Published online Nov 6, 2020. doi: 10.12998/wjcc.v8.i21.5320
Peer-review started: September 10, 2020
First decision: September 23, 2020
Revised: October 1, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: November 6, 2020
Processing time: 57 Days and 7.8 Hours
Nafamostat mesylate (NM) may prove to be one of the key drugs effective against coronavirus disease 2019 (COVID-19) because of its anti-viral properties and the potential to manage coagulopathy. However, NM tends to increase serum potassium levels.
We observed hyperkalemia immediately after NM administration (200 mg/d) in four consecutive patients who were admitted to the Kanazawa University Hospital with severe COVID-19 pneumonia. Urinary potassium excretion decreased after NM administration in three patients who underwent urinalysis.
NM is likely to produce hyperkalemia in patients with COVID-19. Therefore, it is necessary to monitor serum potassium values closely after NM initiation in COVID-19 patients who need respiratory support.
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic is a rapidly evolving situation. Nafamostat mesylate (NM) may prove to effective against COVID-19. However, our case series shows NM-induced hyperkalemia in 4 patients with COVID-19. Monitoring serum potassium levels after NM-initiation is imperative.
- Citation: Okajima M, Takahashi Y, Kaji T, Ogawa N, Mouri H. Nafamostat mesylate-induced hyperkalemia in critically ill patients with COVID-19: Four case reports. World J Clin Cases 2020; 8(21): 5320-5325
- URL: https://www.wjgnet.com/2307-8960/full/v8/i21/5320.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i21.5320
The pathophysiology of coronavirus disease 2019 (COVID-19) is still unclear; however, coagulopathy, an important mechanism of severe respiratory failure, has been observed in many severe COVID-19 cases and is associated with high mortality[1]. Therefore, managing coagulopathy is an important therapeutic target in severe COVID-19 cases.
Nafamostat mesylate (NM) is a serine protease inhibitor that inhibits proteolytic enzymes, such as thrombin, plasmin, and trypsin, and has been used for several decades in Japan for disseminated intravascular coagulation (DIC) and pancreatitis treatment.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been reported to enter epithelial cells via transmembrane protease serine 2 (TMPRSS2), which is inhibited by NM[2]. Additionally, coagulopathy in COVID-19 is characterized by enhanced fibrinolysis, which is treatable with NM[3]. Therefore, NM is anticipated to be a key drug against COVID-19.
However, NM has several side effects, with the most frequently observed side effect being hyperkalemia. We observed hyperkalemia immediately after NM administration in four consecutive patients who were admitted to the Kanazawa University Hospital between April 15 and April 27, 2020 with severe COVID-19 pneumonia and were treated with NM 200 mg/d for intravascular coagulopathy.
Patient 1 suffered from fever, dyspnea and diarrhea. Patient 2 had cough and sore throat. Patient 3 gradually developed cough, loss of appetite and malaise. Patient 4 suffered from fever, cough, sore throat, dyspnea and diarrhea.
As described above.
Patient 2 had hypertension and patient 4 had diabetes mellitus. Others had no history of past illness.
As described above.
Not applicable.
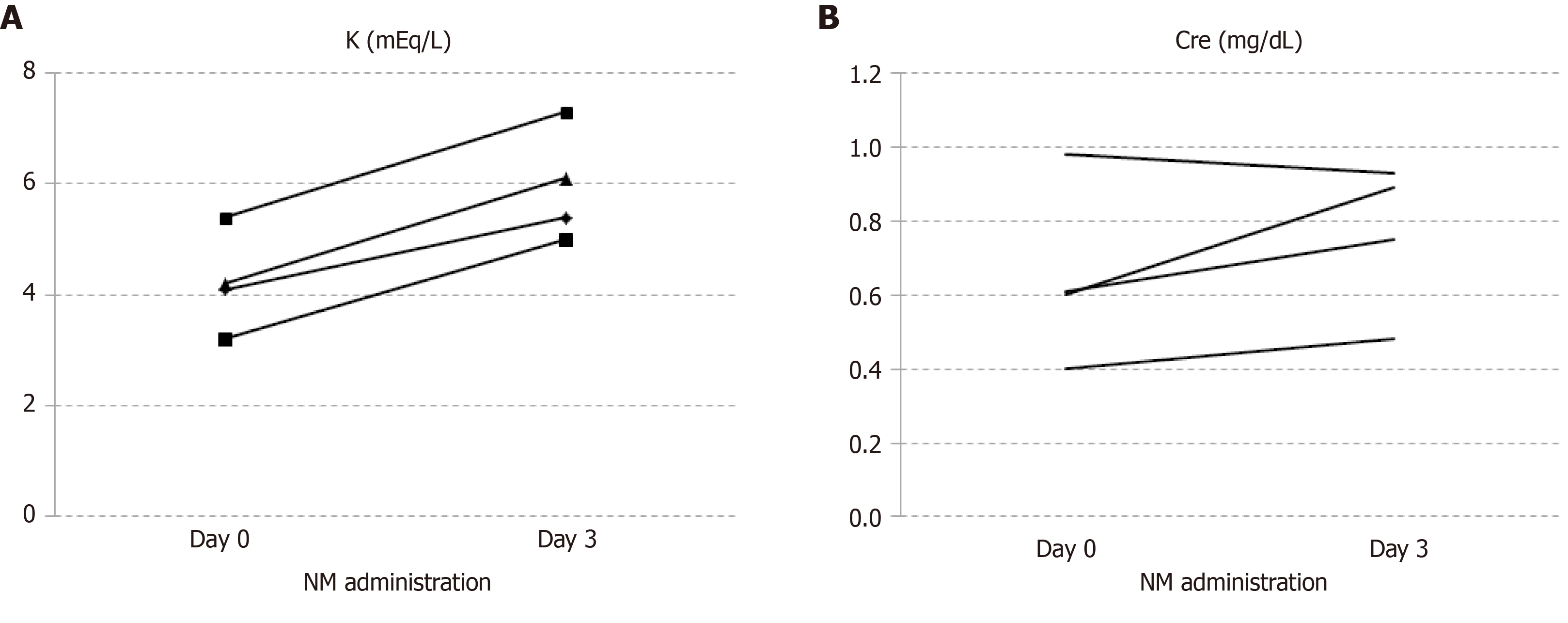
Their characteristics before NM administration are shown in Table 1. Levels of fibrin degradation products, fibrin degradation product D-dimer, and fibrinogen were elevated in all the patients. All required invasive mechanical ventilation, while two patients additionally required veno-venous extracorporeal membrane oxygenation after several days. No patient had chronic kidney disease and diarrhea; one patient was administered an angiotensin II receptor blocker, which is a potent drug that increases serum potassium levels. All four patients developed hyperkalemia (> 6 mEq/L in two patients) immediately after NM administration, without any acute kidney injuries (Figure 1). Three patients underwent urinalysis, and the urinary potassium levels were found to be relatively low; their potassium balance was positive, considering serum potassium levels were high in all of them. In the fourth patient, the transtubular potassium gradient was examined and found to be low (< 6).
| Patient | ||||
| Baseline | 1 | 2 | 3 | 4 |
| Age (yr) | 63 | 58 | 54 | 60 |
| Gender | Male | Female | Male | Female |
| Height (m) | 1.67 | 1.5 | 1.78 | 1.52 |
| Weight (kg) | 75 | 60 | 78.6 | 56 |
| Comorbidity | ||||
| Diabetes mellitus | - | - | + | - |
| Hypertension | - | + | - | - |
| Chronic kidney disease | - | - | - | - |
| Cardiovascular disease | - | - | - | - |
| Before NM initiation | ||||
| Laboratory data at NM initiation | ||||
| WBC (/L) | 11.02 × 109 | 9.92 × 109 | 6.93 × 109 | 4.2 × 109 |
| Lym (%) | 18.8 | 8.3 | 9.5 | 12.8 |
| Hb (g/dL) | 10.2 | 11.2 | 12.4 | 12.9 |
| PLT (/L) | 345 × 109 | 465 × 109 | 129 × 109 | 222 × 109 |
| Lactate (mmol/L) | 1.33 | 0.97 | 1.85 | 0.55 |
| CRP (mg/dL) | 5.99 | 9.71 | 16.41 | 10.33 |
| AST (IU/L) | 110 | 116 | 50 | 52 |
| ALT (IU/L) | 197 | 174 | 26 | 24 |
| LDH (IU/L) | 403 | 469 | 678 | 611 |
| UN (mg/dL) | 17 | 21 | 30 | 6 |
| Cre (mg/dL) | 0.61 | 0.6 | 0.98 | 0.4 |
| APTT (s) | 35.0 | 56.1 | 49.4 | 32.8 |
| PT-INR | 0.97 | 1.23 | 1.22 | 1.05 |
| FDP (mcg/mL) | 19.9 | 21.0 | 6.6 | 5.0 |
| FDP-DD (mcg/mL) | 9.5 | 10.7 | 2.2 | 1.4 |
| Fbg (mg/dL) | 578 | 605 | 531 | 484 |
| p/f | 233 | 200 | 146 | 196 |
| Physical sign | ||||
| Systolic blood pressure (mmHg) | 169 | 167 | 102 | 196 |
| Diastolic blood pressure (mmHg) | 80 | 75 | 53 | 108 |
| Heart rate (/min) | 117 | 95 | 118 | 82 |
| Respiratory rate (/min) | 29 | 20 | 18 | 15 |
| Body temperature (°C) | 37.1 | 37.7 | 38.7 | 37.2 |
| SOFA score | 3 | 2 | 6 | 3 |
| Support | ||||
| Respiratory support | IMV | IMV | IMV | IMV |
| Prone position | + | + | - | - |
| VV-ECMO | - | - | + | + |
| During NM administration | ||||
| NM [mg/(kg·h)] | 0.13 | 0.14 | 0.15 | 0.14 |
| Medication | Favipiravir, Dalteparin, Esomeprazole, Dexmedetomedine, Suvorexant, Ramelteon, Bromhexine, Mosapride | Favipiravir, Dalteparin, Esomeprazole, Suvorexant, Ramelteon, Pitavastatin, Amlodipine, Azilsartane | Dalteparin, Omeprazole, Dexmedetomedine, Propofol, Fentanyl, Midazolam, Bromhexine, Rocuronium, Noradrenaline, CTRX, AZM | Favipiravir, Esomeprazole, Omeprazole, Dexmedetomedine, Propofol, Fentanyl, Nardemedine |
| Intervention | ||||
| K-intake decrease | - | + | - | + |
| Furosemide | - | - | - | + |
| Glucose-Insulin therapy | - | - | + | - |
| K net | ||||
| K-intake [mEq/(kg·d)] | 0.90 | 1.07 | 0.34 | 0.81 |
| K-out (mEq/d) | 49.8 | 8.7 | 16.5 | |
| K-balance (mEq/d) | 7.8 | 31.3 | 51.5 |
Not applicable.
All four patients were diagnosed with NM-induced hyperkalemia.
As indicated in Table 1, all patients required antihyperkalemic therapy including potassium intake reduction, furosemide administration, and glucose-insulin therapy.
Serum potassium levels were normalized after administration of NM was stopped in all patients.
NM may be suitable against COVID-19 infection as it can effectively block the SARS-CoV-2 entry via inhibition of TMPRSS2, and possesses antifibrinolytic qualities that may treat intravascular coagulation with enhanced fibrinolysis observed in COVID-19 patients. However, NM tends to increase serum potassium levels. Generally, NM causes hyperkalemia in 0.19% of patients with pancreatitis and in 4.75% with DIC. The required dose of NM is higher for DIC patients [0.06-0.20 mg/(kg·h)] than pancreatitis patients (10-20 mg/d)[4]. Our four patients received NM at a dose of 0.13-0.16 mg/(kg·h), which is equivalent to the dose used for DIC. However, we observed hyperkalemia immediately after NM administration in all four patients, which is a higher rate than has been previously reported.
NM and its metabolites have been reported to cause hyperkalemia by inhibiting aldosterone, Na-K ATPase activity in urinary tubules, and amiloride-sensitive sodium conductance in the cortical collecting duct, resulting in the reduction of urinary potassium excretion[5]. Our findings also indicate a decrease in urinary potassium excretion after NM administration. SARS-CoV-2 has been reported to be excreted in the urine and exists in the urinary tubules of patients with severe COVID-19[6]. Therefore, SARS-CoV-2 could enhance NM-induced hyperkalemia by inhibiting Na-K ATPase activity or amiloride-sensitive sodium conductance, especially in patients with severe COVID-19 who need respiratory support.
This is the first report to suggest that NM is likely to produce hyperkalemia in patients with COVID-19. Therefore, we need to monitor serum potassium values closely after NM initiation in COVID-19 patients.
We would like for the following collaborating author names from COVSAT-KUH (Special Assistant Team against COVID-19 at the Kanazawa University Hospital) Study Group to be searchable through their individual PubMed records: Endo I, Takashima S, Okada H, Oishi M, Yoshio T and Takago S.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu YC S-Editor: Gao CC L-Editor: A P-Editor: Xing YX
| 1. | Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 835] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 2. | Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, Kinoshita N, Ohmagari N, Gohda J, Semba K, Matsuda Z, Kawaguchi Y, Kawaoka Y, Inoue JI. The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 3. | Asakura H, Ogawa H. Potential of heparin and nafamostat combination therapy for COVID-19. J Thromb Haemost. 2020;18:1521-1522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Nishimura H, Yamaya M. A Synthetic Serine Protease Inhibitor, Nafamostat Mesilate, Is a Drug Potentially Applicable to the Treatment of Ebola Virus Disease. Tohoku J Exp Med. 2015;237:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Muto S, Imai M, Asano Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen Pharmacol. 1995;26:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Nomoto H, Ishikane M, Katagiri D, Kinoshita N, Nagashima M, Sadamasu K, Yoshimura K, Ohmagari N. Cautious handling of urine from moderate to severe COVID-19 patients. Am J Infect Control. 2020;48:969-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |