Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5457
Peer-review started: June 27, 2020
First decision: June 13, 2020
Revised: September 4, 2020
Accepted: September 28, 2020
Article in press: September 28, 2020
Published online: November 6, 2020
Processing time: 156 Days and 6.6 Hours
Kawasaki disease (KD) is an acute systemic vasculitis characterized by unknown etiology.
A 4.5-year-old boy developed an acute abdomen during the onset of incomplete KD. He still had persistent abdominal pain after undergoing exploratory laparotomy and appendectomy. Ultrasound examination at early onset revealed a giant coronary artery aneurysm. The patient developed a myocardial infarction and heart failure accompanied by respiratory and cardiac arrest. He underwent coronary artery revascularization and coronary artery bypass graft using an autologous internal mammary artery. After the operation, the cardiac output increased, and the symptoms of heart failure resolved. Follow-up evaluation at 1 mo after operation showed that the patient's cardiac function had restored to New York Heart Association standard Grade I heart failure, and normal growth was obtained.
Coronary artery revascularization and coronary artery bypass graft is an effective method for treating myocardial ischemia in children with KD complicated with giant coronary artery aneurysm . Nevertheless, some issues still need specific attention.
Core Tip: Large-scale epidemiologic investigations and studies with long-term follow-up about coronary artery bypass graft in patients with Kawasaki disease have been rarely reported. At present, anastomosis of the left internal mammary artery with left anterior descending coronary artery is considered to be the optimal bypass grafting technique, as it yields satisfactory long-term patency and survival rates.
- Citation: Wang T, Wang C, Zhou KY, Wang XQ, Hu N, Hua YM. Incomplete Kawasaki disease complicated with acute abdomen: A case report. World J Clin Cases 2020; 8(21): 5457-5466
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5457.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5457
Kawasaki disease (KD) is an acute systemic vasculitis characterized by unknown etiology. It mainly affects middle-sized arteries, especially the coronary arteries. The pathologic characteristics mainly include vascular wall edema, mononuclear cell infiltration, and progressive fibrosis. Giant coronary artery aneurysms (GCAA) are the most severe sequela of KD, with an incidence of about 0.2%. GCAA do not heal spontaneously without difficulty and are always complicated by myocardial infarction or sudden death[1]. Although KD has been previously reported, the clinical features and cardiovascular surgical treatment for GCAA have not been fully studied. GCAA may be misdiagnosed or missed, especially for patients with incomplete KD. In this study, we report a case of a 4.5-year-old patient with incomplete KD complicated with GCAA and secondary myocardial infarction presenting with acute abdomen. In addition, we explore the treatment and follow-up for the patient.
A 4.5-year-old boy presented to the Emergency Department of our hospi-tal complaining of abdominal pain. During his presentation in the Emergency Department, he experienced an exploratory laparotomy, appendectomy, and peritoneal drainage.
Ten days after exploratory laparotomy, appendectomy, and peritoneal drainage, he was conscious and still reported abdominal pain. He was transferred to the West China Medical Center for further diagnosis and treatment.
The patient was admitted to a community clinic due to fever and abdominal pain for 10 d. The child developed fever accompanied by abdominal pain around the umbilicus 10 d before the visit. His body temperature fluctuated between 38 °C and 39 °C. There were no signs of rash, chapped lips, glossitis, limb swelling, or desquamation. After routine anti-infective treatment, the body temperature returned to normal on day 6 after the onset of the disease. However, the child continued to experience abdominal pain, so he was transferred to a higher-level hospital. He was diagnosed with acute simple appendicitis and treated with anti-infective therapy for 3 d. However, the abdominal pain worsened. He subsequently underwent an exploratory laparotomy, appendectomy, and peritoneal drainage. No abnormal electrocardiogram (ECG) was found before operation. Three days after surgery, he presented with a sudden cardiac arrest and underwent cardiopulmonary resuscitation, ventilator-assisted ventilation, and anti-infective treatment with meropenem combined with metronidazole. Seven days later, the child was conscious and still reported abdominal pain. He was transferred to the West China Medical Center for further diagnosis and treatment.
No personal or family history available.
Physical examination at admission showed that blood pressure was 110/80 mmHg, respiration was 24 per min, and pulse was 80 beats per min. The heart margin extended to the lower left, the heart rhythm was normal, P2 > A2, and II/VI systolic murmur was detected in the mitral valve area. The lung sounds were clear bilaterally, the liver was not palpable, and no edema was noted in the lower extremities.
The auxiliary examination included the following: An abdominal ultrasound (a small amount of ascites and no signs of biliary tract infection or obstruction); routine blood testing [white blood cell (WBC), 11 × 109/L; N, 36%; hemoglobin (Hb), 97 g/L; and platelets (PLT), 485 × 109/L]; inflammation- related parameters [C-reactive protein (CRP), 8 mg/L; erythrocyte sedimentation rate, 25 mm/h; and procalcitonin, 0.07 ng/mL]; myocardial damage (N-terminal brain-type natriuretic peptide, 577 pg/mL; troponin I, 0.61 µg/L; and myoglobin 8.2 µg/L).
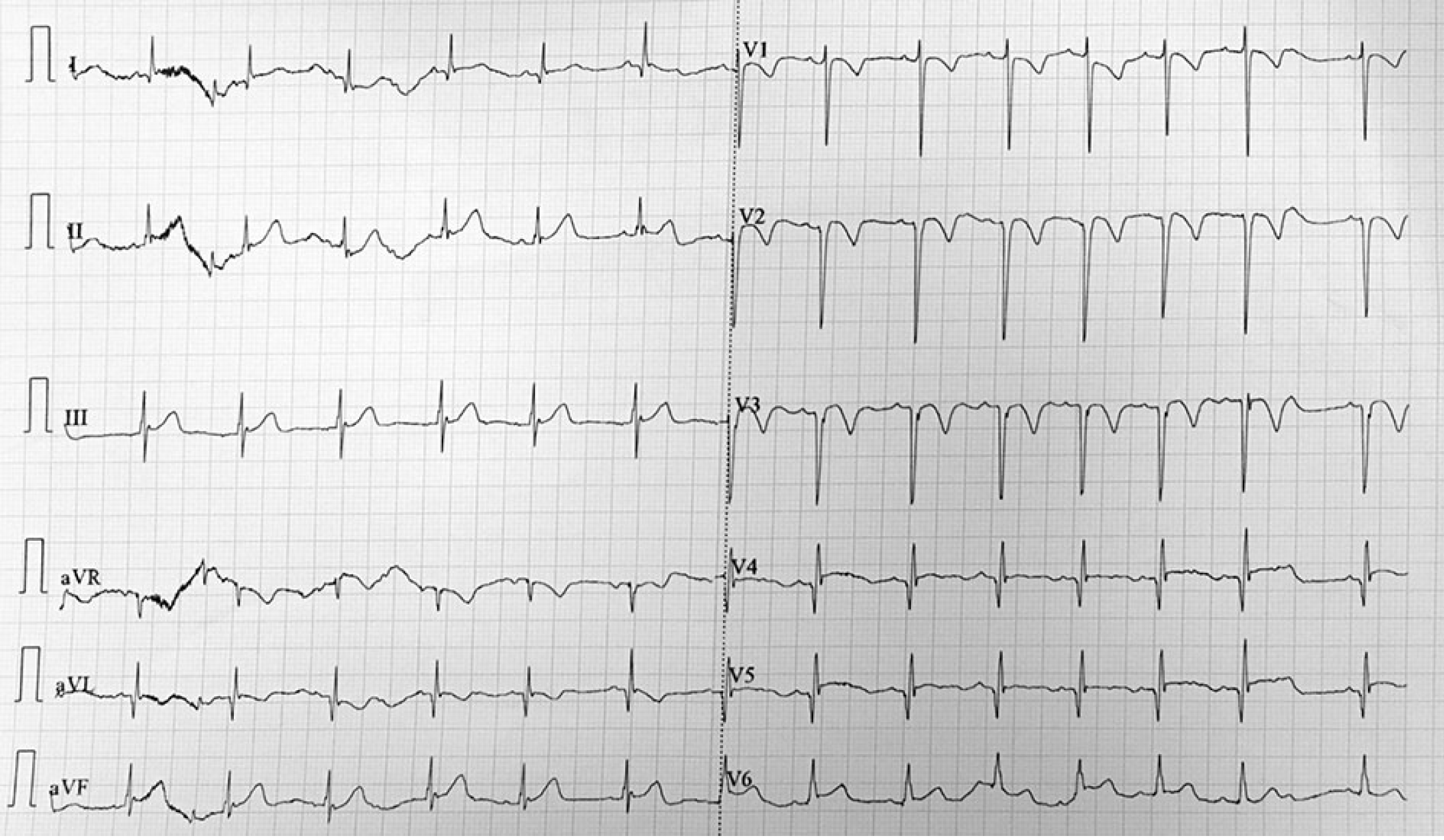
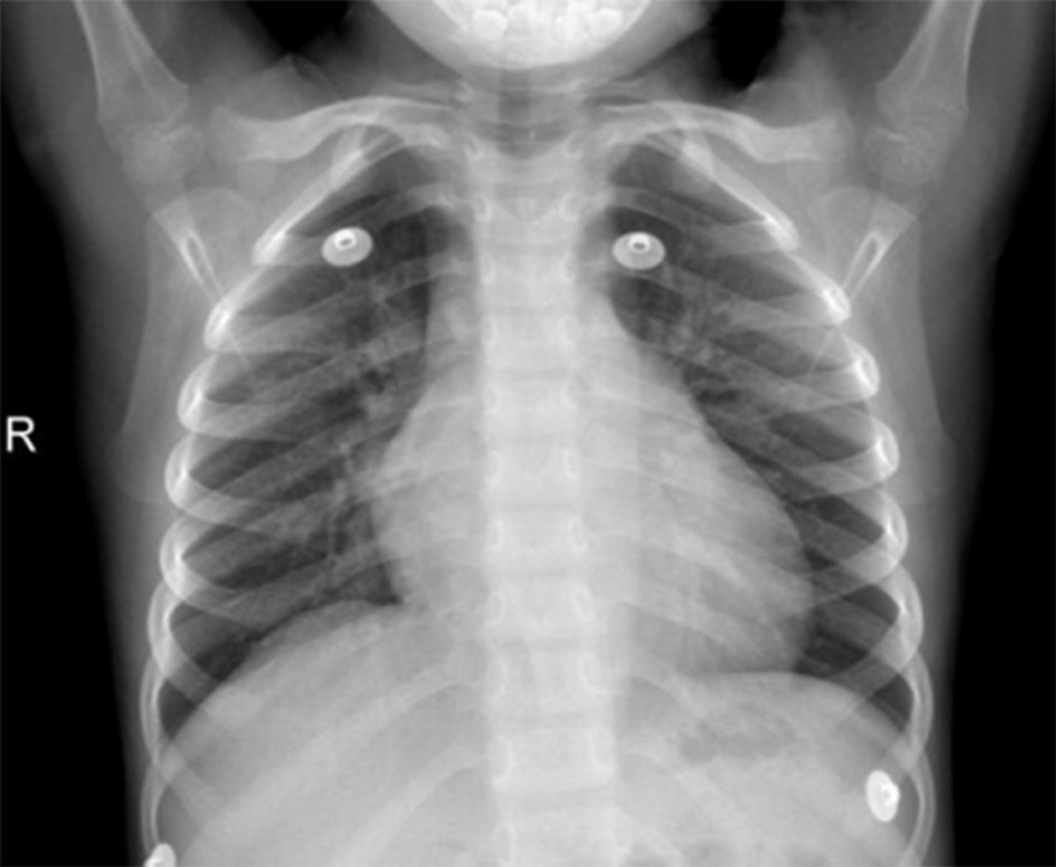
Imaging examinations included the following: Disseminated intravascular coagulation screening (D-dimer, 3.27 mg/L; fibrin and fibrinogen degradation product, 12.6 ug/L; fibrinogen (Fg), 126 mg/dL; prothrombin time (PT), 13.1 s; and activated partial thromboplastin time (APTT), 32.1 s); ECG (sinus tachycardia; non-biased axis; T-wave changes; and abnormal Q-wave; Figure 1); dynamic ECG [sinus tachycardia with sinus arrhythmia; abnormal Q-wave (V2 and V3 in QS type, V4 in QRS type, and V5 in QR type); and T-wave changes (V2-V4 T wave inversion)]; chest X-ray (heart shadow enlargement mainly in the left; Figure 2); echocardiography (tumor-like dilation complicated with a large quantity of mural thrombosis in the left anterior descending branch with a maximal diameter of 17.2 mm; slightly strong echo of 15 mm × 6 mm in size detected on the vascular wall; initial dilation in the right coronary artery with a maximal diameter of 7.5 mm; intermittent abnormal motion of the left ventricular wall; decreased left ventricular systolic function; ejection fraction (EF), 39.5%; fractional shortening (FS), 19.0%; left ventricular interval end-systolic diameter, 3.53 cm; and left ventricular internal end-diastolic diameter, 4.36 cm; Figure 3).
According to the patient's medical history, physical examinations, and auxiliary examinations, the findings met the diagnostic criteria of KD by the American Heart Association. The child was diagnosed with KD, GCAA accompanied with thrombosis, myocardial infarction, ischemic cardiomyopathy, and pericardial effusion.
Low molecular weight heparin, calcium, and warfarin were given to the patient, and clopidogrel and warfarin were given to the patient for anti-coagulant therapy after stabilization. Then, digoxin, captopril, hydrochlorothiazide, and spironolactone were administered to improve heart function. Routine blood re-examination after discharge was as follows: WBC, 6.6 × 109/L; N, 28.5%; Hb, 107 g/L; PLT, 385 × 109/L; CRP, 6 mg/L; coagulation function (disseminated intravascular coagulation screening, 0.94 mg/L; Fg, 162 mg/dL; prothrombin time, 23.8 s; APTT, 47.6 s; and INR, 2.08). The echocardiographic findings were as follows: Left and right coronary artery dilatation complicated with left coronary artery thrombosis; left ventricular wall motion desynchrony; normal low limit for the left ventricular systolic function; EF, 55%; and FS, 29%. After discharge, the patient received long-term oral of warfarin, clopidogrel, and captopril. It was recommended that he conduct monthly outpatient follow-up assessment. When Kawasaki disease is clearly diagnosed, the acute manifestations of Kawasaki disease have disappeared, and the inflammatory indexes have returned to normal. According to the 2017 American Heart Association guidelines for KD, "Those in who never has resolved and laboratory values have normalized and who echocardiograms are normal do not require IVIG treatment", the treatment plan is mainly to prevent thrombus of giant coronary artery aneurysm, and there is no longer gamma globulin shock treatment.
Three months after discharge, the child developed a paroxysmal cough, accompanied with fatigue, shortness of breath, chest tightness, and a pale complexion. He received clopidogrel in combination with warfarin, and captopril combined with betaloc when the symptoms were not mitigated. A routine blood re-examination revealed the following: WBC, 6.6 × 109/L; N, 28.5%; Hb, 107 g/L; PLT, 385 × 109/L; CRP, 6 mg/L; coagulation function (Fg, 230 mg/dL; PT, 26.5 s; APTT, 49.3 s; and international normalization ratio, 2.26); echocardiography (tumor-like dilatation with mural thrombosis in the left anterior descending coronary artery (LAD); proximal dilation up to 21 mm; the right coronary artery dilation for 6 mm; amplitude reduced in the lower ventricular septal and left ventricular apex; abnormal motion of the left ventricular wall; left ventricular enlargement; decreased left ventricular systolic function; EF, 38%; and FS, 18%). Coronary computed tomography (CT) revealed the following: Left and right coronary artery dilation; a giant coronary artery tumor with thrombosis in the left anterior descending artery; obstruction in the distal aneurysm; and smooth blood flow in the left circumflex branch (Figure 4).
Resting myocardial perfusion imaging revealed the following: Radiologic defects in aortic, anterior apical, and inferior apical consistent with obstruction in the middle segment of the anterior descending coronary artery; left ventricular enlargement, and decreased left ventricular systolic function (EF, 51%). Cardiac magnetic resonance imaging (MRI) revealed the following: Delayed sputum-enhanced imaging prompted myocardial enhancement of the left ventricular anterior wall, interventricular septum, and left ventricular posterior wall and left ventricular infarction; nearly no left ventricular apex function (Figure 5). Single photon emission CT and positron emission tomography (PET)/CT revealed the following: F-18-fluordeoxyglycose myocardial metabolic imaging (left ventricular enlargement; no glucose metabolism in the left ventricular apical, anterior wall near the apex and inferior apical myocardium, suggesting myocardial cell necrosis; decreased glucose metabolism in the middle segment of the anterior wall, suggesting survival of partial myocardial cells; and no abnormal myocardial glucose metabolism in the remaining left ventricular walls; Figure 6); coronary angiography [the left coronary opening was normal; a GCAA (21.5 mm × 19.7 mm) was noted in the end of the left main trunk that manifested as visible filling defects, suggesting the formation of thrombosis; no shadow of the left anterior descending artery of the tumor, suggesting chronic occlusion; the left circumflex branch originated from the tumor with smooth blood flow; and no stenosis or dilation of vessels or branches were observed with narrowing and expansion of the diameter and branches]. The opening of the right coronary artery was normal, and a coronary aneurysm was noted at the opening (12.3 mm in length and 6 mm in width). The tumor had a normal inner diameter and smooth blood flow. The distal branch showed formation of multiple collateral branches stretching to the blood supply area of the LAD (Figure 7).
After a general discussion, the surgical plan was determined. On July 8, 2016, CABG was performed under general anesthesia at low temperature (28 °C). The distal end of the left internal mammary artery was anastomosed to the anterior descending branch. Hemodynamic analysis was performed immediately after surgery, and the results showed that the cardiac output was improved when compared to the pre-operative cardiac output. No surgical complications occurred after surgery. He was discharged 2 wk after operation.
Cardiac function was normal, and the symptoms of heart failure were absent at 1-mo post-operative follow-up. The child reported no apparent symptoms, and his daily activities were not limited. The heart function recovered to New York Heart Association class I.
At the 2-year post-operative follow-up evaluation, heart function was maintained at grade I, and the patient had normal growth. CT coronary angiography (CTCA) revealed the CAA in LAD had shrunk significantly, and the artery distal to the aneurysm was patent.
KD is the most common systemic vasculitis affecting middle-sized blood vessels in children. KD can affect each organ in the gastrointestinal system and primarily manifests as non-specific symptoms. The gastrointestinal involvement in patients with systemic vasculitis consistently indicates a poor prognosis. Gámez-González et al[2] reported that up to 91% of patients with KD shock syndrome have gastrointestinal manifestations. In this report, the patient underwent surgical treatment because of the clinical manifestations of acute abdomen. However, the abdominal pain was not alleviated after surgery. Mesenteric thickening in the ileocecal region was consistent with the manifestation of mesenteric vasculitis. In combination with the pathogenesis of KD, the acute abdomen in this case may be correlated with intestinal ischemia, thrombosis, and dysfunction of the intestinal plexus caused by small branch vasculitis of the mesenteric artery.
Abdominal symptoms in patients with systemic vasculitis reflect that intestinal bacterial superantigen or cytokine release may also be the result of mesenteric ischemia caused by hemodynamic failure[3]. Zulian et al[4] reported that a group of patients with KD have abdominal symptoms. Four of 10 patients were initially diagnosed with toxic shock syndrome but were eventually diagnosed with KD. The similarity between KD and toxic shock syndrome in clinical features and immune responses also leads to speculation that these two diseases share common causes[5]. Therefore, if gastrointestinal symptoms were not found in children with surgical acute abdomen, the possibility of KD should be considered[6].
Accurate identification of cardiac coronary lesions depends on various imaging techniques. Two-dimensional echocardiography is still the most commonly utilized technique to identify coronary artery lesions, but it is susceptible to artifacts and operator experience. CTCA is rapidly becoming an effective imaging modality that not only detects dilation, stenosis, and aneurysms in the middle and distal segments of the coronary arteries but also accurately displays the size and morphology of the aneurysms. In the recovery phase of KD, CTCA can diagnose complications, such as segmental stenosis, intra-aneurysm thrombosis, and vessel wall calcification. CTCA is an ideal method for systematic assessment of coronary artery lesions in various segments[7]. Several studies have highlighted the significance of CTCA in the evaluation of coronary artery aneurysms in patients with KD[8-11]. The results have demonstrated that CTCA is superior to two-dimensional echocardiography in the detection of central and distal segmental aneurysms, coronary stenosis, and calcification.
MRI can be utilized to assess the degree of coronary artery disease and myocardial involvement during various stages of KD[7,12]. MRI does not produce radiation, and MRI sequence can be used to analyze the extent of intimal thickening of coronary arteries and thrombus in aneurysms. Myocardial fibrosis can be assessed by MRI of the myocardium. Delayed sputum-enhanced imaging and myocardial perfusion scan can detect and assess myocardial ischemia[12,13].
Radioisotope myocardial perfusion imaging can evaluate myocardial involvement in patients with coronary artery disease and assess the severity of myocardial ischemia and myocardial injury at rest and load status, thereby offering guidance to determine whether or not coronary artery revascularization needs to preform[7]. In addition, the degree of myocardial hypoperfusion in patients with coronary artery disease is associated with non-sustained ventricular tachycardia in the advanced stage. Total and local coronary blood flow can be measured by PET, and vascular inflammation can be assessed by 18F-fluorodeoxyglucose PET/CT[14].
Coronary artery stenosis in patients with KD is constantly accompanied with severe calcification. On the contrary, coronary artery disease in adult is mainly manifested as atherosclerosis. Coronary artery calcification, low body weight, and lack of proper vascular access are the major risk factors for the stent implantation. Therefore, CABG acts as an alternative surgical procedure[15]. CABG is recommended for children with ischemic heart disease and multiple vessel involvement[16].
After CABG in infants and young children with KD, whether or not the vascular grafts are malleable and the flow rate is sufficient to adapt to the gradually increasing activity and body growth is the key to determine the long-term surgical prognosis. Previous studies have demonstrated that the long-term patency rate of arterial grafts is significantly better than that of the great saphenous vein. The 5- and 10-year patency rates of the great saphenous vein are significantly decreased when compared with those of the arterial grafts[17].
The length and diameter of the internal thoracic artery (ITA) can be proportionally prolonged and thickened in the growth of children, whereas the great saphenous vein is adapted to the growth of children mainly by changing the growth curve based on the original length and diameter[18]. The ITA blood flow measurement and hemodynamic studies performed in exercise tests have also demonstrated that ITA can properly adapt to the increasing growth and activity of patients[19]. Consequently, ITA is known as a vital vascular graft, which is increasingly applied to CABG in infants and children.
Currently, it is a controversial issue whether or not prophylactic CABG can be performed in patients with coronary artery aneurysms without stenotic lesions. Some authors have argued that CABG should be adopted to treat patients with coronary artery stenosis, whereas patients with coronary aneurysms without stenosis should be treated with appropriate anticoagulant therapy and regular follow-up[20]. In this case, CABG was performed in a 4.5-year-old child. During the CABG, the giant left coronary aneurysm complicated by severe stenotic lesions was treated, whereas the median coronary artery tumor (maximum diameter of approximately 6 mm) in the right coronary artery trunk was untreated.
The age of onset of KD is small, and cardiac surgery should be performed at an early stage. It has been reported that the average time interval between age of onset and surgical treatment is 9.5 ± 9.4 years[21]. Anastomosis of the internal mammary artery with LAD is the most common bypass graft approach for CABG. During the follow-up period of 21.4 + 32.3 mo, the graft patency rate is 82.4%, and the early and late mortality rates are 0.6% and 3.0%, respectively[21].
Yoshikawa et al[17] reported the long-term clinical efficacy of CABG in treating KD-induced ischemic heart disease and suggested that the age of receiving surgery and whether or not ITA was performed are major factors affecting the long-term survival of patients. The 90-mo follow-up after surgery demonstrated that the survival rate in the ITA group was significantly higher than the great saphenous vein group[17].
In conclusion, large-scale epidemiologic investigations and studies with long-term follow-up about CABG in patients with KD have been rare. At present, anastomosis of the left internal mammary artery with LAD is considered to be the optimal bypass grafting technique, which yields satisfactory long-term patency and survival rates.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pernigo M S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Fukazawa R, Kobayashi T, Mikami M, Saji T, Hamaoka K, Kato H, Suzuki H, Tsuda E, Ayusawa M, Miura M, Ebata R, Kobayashi T, Yashiro M, Ogawa S. Nationwide Survey of Patients With Giant Coronary Aneurysm Secondary to Kawasaki Disease 1999-2010 in Japan. Circ J. 2017;82:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Gámez-González LB, Murata C, Muñoz-Ramírez M, Yamazaki-Nakashimada M. Clinical manifestations associated with Kawasaki disease shock syndrome in Mexican children. Eur J Pediatr. 2013;172:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Taddio A, Rossi ED, Monasta L, Pastore S, Tommasini A, Lepore L, Bronzetti G, Marrani E, Mottolese BD, Simonini G, Cimaz R, Ventura A. Describing Kawasaki shock syndrome: results from a retrospective study and literature review. Clin Rheumatol. 2017;36:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Zulian F, Falcini F, Zancan L, Martini G, Secchieri S, Luzzatto C, Zacchello F. Acute surgical abdomen as presenting manifestation of Kawasaki disease. J Pediatr. 2003;142:731-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Gupta A, Singh S. Kawasaki disease for dermatologists. Indian Dermatol Online J. 2016;7:461-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Anderson E, Gakhar N, Stull C, Caplan L. Gastrointestinal and Hepatic Disease in Vasculitis. Rheum Dis Clin North Am. 2018;44:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Tsuda E, Singhal M. Role of imaging studies in Kawasaki disease. Int J Rheum Dis. 2018;21:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Yu Y, Sun K, Wang R, Li Y, Xue H, Yu L, Chen S, Xi L. Comparison study of echocardiography and dual-source CT in diagnosis of coronary artery aneurysm due to Kawasaki disease: coronary artery disease. Echocardiography. 2011;28:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Duan Y, Wang X, Cheng Z, Wu D, Wu L. Application of prospective ECG-triggered dual-source CT coronary angiography for infants and children with coronary artery aneurysms due to Kawasaki disease. Br J Radiol. 2012;85:e1190-e1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Xing Y, Wang H, Yu X, Chen R, Hou Y. Assessment of coronary artery lesions in children with Kawasaki disease: evaluation of MSCT in comparison with 2-D echocardiography. Pediatr Radiol. 2009;39:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Arnold R, Ley S, Ley-Zaporozhan J, Eichhorn J, Schenk JP, Ulmer H, Kauczor HU. Visualization of coronary arteries in patients after childhood Kawasaki syndrome: value of multidetector CT and MR imaging in comparison to conventional coronary catheterization. Pediatr Radiol. 2007;37:998-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Goo HW. Coronary artery imaging in children. Korean J Radiol. 2015;16:239-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Vijarnsorn C, Noga M, Schantz D, Pepelassis D, Tham EB. Stress perfusion magnetic resonance imaging to detect coronary artery lesions in children. Int J Cardiovasc Imaging. 2017;33:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Suda K, Tahara N, Kudo Y, Yoshimoto H, Iemura M, Ueno T, Kaida H, Ishibashi M, Imaizumi T. Persistent coronary arterial inflammation in a patient long after the onset of Kawasaki disease. Int J Cardiol. 2012;154:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Ochi M. Review: surgical treatment of giant coronary aneurysms in pediatric patients with Kawasaki disease. Gen Thorac Cardiovasc Surg. 2018;66:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Muta H, Ishii M. Percutaneous coronary intervention versus coronary artery bypass grafting for stenotic lesions after Kawasaki disease. J Pediatr. 2010;157:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Yoshikawa Y, Yagihara T, Kameda Y, Taniguchi S, Tsuda E, Kawahira Y, Uemura H, Kitamura S. Result of surgical treatments in patients with coronary-arterial obstructive disease after Kawasaki disease. Eur J Cardiothorac Surg. 2000;17:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Kameda Y, Kitamura S, Taniguchi S, Kawata T, Mizuguchi K, Nishioka H, Sakaguchi H. Differences in adaptation to growth of children between internal thoracic artery and saphenous vein coronary bypass grafts. J Cardiovasc Surg (Torino). 2001;42:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 19. | Kitamura S. A new arena in cardiac surgery: Pediatric coronary artery bypass surgery. Proc Jpn Acad Ser B Phys Biol Sci. 2018;94:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Pahlavan PS, Niroomand F. Coronary artery aneurysm: a review. Clin Cardiol. 2006;29:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Yuan SM. Cardiac surgical procedures for the coronary sequelae of Kawasaki disease. Libyan J Med. 2012;7:19796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |