Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.5057
Peer-review started: July 16, 2020
First decision: August 21, 2020
Revised: August 22, 2020
Accepted: September 5, 2020
Article in press: September 5, 2020
Published online: October 26, 2020
Processing time: 101 Days and 23.7 Hours
Hypersensitivity pneumonitis (HP) is an immune-mediated syndrome caused by allergen inhalation. High-resolution computed tomography (HRCT) of HP may show diffuse ground-glass opacity, centrilobular ground-glass nodules, areas of air-trapping, thin-walled cysts, or fibrotic changes.
A 47-year-old male patient went to the hospital complaining of cough and gradual aggravation of shortness of breath. HRCT of the lung showed that multiple nodules and ground-glass high-density shadows were present in both lungs. In addition, circular high-density shadows of various sizes were widely distributed in both lungs with relatively normal lung markings inside them. But other tests did not have a positive finding that can clarify the cause. Therefore, the patient underwent a lung biopsy. The pathological results showed that the lesions tended to be HP. After 4 mo of follow-up, the lesions in the patient's lungs were absorbed spontaneously, and the symptoms of cough and shortness of breath have disappeared. The review results suggested that the patient’s disease was self-healing, which was consistent with the characteristics of HP.
For some patients with HP, abnormal HRCT findings, such as the lesions in the lungs, can be absorbed on their own, which is an important clue in the diagnosis of the disease. Early diagnosis by lung biopsy is necessary when antigen exposure is unknown.
Core Tip: We report a case of hypersensitivity pneumonitis (HP) with special high-resolution computed tomography (HRCT) findings. Circular high-density shadows of various sizes were widely distributed in both lungs with lung markings inside them. For some patients with HP, abnormal HRCT findings, such as the lesions in the lungs, can be absorbed on their own, which is an important clue in the diagnosis of the disease.
- Citation: Wang HJ, Chen XJ, Fan LX, Qi QL, Chen QZ. Rare imaging findings of hypersensitivity pneumonitis: A case report. World J Clin Cases 2020; 8(20): 5057-5061
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/5057.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.5057
The diagnosis of hypersensitivity pneumonitis (HP) requires a combination of medical history, clinical manifestations, radiological findings, pulmonary function, bronchoalveolar lavage (BAL) findings, and histopathological characteristics[1]. However, HP is sometimes difficult to diagnose without a clear history of specific allergen exposure. High-resolution computed tomography (HRCT) is recommended for the diagnosis of HP. For patients suspected of having HP who have no known antigen exposure and whose HRCT findings and other examination lack definitive results, early diagnosis by lung biopsy is necessary[2].
A 47-year-old male patient went to the hospital complaining of cough and gradual aggravation of shortness of breath.
One month before, he once had mild fever for 1 wk (Tmax < 37.5°C). The patient disclosed that he used an air humidifier 1 mo before the onset of symptoms, but no other specific antigen exposure history was found.
The patient had a free previous medical history.
Before bronchoscopy, the patient’s temperature was 36.7 °C, heart rate was 80 bpm, respiratory rate was 22 breaths per minute, blood pressure was 117/69 mmHg, and oxygen saturation in room air was 98%.
Further laboratory examination revealed an antinuclear antibody titer of 1:80, erythrocyte sedimentation rate (ESR) of 25 mm/h, immunoglobulin G level (IgG) of 5.67 g/L, and cytokeratin 19 fragment of 2.72 ng/mL. The results of blood gas analysis, blood count, procalitonin, interleukin 6 (IL-6), G test, GM test, IgE, eosinophil count, T-SPOT, tuberculosis antibody, and rheumatoid factor were all within normal limits.
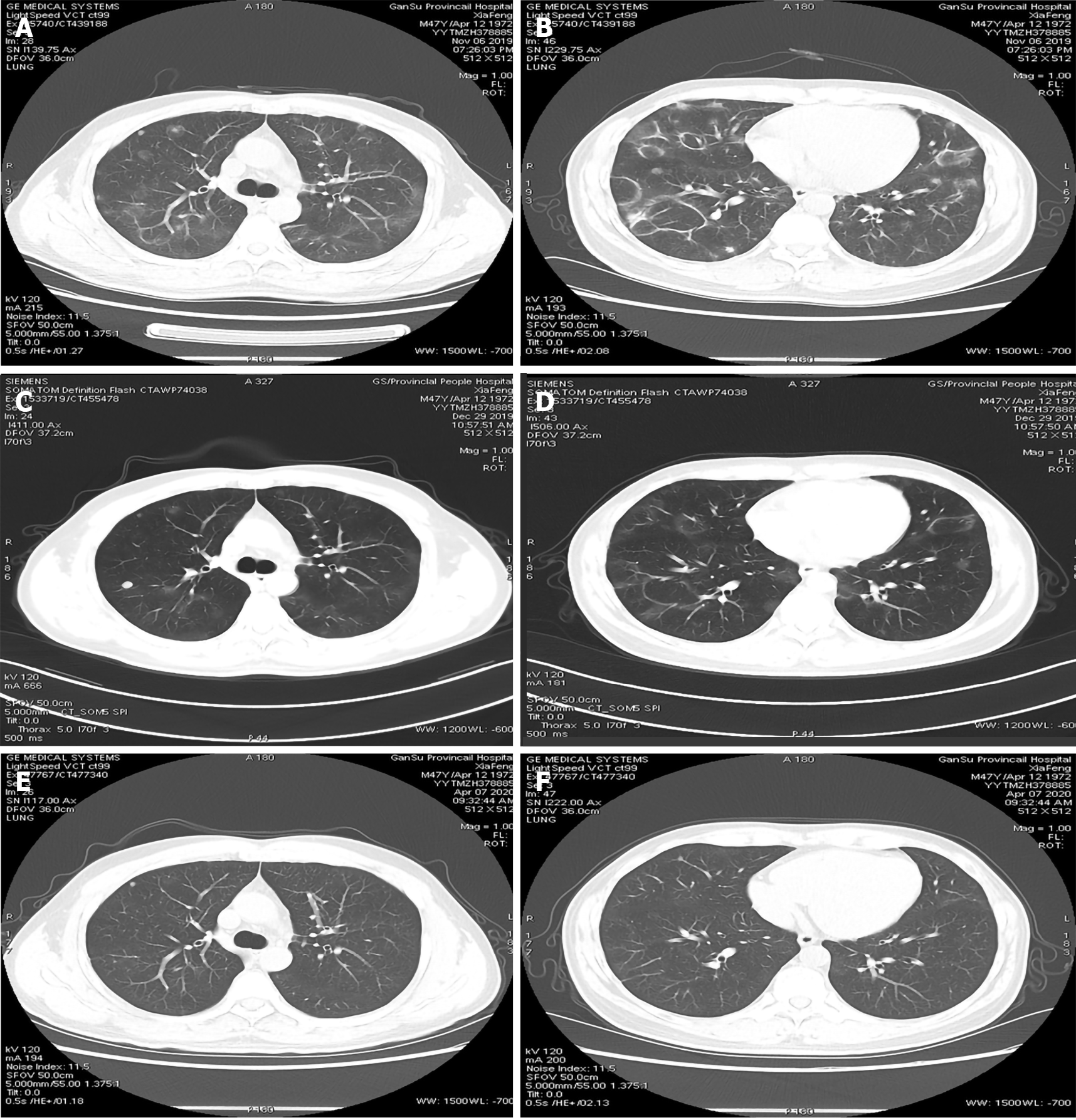
HRCT of the lung showed that multiple nodules and ground-glass high-density shadows were present in both lungs (Figure 1A). In addition, circular high-density shadows of various sizes were widely distributed in both lungs with relatively normal lung markings inside them (Figure 1B).
Bronchoscopy revealed no obvious abnormalities. Acid-fast bacillus was identified by smear of brush biopsy, whereas Gene Xpert and Mycobacterium liquid culture were both negative in the BAL fluid. No acid-fast bacillus was found in three other sputum smears.
The patient had no classical symptoms of tuberculosis, such as chronic cough with blood-containing mucus, fever, night sweats, and weight loss, and the imaging findings were not consistent with tuberculosis. Thus, the acid-fast bacilli detected by bronchoscopy may have been caused by laboratory contamination. The patient was transferred to another teaching Hospital (China-Japan Friendship Hospital) for further examination.
Further examination including acid-fast staining, Gene Xpert, and Mycobacterium liquid culture of BAL lavage and lung biopsy specimens revealed no evidence of tuberculosis and other specific pathogens. Pulmonary function test results suggested a mild decrease in diffusion (DLCO SB 75.8%). The pathological results showed that the alveolar interval was slightly widened. A high level of lymphocyte infiltration was seen in the interval and around the small blood vessels. Few loose fibroblast clusters were seen in the focal alveolar cavity. Small granuloma formation was observed around individual small blood vessels. Immunohistochemical results were as follows: CK7 (+), KP-1(+), CD3 (+), and CD20 (L26) (+). Special staining results were as follows: Masson (+), PAS (-), acid fast (-), and silver stain (-). An increase in the proportion of lymphocytes and neutrophils was observed in the alveolar lavage fluid, which did not show any growth of pathogenic bacteria. According to the above mentioned examination results, the occurrence of HP was highly possible. Given that the symptoms of the patient were not severe and acid-fast bacilli were once positive, corticosteroid was not administered, and getting rid of possible allergens and a return visit were requested.
The final diagnosis of the presented case was HP.
Given that the symptoms of the patient were not severe and acid-fast bacilli were once positive, corticosteroid was not administered.
One month later, the patient underwent lung HRCT and pulmonary function test. HRCT displayed that the original ground glass opacity and nodular shadows were thinner than before (Figure 1C and D). The spirometry results showed that the diffusion function of the lungs returned to normal. The symptoms of cough and shortness of breath have disappeared. The review results suggested that the patient’s disease was self-healing, which was consistent with the characteristics of HP.
Three months later, the patient underwent lung HRCT again, which revealed that the lesions in the lungs have been absorbed (Figure 1E and F). All these finding contributed to the confirmation of HP diagnosis.
HP is a complex syndrome involving diffuse parenchymal lung disease caused by inhalation of and sensitization to an antigen[3]. However, a clear history of allergen exposure may not exist for patients with HP, especially for those with subacute and chronic HP. A large number of substances can cause HP when inhaled as fine particles. HP is sometimes difficult to diagnose if exposure to an antigenic agent is unknown. In this case, it is necessary to combine clinical manifestations, imaging examination results, fiberoptic bronchoscopic findings, and even pathological examination to achieve a diagnosis.
HRCT is recommended for the diagnosis of HP. The HRCT patterns typical for HP include the following: A centrilobular diffuse micronodular pattern, ground-glass opacification, mosaic attenuation, areas of air-trapping, thin-walled cysts, and fibrosis (reticulation, architectural distortion, and traction bronchiectasis with or without honeycomb change)[4-6]. This patient’s CT results showed circular high-density shadows of various sizes, multiple nodules, and ground-glass high-density shadows in both lungs. These results may be due to vasodilation caused by allergies. However, we cannot yet explain why vasodilation was distributed in a circular shape. This pattern is not consistent with those of common HP. These CT findings need to be distinguished from those of cryptogenic organizing pneumonia (COP). Thus, the patient underwent CT-guided needle biopsy of the lung via rigid bronchoscopy under CT guidance.
In acute HP, histopathology shows peribronchovascular fibrin deposition and interstitial accumulation of neutrophils, lymphocytes, plasma cells, and macrophages. The histologic changes of subacute HP consist of a classic triad of lymphocytic interstitial infiltrate, cellular bronchiolitis, and poorly formed non-necrotizing granulomas. Chronic HP often manifests fibrotic changes, such as honeycombing and reticular opacities[3,7]. This case’s pathological characteristics are consistent with subacute HP. However, we have to emphasize that histopathological evaluation is only recommended for cases in which a diagnosis cannot be reached by other means, especially when information on possible antigen exposure is lacking.
Acid-fast bacillus was found by smear of brush biopsy in the first bronchoscopic examination, but no growth of Mycobacterium tuberculosis and non-tuberculous mycobacteria was observed after the culture. Other tuberculosis-related tests showed negative results, and the lung lesions were absorbed on their own. The initial bronchoscopic examination performed on the patient was the first examination performed in the bronchoscopy room on that day, and the specimen was immediately taken to the laboratory for examination. Therefore, we considered that the detection of acid-fast bacilli was caused by laboratory contamination. For patients with HP, early diagnosis and corticosteroid therapy might prevent the progression of the disease to lung fibrosis. Therefore, it is important to reduce laboratory contamination and provide accurate reference results for the clinician.
We report a case of HP without a clear history of allergen exposure, which has special imaging findings of HP. For some patients with HP, abnormal HRCT findings, such as the lesions in the lungs, can be absorbed on their own, which is an important clue in the diagnosis of the disease. Early diagnosis by lung biopsy is necessary when antigen exposure is unknown.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair):
Grade E (Poor): 0
P-Reviewer: Bordin DS S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Vasakova M, Selman M, Morell F, Sterclova M, Molina-Molina M, Raghu G. Hypersensitivity Pneumonitis: Current Concepts of Pathogenesis and Potential Targets for Treatment. Am J Respir Crit Care Med. 2019;200:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Leone PM, Richeldi L. Current Diagnosis and Management of Hypersensitivity Pneumonitis. Tuberc Respir Dis (Seoul). 2020;83:122-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Magee AL, Montner SM, Husain A, Adegunsoye A, Vij R, Chung JH. Imaging of Hypersensitivity Pneumonitis. Radiol Clin North Am. 2016;54:1033-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Sahin H, Kaproth-Joslin K, Hobbs SK. Hypersensitivity Pneumonitis. Semin Roentgenol. 2019;54:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Jędrych ME, Szturmowicz M, Bestry I, Kuś J. [Hypersensitivity pneumonitis: Diagnostic criteria, treatment, prognosis and prevention]. Med Pr. 2016;67:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Vasakova M, Morell F, Walsh S, Leslie K, Raghu G. Hypersensitivity Pneumonitis: Perspectives in Diagnosis and Management. Am J Respir Crit Care Med. 2017;196:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 283] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 7. | Torres PP, Moreira MA, Silva DG, da Gama RR, Sugita DM, Moreira MA. High-resolution computed tomography and histopathological findings in hypersensitivity pneumonitis: a pictorial essay. Radiol Bras. 2016;49:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |