Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5439
Peer-review started: June 28, 2020
First decision: July 24, 2020
Revised: August 14, 2020
Accepted: September 29, 2020
Article in press: September 29, 2020
Published online: November 6, 2020
Processing time: 130 Days and 23.3 Hours
Anaplastic large cell lymphoma (ALCL) is a rare and heterogeneous malignant tumor, which is classified as anaplastic lymphoma kinase (ALK)positive ALCL and ALK- ALCL. Many patients are diagnosed with ALCL at the stage of bone marrow involvement. However, ALCL patients with clinical manifestations consistent with acute leukemia are relatively rare.
In this report, the patient did not receive appropriate diagnosis and treatment despite a two-year history of lymph node enlargement. Hereafter, she was admitted for B symptoms and was diagnosed as ALK-ALCL by lymph node biopsy. Then, the disease progressed to leukemia without any treatment after 2 mo. The proportion of lymphoma cells in bone marrow was as high as 96%, and the proportion of peripheral blood was 84%. She also had clinical manifestations similar to acute leukemia. After completion of chemotherapy, she developed granulocytopenia and fever and died from septicemia.
ALCL with leukemic presentation is a late manifestation of lymphoma with low chemotherapy tolerance and poor prognosis.
Core Tip: Anaplastic large cell lymphoma (ALCL) is a rare and heterogeneous malignant tumor. Many patients are diagnosed with ALCL at the stage of bone marrow involvement. However, ALCL patients with clinical manifestations consistent with acute leukemia are relatively rare. Here, we reported an anaplastic lymphoma kinase negative ALCL patient with a two-year history of lymph node enlargement who did not receive the appropriate diagnosis and treatment and rapidly progressed to acute leukemia. This suggested that ALCL with leukemic presentation is a late manifestation of lymphoma with low chemotherapy tolerance and poor prognosis.
- Citation: Zhang HF, Guo Y. Acute leukemic phase of anaplastic lymphoma kinase-anaplastic large cell lymphoma: A case report and review of the literature. World J Clin Cases 2020; 8(21): 5439-5445
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5439.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5439
Anaplastic large cell lymphoma (ALCL) is a relatively rare heterogeneous malignancy. According to the World Health Organization 2008 Classification for ALCL, the systematic ALCL is classified as anaplastic lymphoma kinase positive (ALK+) ALCL and anaplastic lymphoma kinase negative (ALK-) ALCL based on expression of ALK[1]. Previously, ALK- ALCL was considered as a provisional entity. In 2016, ALK- ALCL was first recognized as a definite entity characterized by high CD30 (Ki-1) expression, large cell proliferation and no ALK protein expression[2]. ALK- ALCL, which accounts for 2%-3% of non-Hodgkin lymphoma and 12% of T-cell non-Hodgkin lymphoma cases, can occur in all age groups with a peak at 40 to 65 years old, and it is more common in males[1]. The ALK- ALCL has a worse prognosis than ALK+ ALCL though their morphology is considered to be similar.
ALK- ALCL may be presented as various clinical manifestations. Besides peripheral and/or abdominal lymphadenopathy, patients with ALK- ALCL often have aggressive clinical symptoms, including B symptoms, elevated serum lactate dehydrogenase (LDH) levels and high International Prognostic Index score[3]. Unlike the ALK+ ALCL, ALK- ALCL involves less extranodal tissues[4]. Most patients are diagnosed with ALCL at the stage of bone marrow involvement. However, ALCL patients with clinical manifestations consistent with acute leukemia are relatively rare. Herein, we reported a case of untreated ALK-ALCL that rapidly progressed to acute leukemia and performed a literature review.
A 49-year-old female presented with bilateral submental and inguinal lymphadenopathy without pain or other discomfort.
The patient was admitted two years ago presenting with bilateral submental and inguinal lymphadenopathy without pain or other discomfort. Thereafter, the size of the involved lymph nodes was progressively growing and presented as redness in appearance. Meanwhile, she had fatigue and fever.
None.
No personal and family history available.
Swollen lymph nodes in the bilateral armpits, bilateral neck, supraclavicular, and groin area.
The serum LDH level was 554.00 U/L. Peripheral blood routine results: White blood cell, 3.02 × 109/L; lymphocytes, 0.16 × 109/L; hemoglobin, 132 g/L; and platelet count, 135 × 109/L.
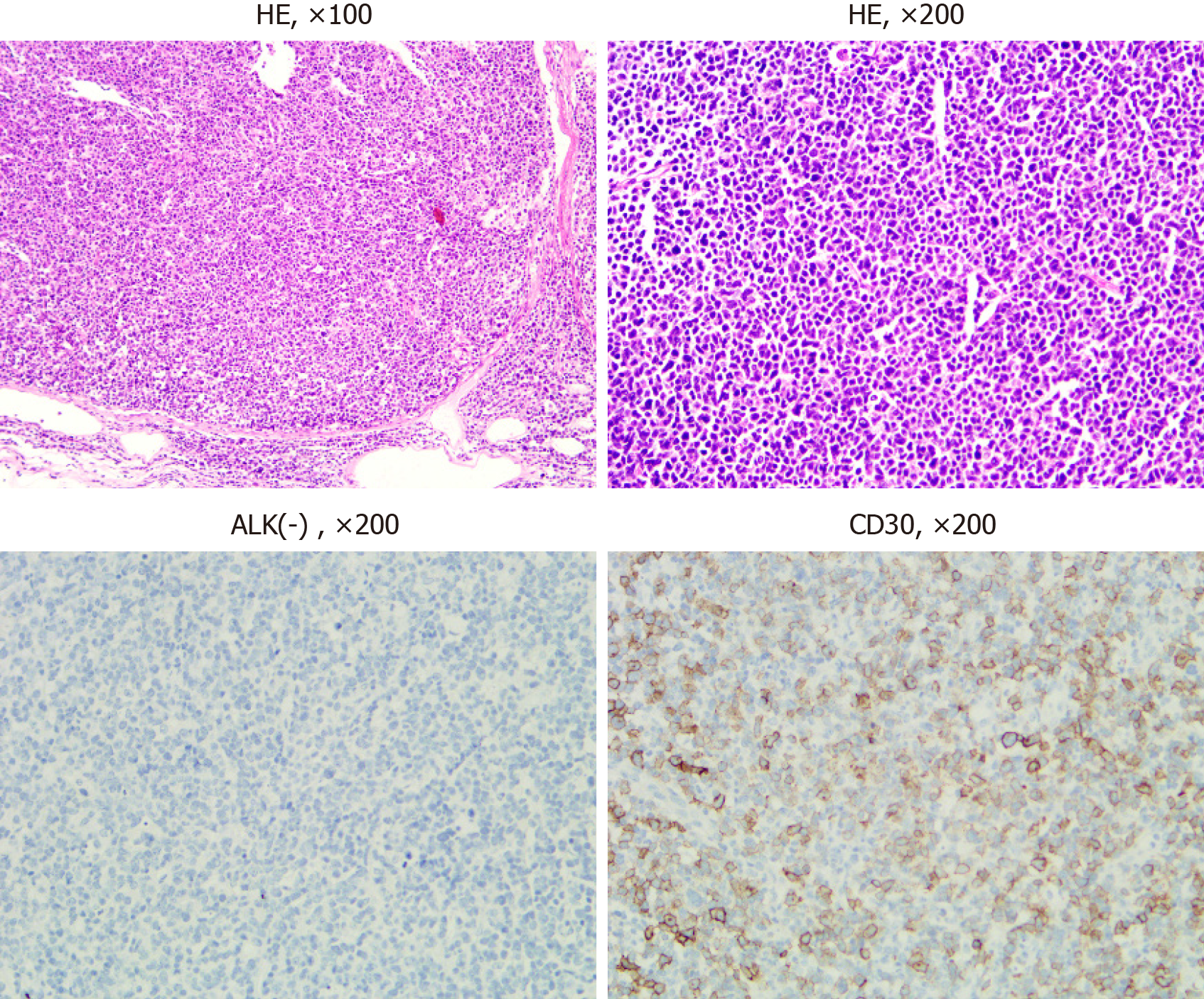
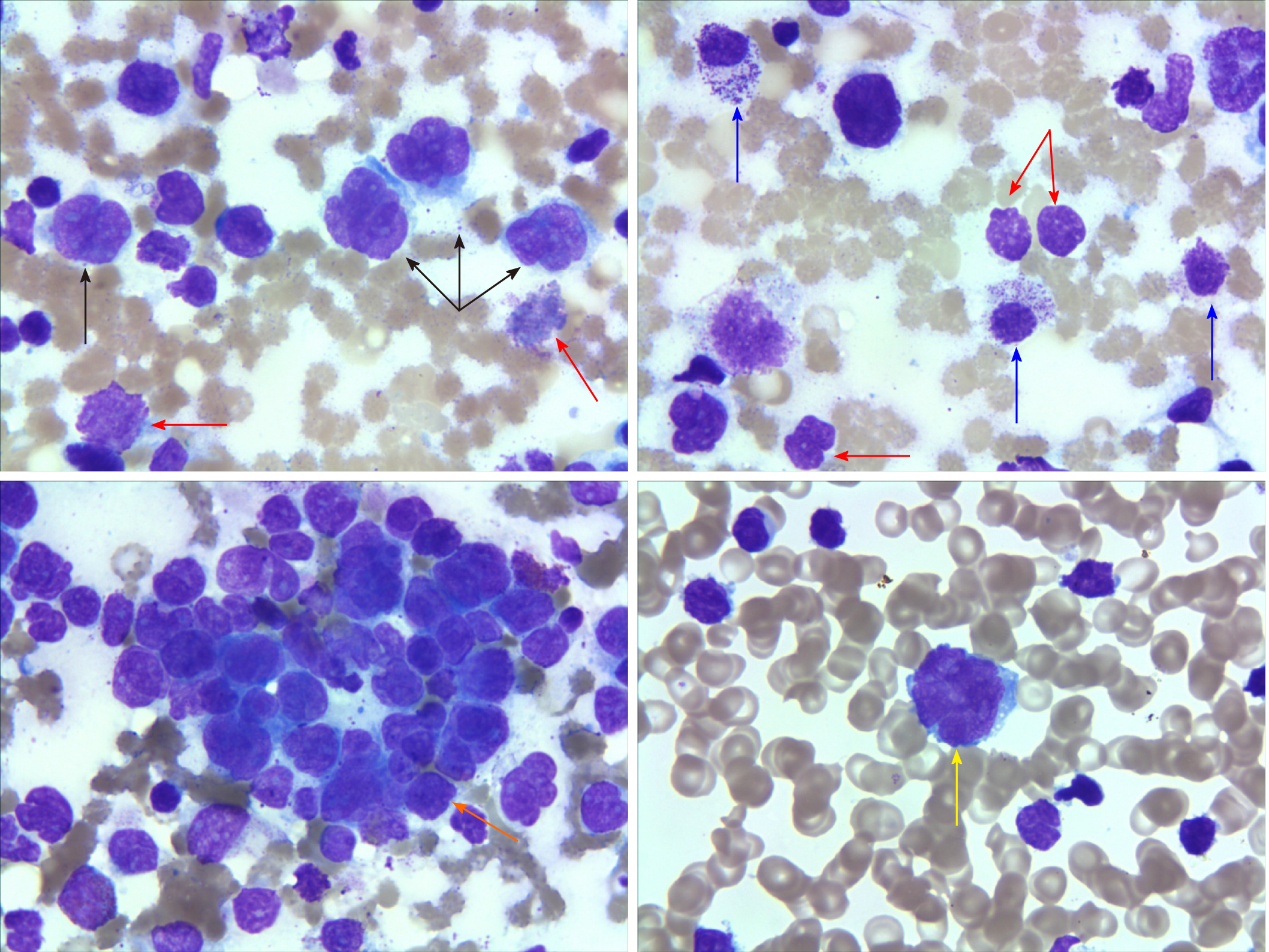
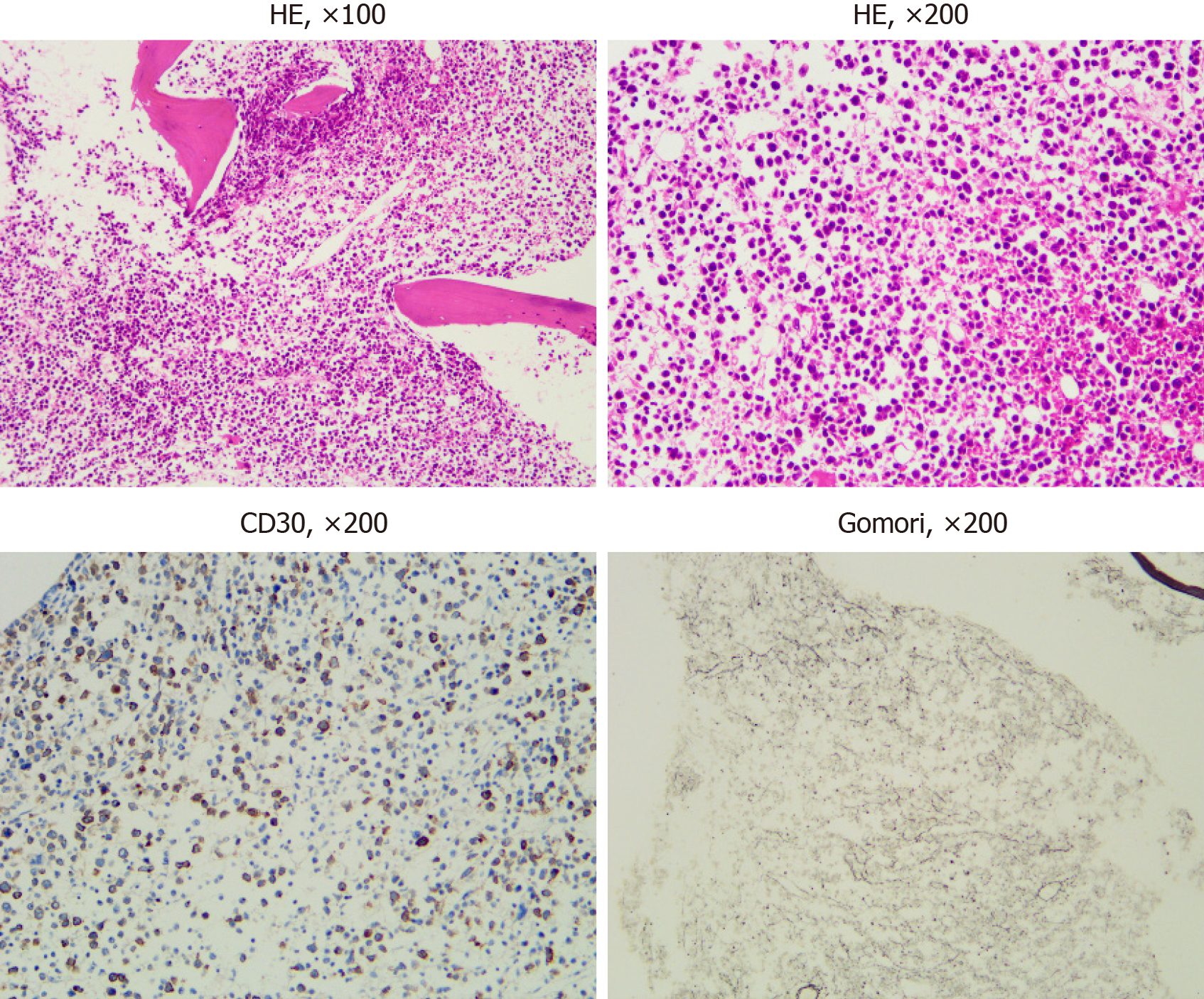
Chest and abdomen computed tomography scans showed splenomegaly and lymphadenopathy in hilum of the right lung, mediastinum and bilateral axilla, abdominal cavity, and retroperitoneum. Immunohistochemistry of left axillary lymph node biopsy showed anaplastic large cell lymphoma with CD30 (+), ALK (-), CD3 (part +), CD5 (part +), CD7 (individual +), EBER (-), CD20 (-), CD10 (-), Bcl-2 (-), Bcl-6 (-), Mum-1 (individual +), C-myc (-), and Ki-67 (70%) (Figure 1). She refused to receive bone marrow biopsy and chemotherapy and was discharged. After 2 mo, she was readmitted, with presentations of fatigue, poor appetite, and fever. Laboratory tests: White blood cell, 92.55 × 109/L; lymphocytes, 24.99 × 109/L; hemoglobin, 93.0 g/L; Platelet count, 33 × 109/L; lactate dehydrogenase, 4968.00 U/L; and Serum sodium 112.0 mmol/L. The results of a bone marrow smear (Figure 2) showed 96% medium to large lymphoma cells with convoluted nuclei and small nucleoli. In peripheral blood smears, lymphoma cells accounted for 84%. Bone marrow biopsy showed that there were many CD30 (+) tumor cells among the trabecular bones (Figure 3) and ALK (-), EBER (-), and lack of B lymphocyte immunolabeling. Reticular fiber staining was 2+ - 3+.
Combined with all the above examinations, the patient was clearly diagnosed as ALK (-) ALCL, leukemic phase.
The patient was given chemotherapy with DA-EPOCH regimen.
At 4 d after chemotherapy, she developed granulocytopenia and fever. Blood culture showed Klebsiella pneumoniae. Although given antibiotics, unfortunately, septic shock and death occurred in the short term.
ALCL is an aggressive CD30+ T-lymphocyte proliferative disease. Stein et al[5] first identified ALCL. Morris et al[6] studied the gene pathogenesis of ALCL and found the recurrent t(2;5)(p23;q35) translocation, which led to the fusion of ALK gene and the nucleophosmin gene. The diagnosis of ALCL is mainly dependent on the morphology and immunohistochemistry results of a lymph node biopsy. In the World Health Organization 2008 Classification, while ALK+ ALCL was recognized as a definite entity, systemic ALK- ALCL remained as a provisional entity due to lack of definite criteria to distinguish it from other CD30+ peripheral T-cell lymphomas (PTCLs)[1]. Previous studies have shown that the clinical manifestation, efficacy, and prognosis of ALK- ALCL were significantly different from those of ALK+ ALCL and peripheral T-cell lymphomas[7,8]. In the World Health Organization 2016 Classification, ALK- ALCL was recognized as a definite entity[2].
ALK- ALCL is often accompanied by systemic disseminated lesions and presented as B symptoms. The lesions may occur within lymph nodes or in extranodal organs including bone, soft tissue, or skin. Most patients with ALK- ALCL are diagnosed at clinical stage III or IV due to atypical and uncommon acute leukemic presentations[3]. ALK- ALCL is common in middle-aged and elderly males, while ALK+ ALCL is more common in young people less than 30 years old or children. Except for ALK, the morphology and immunohistochemistry of ALK- ALCL are similar to ALK+ ALCL. However, ALK- ALCL shows a different prognosis according to different gene rearrangements, which can be divided into DUSP22 (6p25.3), TP63 (3q28) rearrangement, or all-negative patients. The five-year overall survival rate in DUSP22 rearrangement patients can reach 90%, while is 17% in TP63 rearrangement patients[9]. The average overall survival rate is 40%-60% in ALK- ALCL patients, which is much lower than that in ALK+ ALCL[9].
For ALK+ ALCL patients, the bone, subcutaneous tissue, and spleen involvement are common. However, ALK- ALCL mainly involves the skin, liver, and gastrointestinal tract[7]. Villamor et al[10] first reported that patients with a small-cell variant of ALCL with T-cell phenotype and ALK-1 positive developed a rapid leukemic phase. A previous study[11] has shown that ALK+ ALCL patients may have presentations of leukemia at any stage of ALCL development. Leukemia phase in ALK+ ALCL is usually associated with small-cell variant and t(2;5) (p23;q35) translocation, which is more common in stage III or IV patients, indicting a poor prognosis[12-18]. Furthermore, ALK+ ALCL patients with presentations of leukemia always have complex secondary chromosome abnormalities including 1q21, 10q24, t(3; 8)(q26.2; q24) and overexpression of MCL1, HOX11/TCL3, or C-myc, which were also associated with a poor prognosis[19-21].
However, leukemia phase was uncommon in patients with ALK- ALCL[3]. In 2005, Dalal et al[22] reported a case of ALK- ALCL with leukemia presentations. This case had 12%-20% irregularly shaped blast-like cells in peripheral blood film and had no evidence of the t(2;5) translocation but a complex karyotypic abnormality with involvement of chromosomes 1, 2, 5, 7, 8, and 15[22]. Wong et al[23] also reported a ALK- ALCL case with complex karyotypes that had leukemia phase. Leukemia phase is relatively rare in ALK- ALCL, although bone marrow involvement is common. Therefore, there are few case reports concerning ALK- ALCL with leukemia phase.
Whether the difference in terms of prognosis between ALK- and ALK+ ALCL patients is related to the presence of the ALK fusion protein has been controversial. In addition to age < 40 years, β-2 microglobulin is a prognostic factor for overall survival of ALK+ ALCL and ALK- ALCL[24]. Liver involvement, albumin levels, and International Prognostic Index are prognostic factors for ALK- ALCL. Regardless of ALK expression, bone marrow infiltration appears to be associated with poor prognosis in ALCL[25]. In our report, the patient had enlarged lymph nodes for two years but did not receive systematic diagnosis and treatment. Unfortunately, bone marrow aspiration was not performed at the first admission. Thus, we cannot clarify the exact time of bone marrow involvement. The pathological results of the lymph nodes was EBER (-), and no other B cells were observed. There was currently no evidence that the leukemia was transformed from B-cell lymphoma. The patient was then diagnosed as ALK- ALCL by lymph node biopsy and rapidly progressed to lymphoma leukemia after 2 mo of noncompliance to treatments. The proportion of lymphoma cells in bone marrow reached 96%, which may mistakenly indicate an acute leukemia. After progressing to lymphoma leukemia, she died in the short term. It is a limitation that we did not perform a chromosome test for this patient during disease progression.
In conclusion, ALCL with leukemic presentation is a late manifestation of lymphoma with low chemotherapy tolerance and poor prognosis. We thought that untreated interstitial large cell lymphoma would eventually progress to the leukemia phase. Risk factors associated with leukemia transformation and post-transformation treatment strategies deserve further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kohei S, Saito M S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Swerdlow S, Campo E, Harris N. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2008. |
| 2. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5412] [Article Influence: 601.3] [Reference Citation Analysis (0)] |
| 3. | Ferreri AJ, Govi S, Pileri SA, Savage KJ. Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol. 2013;85:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Querfeld C, Khan I, Mahon B, Nelson BP, Rosen ST, Evens AM. Primary cutaneous and systemic anaplastic large cell lymphoma: clinicopathologic aspects and therapeutic options. Oncology (Williston Park). 2010;24:574-587. [PubMed] |
| 5. | Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, Gatter K, Falini B, Delsol G, Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848-858. [PubMed] |
| 6. | Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, Look AT. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263:1281-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1698] [Cited by in RCA: 1706] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 7. | Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, Rimsza L, Pileri SA, Chhanabhai M, Gascoyne RD, Armitage JO, Weisenburger DD; International Peripheral T-Cell Lymphoma Project. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496-5504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 617] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 8. | ten Berge RL, de Bruin PC, Oudejans JJ, Ossenkoppele GJ, van der Valk P, Meijer CJ. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology. 2003;43:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Villamor N, Rozman M, Esteve J, Aymerich M, Colomer D, Aguilar JL, Campo E, Montserrat E. Anaplastic large-cell lymphoma with rapid evolution to leukemic phase. Ann Hematol. 1999;78:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Nguyen JT, Condron MR, Nguyen ND, De J, Medeiros LJ, Padula A. Anaplastic large cell lymphoma in leukemic phase: extraordinarily high white blood cell count. Pathol Int. 2009;59:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Onciu M, Behm FG, Raimondi SC, Moore S, Harwood EL, Pui CH, Sandlund JT. ALK-positive anaplastic large cell lymphoma with leukemic peripheral blood involvement is a clinicopathologic entity with an unfavorable prognosis. Report of three cases and review of the literature. Am J Clin Pathol. 2003;120:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Gadage VS, Subramanian PG, Galani KS, Sehgal KK, Johari SP, Ghogale VS, Badrinath Y, Ashok K, Gujral S. Leukemic phase of anaplastic lymphoma kinase positive, anaplastic large cell lymphoma. Indian J Pathol Microbiol. 2011;54:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | He R, Viswanatha DS. Leukemic phase of ALK-positive anaplastic large cell lymphoma. Blood. 2013;121:1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Vawda A, Davis J, Vercauteren S. Leukemic transformation of anaplastic large-cell lymphoma. Blood. 2011;118:3763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Liu H, Hussein S. Small-cell variant of ALK+ anaplastic large-cell lymphoma with a leukemic phase. Blood. 2014;124:3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Spiegel A, Paillard C, Ducassou S, Perel Y, Plantaz D, Strullu M, Eischen A, Lutz P, Lamant L, Le Deley MC, Brugières L. Paediatric anaplastic large cell lymphoma with leukaemic presentation in children: a report of nine French cases. Br J Haematol. 2014;165:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Jiang X, Zhou D. ALK+ small cell variant of anaplastic large cell lymphoma with leukemic presentation. Blood. 2018;131:1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lones MA, Heerema NA, Le Beau MM, Perkins SL, Kadin ME, Kjeldsberg CR, Sposto R, Meadows A, Siegel S, Buckley J, Finlay J, Abromowitch M, Cairo MS, Sanger WG. Complex secondary chromosome abnormalities in advanced stage anaplastic large cell lymphoma of children and adolescents: a report from CCG-E08. Cancer Genet Cytogenet. 2006;171:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Monaco S, Tsao L, Murty VV, Nandula SV, Donovan V, Oesterheld J, Bhagat G, Alobeid B. Pediatric ALK+ anaplastic large cell lymphoma with t(3;8)(q26.2;q24) translocation and c-myc rearrangement terminating in a leukemic phase. Am J Hematol. 2007;82:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Wu SJ, Yao M, Tien HF, Tang JL, Lin CW, Chen YC. Anaplastic large cell lymphoma in leukemic transformation: successful treatment by transplantation. J Clin Oncol. 2007;25:4490-4492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Dalal BI, Chhanabhai M, Horsman DE, LeHuquet J, Coupland R. Anaplastic large-cell lymphoma presenting as acute leukemia. Am J Hematol. 2005;79:164-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Wong WS, Liu BW, Lam FS, Wong KF. ALK-negative anaplastic large cell lymphoma in leukemic phase with near-pentaploidy. Leuk Lymphoma. 2010;51:1927-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Proceedings of the 11th International Conference on Malignant Lymphoma, 15-18 June 2011, Lugano, Switzerland. Ann Oncol. 2011;22 Suppl 4:iv5-282. [PubMed] |
| 25. | Pfreundschuh M, Trümper L, Kloess M, Schmits R, Feller AC, Rübe C, Rudolph C, Reiser M, Hossfeld DK, Eimermacher H, Hasenclever D, Schmitz N, Loeffler M; German High-Grade Non-Hodgkin's Lymphoma Study Group. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (0)] |