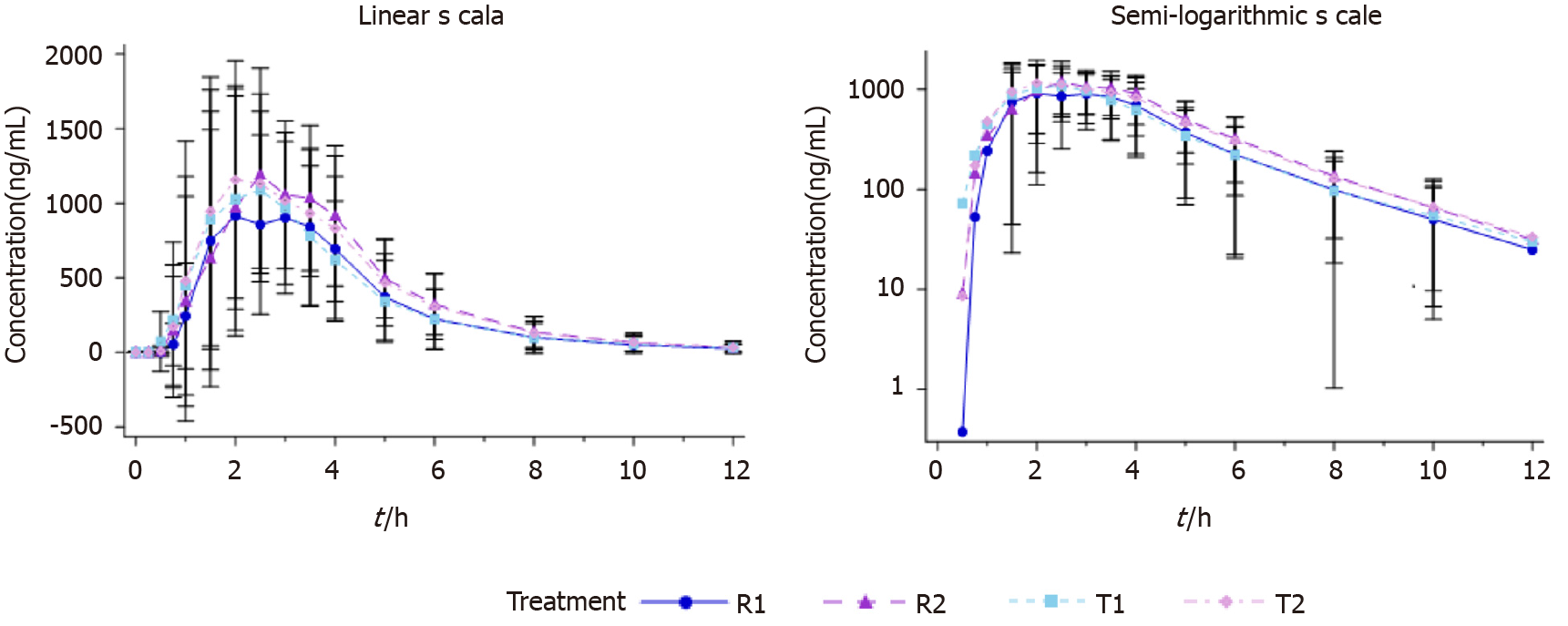
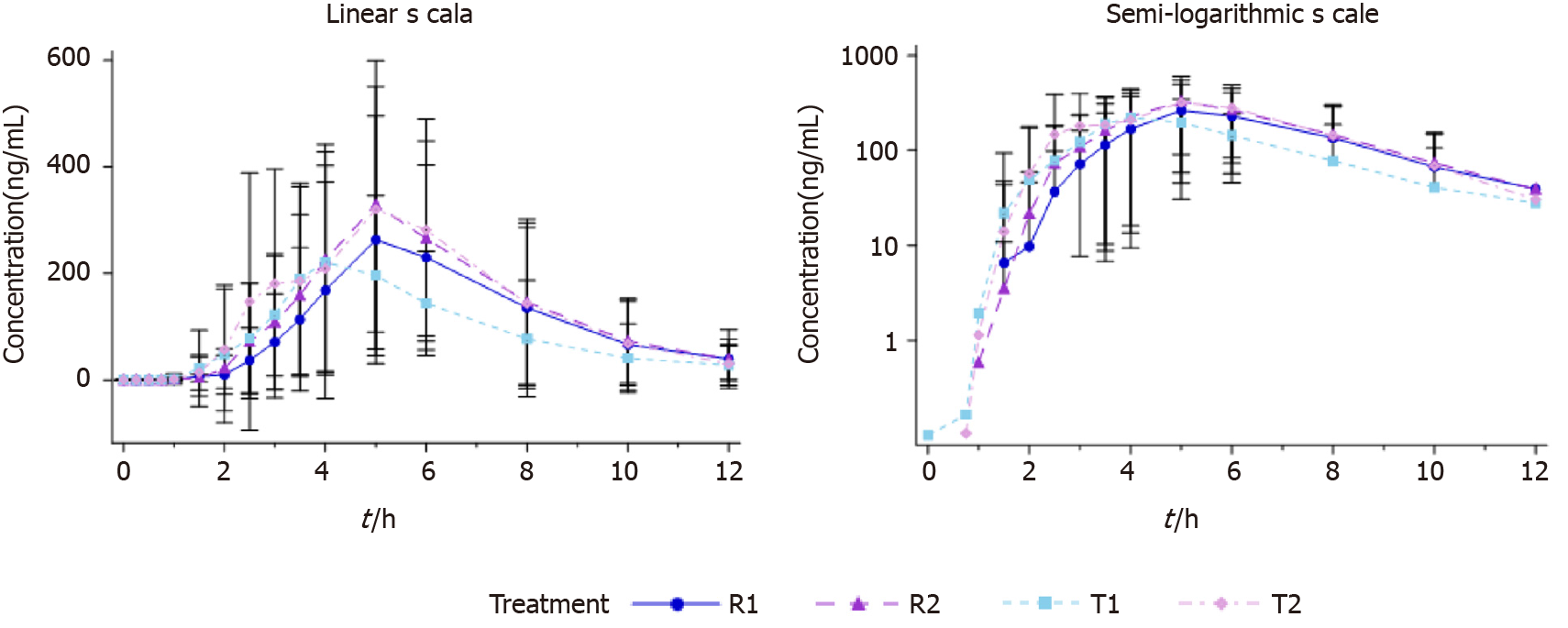
All subjects who completed the study were analyzed for PK data (n = 64). The subjects in both fasting and fed arms took a single oral dose of the test formulation and the reference formulation of 40 mg Eso enteric-coated capsules. The plasma drug concentration-time curves are shown in Figure 2 and 3.
The in vivo processes of esomeprazole test and reference formulations were consistent under both fasting and fed status. Tmax of esomeprazole in the fed arm was slightly extended compared with the fasting group. The rest PK parameters were basically consistent between the two arms (Table 1 and 2).
| PK parameters (unit) | mean ± SD (CV%), 1n = 32 | |||
| 2n | Test formulation | 2n | Reference formulation | |
| Cmax (ng/mL) | 64 | 1709.563 ± 650.8205 (38.07%) | 64 | 1635.875 ± 591.2969 (36.15%) |
| AUC0-t (hr*ng/mL) | 64 | 4328.5196 ± 2109.6280 (48.74%) | 64 | 4132.7124 ± 1991.9727 (48.20%) |
| AUC0-inf (hr*ng/mL) | 64 | 4395.5223 ± 2173.2633 (49.44%) | 64 | 4187.7795 ± 2046.3196 (48.86%) |
| Tmax (h) | 64 | 2.000 (1.00, 4.00) | 64 | 2.500 (1.00, 5.00) |
| %AUCex | 64 | 1.135 ± 1.8793 (165.54%) | 64 | 1.040 ± 1.1709 (112.60%) |
| λz (1/h) | 64 | 0.5685 ± 0.1969 (34.64%) | 64 | 0.5757 ± 0.1967 (34.16%) |
| t1/2 (h) | 64 | 1.366 ± 0.4855 (35.53%) | 64 | 1.329 ± 0.3972 (29.89%) |
| CL/F (L/h) | 64 | 12.3599 ± 8.6012 (69.59%) | 64 | 13.2105 ± 9.5635 (72.39%) |
| Vd/F (L) | 64 | 20.3287 ± 6.4901 (31.93%) | 64 | 21.3166 ± 8.6636 (40.64%) |
| PK parameters (unit) | mean ± SD (CV%), 1n = 32 | |||
| 2n | Test formulation | 2n | Control formulation | |
| Cmax (ng/mL) | 64 | 360.373 ± 249.7500 (69.30%) | 64 | 390.725 ± 257.6718 (65.95%) |
| AUC0-t (hr*ng/mL) | 64 | 1285.9846 ± 965.7697 (75.10%) | 64 | 1363.9129 ± 887.0435 (65.04%) |
| AUC0-inf (hr*ng/mL) | 63 | 1366.4590 ± 1014.866 (74.27%) | 58 | 1497.9755 ± 979.5204 (65.39%) |
| Tmax (h) | 64 | 5.000 (2.00, 8.00) | 64 | 5.000 (3.00, 10.00) |
| %AUCex | 63 | 4.154 ± 6.7878 (163.39%) | 58 | 4.191 ± 5.6377 (134.53%) |
| λz (1/h) | 63 | 0.5766 ± 0.1851 (32.11%) | 58 | 0.5529 ± 0.1602 (28.98%) |
| t1/2 (h) | 63 | 1.454 ± 0.9882 (67.97%) | 58 | 1.408 ± 0.5896 (41.89%) |
| CL/F (L/h) | 63 | 54.4431 ± 60.2376 (110.64%) | 58 | 47.2423 ± 47.5796 (100.71%) |
| Vd/F (L) | 63 | 101.5421 ± 111.3586 (109.67%) | 58 | 93.9881 ± 113.9048 (121.19%) |
In the fasting status, the within-subject standard deviation Swr of the primary PK parameters Cmax, AUC0-t, and AUC0-inf of esomeprazole magnesium enteric-coated capsule reference formulation were 0.2067, 0.2199 and 0.2175, respectively, all of which were smaller than 0.294. Therefore, the average bioequivalence method was used to evaluate bioequivalence. Cmax was calculated to evaluate the bioequivalence of test and reference formulations. The ratio of geometric means of the Cmax was 104.15%, with 90% CI of 98.20%-110.46%. AUC was calculated to evaluate the bioequivalence of test and reference formulations. The ratio of geometric means of AUC0-t was 105.26%, with 90% CI of 99.80%-111.01%. The ratio of geometric means of AUC0-inf was 105.37%, with 90% CI of 99.97%-111.06%.
In the fed status, the within-subject standard deviation Swr of the primary PK parameters Cmax, AUC0-t, and AUC0-inf of esomeprazole magnesium enteric-coated capsule reference formulation were 0.5690, 0.4776 and 0.4754, respectively, all larger than 0.294. Therefore, the reference-scale average bioequivalence method was used to evaluate the bioequivalence. Cmax was calculated to evaluate the bioequivalence of test and reference formulations. The upper limit of 95%CI of the cutoff value Formula was -0.1689 (≤ 0), and the point estimate was 0.9509 (within the range of 0.80-1.25). AUC0-t was calculated to evaluate the bioequivalence of test and reference formulations. The upper limit of 95%CI of the cutoff value Formula was 0.1015 (≤ 0), and the point estimate was 0.9003 (within the range of 0.80-1.25). AUC0-inf was calculated to evaluate the bioequivalence of test and reference formulations. The upper limit of 95%CI of the cutoff value Formula was 0.0593 (≤ 0), and the point estimate was 0.8453 (within the range of 0.80-1.25).
The healthy Chinese subjects received the test formulation and reference formulation of 40 mg esomeprazole magnesium enteric-coated capsule under either fasting or fed status. The 90% CI of ratios of geometric means of the primary PK parameters Cmax, AUC0-t, and AUC0-inf of plasma esomeprazole are shown in Table 3 and 4. As shown in the tables, regardless of fasting or fed status, the 90% CI of ratios of geometric means of esomeprazole all fell within the acceptable equivalence range of 80.00%-25.00%, meeting the criteria of bioequivalence.
| Average bioequivalence | Reference-scaled average bioequivalence | Intra-subject variability (%) | |||||||||
| Parameters | n | GLSmean T | GLSmean R | Ratio (%) (T vs R) | 90%CI (%) | S2wr | Swr | Point estimate (0.8, 1.25) | Criteria bound (≤ 0) | CVwt | CVwr |
| Cmax (ng/mL) | 32 | 1591.275 | 1527.891 | 104.15 | (98.20, 110.46) | 0.0427 | 0.2067 | 1.0415 | -0.0193 | 15.04 | 20.89 |
| AUC0-t (h*ng/mL) | 32 | 3786.532 | 3597.451 | 105.26 | (99.80, 111.01) | 0.0484 | 0.2199 | 1.0526 | -0.0228 | 18.39 | 22.26 |
| AUC0-inf (h*ng/mL) | 32 | 3830.746 | 3635.508 | 105.37 | (99.97, 111.06) | 0.0473 | 0.2175 | 1.0537 | -0.0222 | 18.31 | 22.01 |
| Average bioequivalence | Reference-scaled average bioequivalence | Intra-subject variability (%) | |||||||||
| Parameters | n | GLSmean T | GLSmean R | Ratio (%) (T vs R) | 90%CI (%) | S2wr | Swr | Point estimate (0.8, 1.25) | Criteria bound (≤ 0) | CVwt | CVwr |
| Cmax(ng/mL) | 32 | 284.060 | 298.718 | 95.09 | (80.92, 111.75) | 0.3238 | 0.5690 | 0.9509 | -0.1689 | 53.05 | 61.84 |
| AUC0-t (h*ng/mL) | 32 | 958.8895 | 1065.110 | 90.03 | (79.29, 102.22) | 0.2281 | 0.4776 | 0.9003 | -0.1015 | 39.91 | 50.62 |
| AUC0-inf (h*ng/mL) | 26 | 1074.615 | 1271.273 | 84.53 | (72.99, 97.90) | 0.2260 | 0.4754 | 0.8453 | -0.0593 | 42.76 | 50.36 |
Out of the 32 subjects in the fasting arm, 4 subjects experienced AEs during the study. Four AEs were observed (3 AEs with the test formulation and 1 AE with reference formulation), including grade 1 atrioventricular block, toothache, sinus bradycardia, and sinus tachycardia. Out of the 32 subjects in the fed arm, 5 subjects experienced AEs during the study, and a total of 7 AEs were observed (1 AE with test formulation and 6 AEs with reference formulation): Diarrhea, abdominal pain, nausea, increased alanine aminotransferase levels and positive occult blood, which were all grade 1. All emergent AEs were recovered. All subjects in both fasting and fed arms were in good condition during the study, with stable vital signs and no severe AEs reported. The safety results of the test formulation were comparable to those of the reference formulation, and both formulations can be used within the ordinary doses.
The FDA guidance on esomeprazole recommends that the bioequivalence study should be performed in both fasting and fed states. However, the pharmacokinetics and bioequivalence of esomeprazole have not been studied in healthy Chinese subjects under either condition. Therefore, in order to compare the pharmacokinetics and safety of two formulations of esomeprazole in the healthy subjects in China, we designed a single-center, open-label, single-dose, randomized, repeated, four-cycle crossover bioequivalence study in healthy subjects under fasting and fed states. The healthy Chinese subjects took the test or reference formulation of 40 mg esomeprazole magnesium enteric coated capsules orally under either fasting or fed status. The results showed that the 90% CI of the ratios of geometric means of the primary PK parameters Cmax, AUC0-t, and AUC0-inf of esomeprazole in plasma all fell within the acceptable equivalence range of 80.00%-125.00%, which was within the bioequivalence criteria set by the FDA.
It is worth noting that food intake may affect some PK parameters and, in turn, change the absorption of oral drugs. Food may also change drug clearance through changing plasma protein binding and blood flow[34]. This food-drug interaction may affect the pharmacokinetics of the drug, thereby affecting efficacy and toxicity[34]. Therefore, in the research of pharmacokinetics of esomeprazole, the simultaneous administration of the drug with food is essential to determine the optimal administration time. In this study, Tmax was slightly different between fed and fasting status, indicating that food may delay and reduce esomeprazole absorption.
In summary, the pharmacokinetic characteristic and bioequivalence of the two types of single oral dose esomeprazole magnesium enteric coated capsules were assessed. After oral administration, the 90% CI of the ratios of geometric means of the primary pharmacokinetic parameters, Cmax, AUC0-t, and AUC0-inf, all fell within the acceptable limits of 80.00%-125.00%. In addition, although the meal was able to extend drug absorption, it had no impact on Cmax, AUC0-t, or AUC0-inf, of either of the formulations under the same status. Furthermore, no significant differences in safety issues were observed between the two formulations. Therefore, the two formulations of esomeprazole magnesium enteric coated capsules are considered bioequivalent.
Gastroesophageal reflux disease is the most common acid-related disease and also the most commonly diagnosed acid-related disease in the United States. The typical symptoms include heartburn and/or reflux. Without effective treatment, patients can develop serious complications, such as esophageal stricture, ulcers, or Barrett’s esophagus.
Esomeprazole is a new generation of proton pump inhibitors with faster absorption and a more vital ability to inhibit gastric acid secretion. The drug inhibits gastric acid secretion by explicitly inhibiting the H+/K+-ATPase in the gastric parietal cells, and is an alternative for proton pump inhibitors. At present, the pharmacokinetics and bioequivalence of esomeprazole in healthy Chinese subjects and the effects of food on the pharmacokinetics have not been well studied.
To observe the bioequivalence, tolerability, and safety of esomeprazole in healthy Chinese people.
Thirty-two healthy subjects in a fasting state and 32 in a fed state took the test or reference formulation Eso enteric-coated capsule by a four-cycle, two-sequence crossover of fasting/fed, self-controlled method. The liquid chromatography-mass spectrometry was used to determine the drug plasma concentration at 16 different time points within 12 h after drug administration. The pharmacokinetic parameters Cmax, area under the curve AUC0-t, and AUC0-inf were calculated to evaluate the bioequivalence.
Pharmacokinetic parameters were evaluated after the subjects took the test formulation and control formulation under the fasting status. The ratio of the geometric means of Cmax was 104.15%, with a CI of 98.20%-110.46%. The ratio of the geometric means of AUC0-t was 105.26%, with a CI of 99.80%-111.01%. The ratio of the geometric means of AUC0-inf was 105.37%, with a CI of 99.97%-111.06%. The pharmacokinetic parameters were also evaluated after the subjects took the reference formulation of the esomeprazole magnesium enteric-coated capsule after eating. The upper limit of the 95% confidence interval (CI) of the geometric mean ratio of the pharmacokinetic parameters of esomeprazole magnesium enteric-coated capsules in the postprandial state Cmax was -0.1689, and the point estimate was 0.9509 (0.80-1.25). The upper limit of the 95% confidence interval (CI) of the geometric mean ratio of the pharmacokinetic parameters of esomeprazole magnesium enteric-coated capsules in the postprandial state AUC0-t was -0.1015 (≤ 0), and the point estimate was 0.9003 (0.80-1.25). The upper limit of the 95% confidence interval (CI) of the geometric mean ratio of the pharmacokinetic parameters of esomeprazole magnesium enteric-coated capsules in the postprandial state AUC0-inf was -0.0593 (≤ 0), and the point estimate was 0.8453 (0.80-1.25). The results indicated that the two formulations were bioequivalent under both fasting and fed states.
The pharmacokinetic characteristics and bioequivalence of the two types of single-oral dose esomeprazole magnesium enteric-coated capsules were assessed. After oral administration, the 90% CI of the ratios of the geometric means of the primary pharmacokinetic parameters Cmax, AUC0-t, and AUC0-inf all fell within the acceptable limits of 80.00%-125.00%. In addition, although the meal extended the drug absorption, it had no impact on the Cmax, AUC0-t, or AUC0-inf of either of the formulations under the same status. Furthermore, no significant differences in safety issues were observed between treatment with the two formulations. Therefore, the two formulations of Eso enteric-coated capsules are considered bioequivalent.
The test formulation of the Eso enteric-coated capsule is equivalent to the reference formulation under both the fasting and fed states. Furthermore, no significant differences in safety issues were observed between treatments with the two formulations.
We thank all medical staff who agreed to participate in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goikoetxea N, Rombouts K S-Editor: Gao CC L-Editor: MedE-Ma JY P-Editor: Zhang YL
| 1. | Joelsson B, Johnsson F. Heartburn--the acid test. Gut. 1989;30:1523-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1263] [Article Influence: 114.8] [Reference Citation Analysis (2)] |
| 3. | Triantos C, Koukias N, Karamanolis G, Thomopoulos K. Changes in the esophageal mucosa of patients with non erosive reflux disease: How far have we gone? World J Gastroenterol. 2015;21:5762-5767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Miner P. Review article: relief of symptoms in gastric acid-related diseases--correlation with acid suppression in rabeprazole treatment. Aliment Pharmacol Ther. 2004;20 Suppl 6:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Huang JQ, Hunt RH. pH, healing rate, and symptom relief in patients with GERD. Yale J Biol Med. 1999;72:181-194. [PubMed] |
| 6. | Iwakiri K, Kinoshita Y, Habu Y, Oshima T, Manabe N, Fujiwara Y, Nagahara A, Kawamura O, Iwakiri R, Ozawa S, Ashida K, Ohara S, Kashiwagi H, Adachi K, Higuchi K, Miwa H, Fujimoto K, Kusano M, Hoshihara Y, Kawano T, Haruma K, Hongo M, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for gastroesophageal reflux disease 2015. J Gastroenterol. 2016;51:751-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 7. | Zhang C, Kwong JS, Yuan RX, Chen H, Xu C, Wang YP, Yang GL, Yan JZ, Peng L, Zeng XT, Weng H, Luo J, Niu YM. Effectiveness and Tolerability of Different Recommended Doses of PPIs and H2RAs in GERD: Network Meta-Analysis and GRADE system. Sci Rep. 2017;7:41021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | DeVault KR, Castell DO; American College of Gastroenterology. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. [PubMed] [DOI] [Full Text] |
| 9. | Robinson M. Proton pump inhibitors: update on their role in acid-related gastrointestinal diseases. Int J Clin Pract. 2005;59:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash). 2000;40:52-62; quiz 121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Welage LS. Pharmacologic properties of proton pump inhibitors. Pharmacotherapy. 2003;23:74S-80S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 13. | Vanderhoff BT, Tahboub RM. Proton pump inhibitors: an update. Am Fam Physician. 2002;66:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Lind T, Rydberg L, Kylebäck A, Jonsson A, Andersson T, Hasselgren G, Holmberg J, Röhss K. Esomeprazole provides improved acid control vs. omeprazole In patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2000;14:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Wilder-Smith C, Ro¨hss K, Lundin C, Rydholm H. Esomeprazole(E) 40 mg provides more effective acid control than pantoprazole(P) 40 mg. Gastroenterology. 2000;118:A22–23. [DOI] [Full Text] |
| 16. | Thomson A, Claar-Nilsson C, Hasselgren G, Niazi M, R o¨hss K, Nyman L. Esomeprazole 40 mg provides more effectiveacid control than lansoprazole 30 mg during single and repeated administration. Gut. 2000;47 Suppl 3:A63. |
| 17. | Wilder-Smith C, Claar-Nilsson C, Hasselgren G, Ro¨hss K. Esomeprazole 40 mg provides faster and more effective acid control than rabeprazole 20 mg in patients with symptoms of GERD. Am J Gastroenterol. 2002;17 Suppl:A612. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Röhss K, Hasselgren G, Hedenström H. Effect of esomeprazole 40 mg vs omeprazole 40 mg on 24-hour intragastric pH in patients with symptoms of gastroesophageal reflux disease. Dig Dis Sci. 2002;47:954-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Saccar CL. The pharmacology of esomeprazole and its role in gastric acid related diseases. Expert Opin Drug Metab Toxicol. 2009;5:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Scott LJ, Dunn CJ, Mallarkey G, Sharpe M. Esomeprazole: a review of its use in the management of acid-related disorders. Drugs. 2002;62:1503-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64:935-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 22. | Fellenius E, Berglindh T, Sachs G, Olbe L, Elander B, Sjöstrand SE, Wallmark B. Substituted benzimidazoles inhibit gastric acid secretion by blocking (H+ + K+) ATPase. Nature. 1981;290:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 448] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 23. | Castell DO, Kahrilas PJ, Richter JE, Vakil NB, Johnson DA, Zuckerman S, Skammer W, Levine JG. Esomeprazole (40 mg) compared with lansoprazole (30 mg) in the treatment of erosive esophagitis. Am J Gastroenterol. 2002;97:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 291] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Andersson T, Bredberg E, Sunzel M, Antonsson. Pharmacokinetics and effect on pentagastrin stimulated peak acid output (PAO)of omeprazole and its 2 optical isomers, S-omeprazole/esomeprazole and R-omeprazole. Gastroenterology. 2000;118:A1210. [DOI] [Full Text] |
| 25. | Andersson T, Hassan-Alin M, Hasselgren G, Röhss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet. 2001;40:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 26. | Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Adv Drug Deliv Rev. 2008;60:717-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 283] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Harris RZ, Jang GR, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42:1071-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | U.S. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Guidance for Industry: Foodeffect bioavailability and fed bioequivalence studies. 2002. Last accessed November 15, 2018. Available form: http://www.fda.gov/downloads/regulatory information/guidances/ucm126833.pdf. |
| 29. | Devineni D, Murphy J, Wang SS, Stieltjes H, Rothenberg P, Scheers E, Mamidi RN. Absolute oral bioavailability and pharmacokinetics of canagliflozin: A microdose study in healthy participants. Clin Pharmacol Drug Dev. 2015;4:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | China Food and Drug Administration. (2016) Technical guidelines for the bioequivalence study of generic chemical drugs with pharmacokinetics parameters as the final evaluation index. |
| 31. | U.S. Food and Drug Administration. (2013) Guidance for Industry: Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Draft Guidance. |
| 32. | Iqbal M, Ezzeldin E, Al-Rashood KA, Asiri YA, Rezk NL. Rapid determination of canagliflozin in rat plasma by UHPLC-MS/MS using negative ionization mode to avoid adduct-ions formation. Talanta. 2015;132:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Food and Drug Administration. Draft Guidance for Industry on Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an Abbreviated New Drug Application. [cited May 12 2013]. Available from: https://www.federalregister.gov/d/2013-29081. |
| 34. | Williams L, Hill DP Jr, Davis JA, Lowenthal DT. The influence of food on the absorption and metabolism of drugs: an update. Eur J Drug Metab Pharmacokinet. 1996;21:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |