Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.5036
Peer-review started: May 27, 2020
First decision: June 13, 2020
Revised: June 18, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: October 26, 2020
Processing time: 152 Days and 7.4 Hours
Choriocarcinoma is a highly malignant trophoblastic tumor that presents with early symptoms similar to those of an ectopic pregnancy. Here we present a patient with suspected ectopic pregnancy diagnosed by laparoscopic surgery in our hospital. The patient was found to have choriocarcinoma that had metastasized to the lumbar muscle and presented with symptoms similar to those of an ectopic pregnancy.
The patient was a 34-year-old female who complained of amenorrhea lasting 53 d, 7 d of right lower back pain, and 3 d of right lower abdominal pain. Transvaginal ultrasonography revealed the absence of a gestational sac in the uterus and a mass in the left adnexa. After 6 d of re-examination, ultrasound and computed tomography (CT) examination were performed on the mass located in the left adnexa area. We also noted that the patient’s serum β-human chorionic gonadotropin (hCG) level was increased. Considering an ectopic pregnancy, we performed a laparoscopy and hysteroscopy. During the operation, a left ovarian mixed echogenic mass approximately 2.5 cm × 2.0 cm with no villous tissue was found. Postoperative levels of serum hCG continued to increase. Lung CT examination showed lung nodules. Both CT and magnetic resonance imaging showed a mixed echogenic mass in the lumbar muscle. Considering lumbar metastasis of choriocarcinoma, six courses of cisplatin, dactinomycin, and etoposide chemotherapy were given after surgery. The patient’s serum β-hCG level decreased to normal and the mixed echogenic mass in the lumbar muscle decreased in size after the fifth course of chemotherapy. All symptoms subsequently disappeared after treatment.
In summary, lumbar metastasis from choriocarcinoma is extremely rare. Appropriate chemotherapy can successfully treat these metastasized tumors.
Core Tip: Choriocarcinoma is a highly malignant trophoblastic tumor. Depending on the disease site, the clinical manifestations of choriocarcinoma are diverse and unique in each case, making diagnosis a challenge. We report the case of a patient with choriocarcinoma that metastasized to the lumbar muscle and caused symptoms similar to those of an ectopic pregnancy. Considering the lumbar metastasis of choriocarcinoma, 6 courses of cisplatin, dactinomycin, and etoposide chemotherapy were given after surgery. The patient's serum β-human chorionic gonadotropin level decreased to normal and the mixed echogenic mass in the lumbar muscle decreased after the 5th course of chemotherapy, and her symptoms disappeared.
- Citation: Pang L, Ma XX. Choriocarcinoma with lumbar muscle metastases: A case report. World J Clin Cases 2020; 8(20): 5036-5041
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/5036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.5036
Choriocarcinoma is a highly malignant tumor originating from trophoblastic tissue, which may be secondary to any type of pregnancy, a hydatidiform mole, or intrauterine or ectopic pregnancy[1,2]. Choriocarcinoma tends to metastasize to the lung, vagina, pelvis, or liver in more than 50% of patients[3-7]. Depending on the disease site, the clinical manifestations of choriocarcinoma are diverse and unique in each case, making diagnosis a challenge. For example, lung lesions can cause coughing and hemoptysis, brain lesions can cause headaches and visual impairment, metastasis to the uterus may cause vaginal bleeding, abdominal pain, intra-abdominal bleeding, and clinical symptoms similar to an ectopic pregnancy[8]. The most common clinical symptoms of choriocarcinoma are reported to be cardiopulmonary discomfort (20.66%), followed by symptoms of the gastrointestinal tract (18.43%), and the central nervous system (17.67%). In addition, 9.91%, 5.28%, and 4.95% of cases involved pregnancy bleeding, renal manifestations, and ocular manifestations, respectively[9]. In the current case presented here, the primary lesion was located in the lumbar region, which caused back and lower abdominal pain. This metastasis site is extremely rare.
The findings of this report demonstrate that if a patient’s serum human chorionic gonadotropin (hCG) levels are elevated, physicians should pay attention to patient complaints and symptoms. If these clinical manifestations are not due to pregnancy, it may lead to an early tumor diagnosis; this can help physicians provide treatment in a timely manner to improve clinical prognosis. With early diagnosis and appropriate chemotherapy treatment, tumors can be treated effectively. To our knowledge, this is the first report of choriocarcinoma metastasizing to the lumbar muscles.
A 34-year-old female was admitted to The Second Shengjing Hospital of China Medical University (Shenyang, China) complaining of amenorrhea lasting 53 d, 7 d of right lower back pain, and 3 d of right lower abdominal pain.
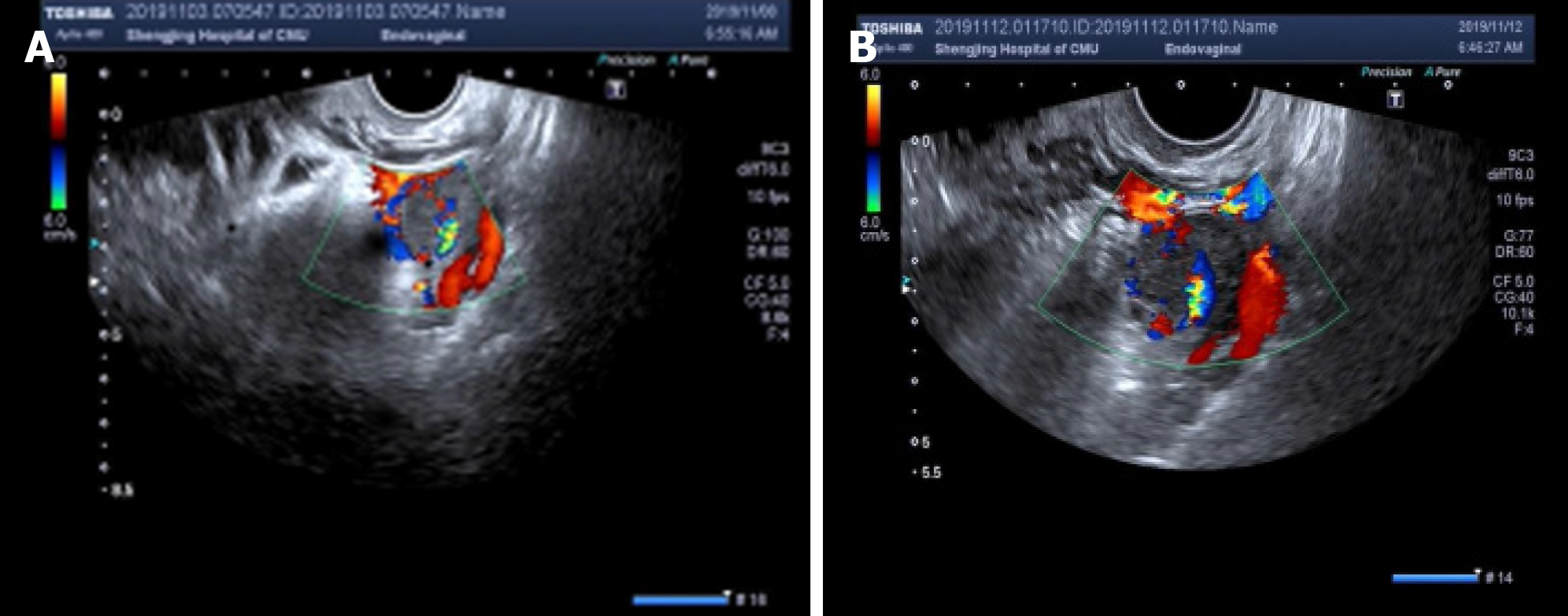
Transvaginal ultrasonography scans performed upon admission revealed a thickened endometrium of approximately 1.4 cm with an uneven echo. A 1.9 cm × 1.4 cm mass was seen in the left adnexa area, which was irregular in shape and had a mixed echo of low and medium. A circular blood flow signal was detected around the mass. No obvious space-occupying lesions were seen on the uterine wall (Figure 1A). The patient’s serum β-human chorionic gonadotropin (β-hCG) level was 33541 mIU/mL at admission.
The patient had a history of three pregnancies. One was an ectopic pregnancy, which resulted in a laparoscopically resected right fallopian tube in 2016; the last pregnancy was aborted. Her menstrual history was unremarkable with her last menstrual period occurring 53 d before this hospital admission.
There were no abnormalities noted on physical examination.
Her serum β-hCG level had increased to 35377 mIU/mL and 46553 mIU/mL on the second and sixth day after admission, respectively. However, all other blood test results were normal.
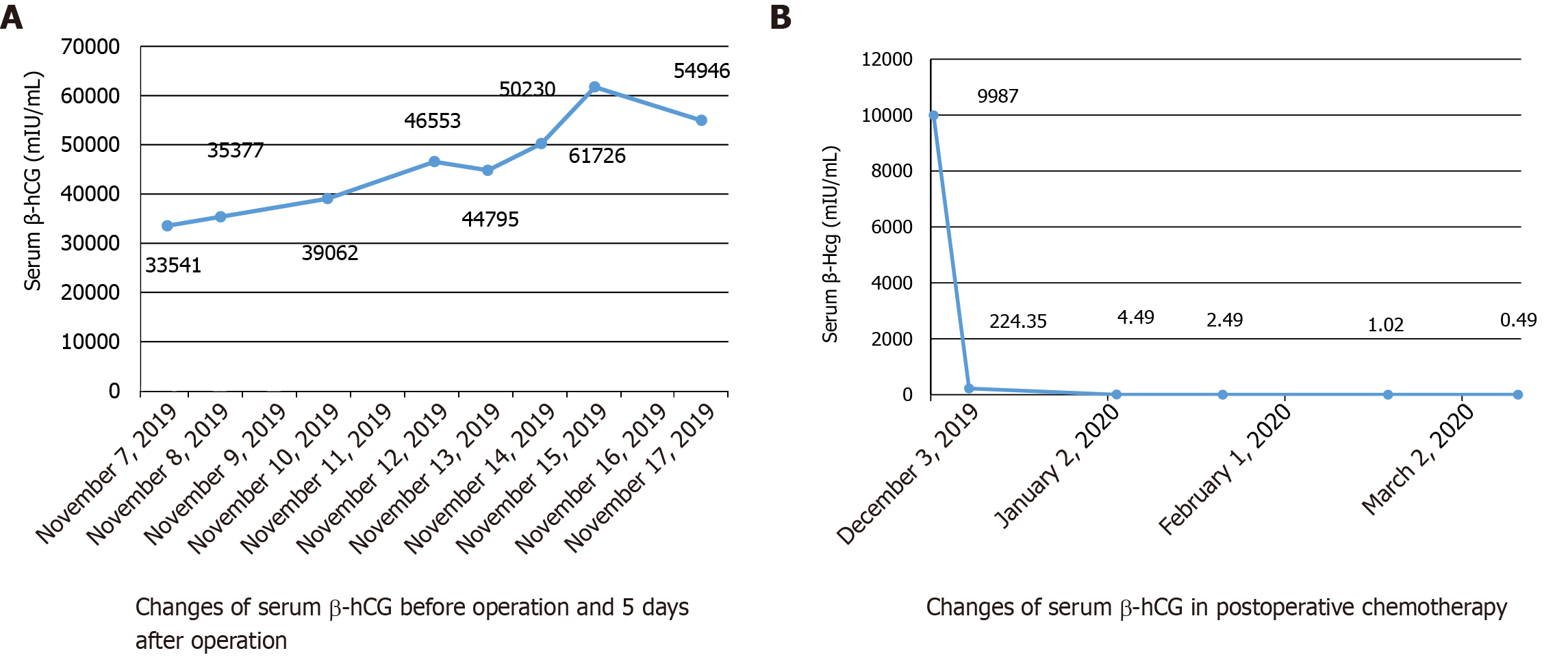
After 6 d, she was re-examined by transvaginal ultrasonography and computed tomography (CT). These images revealed that the mixed echogenic mass in the left adnexa area had increased to 2.5 cm × 2.1 cm (Figure 1B). CT examination also revealed a 2.9 cm × 3.1 cm mixed echogenic mass in front of the right lumbar muscle. Surgical consultation was not sought at that time. An ectopic pregnancy was suspected based on these findings and an emergency laparoscopy was performed under general anesthesia, along with a hysteroscopy adhesion separation and endometrial biopsy. The laparoscopy revealed a cystic projection measuring 2.5 cm × 2 cm located on the left ovary. No abnormalities were observed in the left fallopian tube and right ovary, and the right fallopian tube was absent. The uterus was slightly larger and smoother than average. A left ovary mass was excluded but no pregnancy tissues were visible to the naked eye. Hysteroscopy revealed an adhesion measuring 4 cm located on the anterior and posterior walls of the uterine cavity and the appearance of decidual changes in the uterine cavity. The adhesions and a small amount of intrauterine tissue were sent for pathological analysis and were reported as decidua. During the operation, the ultrasound director requested abdominal ultrasound of the pelvic cavity. However, no pelvic mass was found. Laparoscopy also did not reveal a mass in the pelvic or abdominal cavity. The patient’s serum β-hCG level was 44795 mIU/mL during the operation and persisted at a high-level following surgery, with changes in serum β-hCG before surgery and 5 d after surgery (Figure 2A).
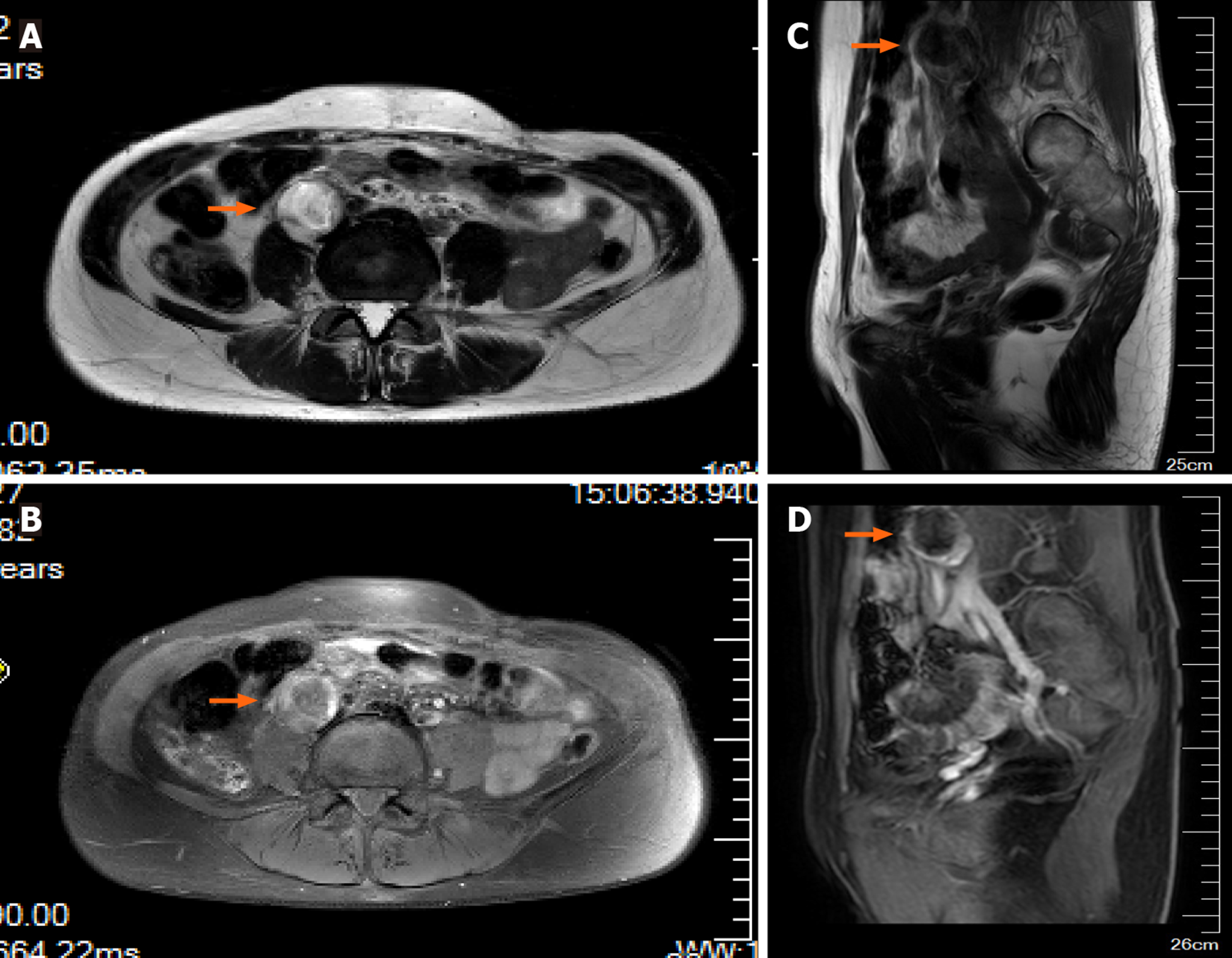
Histopathological examination identified the uterine contents as decidual tissue, and the left ovarian surface mass was luteal tissue. CT examination demonstrated small nodules in the right upper lobe subpleural tip one day after surgery. Lung metastasis was detected on CT and enhanced pelvic magnetic resonance imaging (MRI) demonstrated a cystic solid mass in front of the right lumbar muscle approximately 2.5 cm × 2.1 cm in size. The parenchymal signal of the lesion was slightly mixed, showing a long T2 signal, an equal and slightly longer T1 signal. On enhancing the scan edge, the boundary between the lumbar muscles was not clear. There was no abnormal strengthening of the myometrium (Figure 3). Whole abdominal CT demonstrated a cystic solid mass approximately 2.9 cm × 3.1 cm in size, which was in the right lumbar muscle with an unclear border and thick wall. The wall of the enhanced scan was significantly strengthened.
Based on these findings, the patient was diagnosed with choriocarcinoma (IV:9).
The patient underwent six cycles of cisplatin, dactinomycin, and etoposide therapy.
Complete disappearance of the metastatic lesion in the lung was confirmed by CT after four cycles of chemotherapy; the lumbar muscle mass disappeared after five cycles. The patient’s serum β-hCG level was normal after the second cycle of chemotherapy (Figure 2B).
Gestational trophoblastic neoplasia is a group of malignant tumors related to pregnancy. Histologically derived from placental trophoblasts, the group is divided into either an invasive mole, choriocarcinoma, placental site trophoblastic tumor, or epithelioid trophoblastic tumor. Choriocarcinoma is a common gestational trophoblastic tumor with significant ethnic and regional differences[10]. In China, the incidence of choriocarcinoma is 1/2882 pregnancies, which makes this a high incidence area[11]. Due to the highly vascular nature of choriocarcinoma, hematogeneous metastasis can occur early and affect various organs and tissues throughout the body. Many patients only show symptoms of metastatic lesions. More than 60% of patients have pulmonary metastases at diagnosis[12]. It is the only gynecologic tumor recognized by the International Federation of Obstetrics and Gynecology and the International Gynecological Oncology Association that can be clinically diagnosed without histopathological evidence[12].
In this case report, we noted that the serum β-hCG levels in our patient continued to increase and her lower back pain symptoms persisted and gradually worsened. A color Doppler ultrasound was performed three times after admission, which showed that the left attachment area mass gradually increased. Concurrently, her β-hCG levels continued to increase which led to the diagnosis of ectopic pregnancy. CT showed a cystic solid mixed mass in the anterior psoas muscle before operation, although we were not concerned with this finding. During the operation, no villi were found in her pelvic or abdominal cavity. After careful examination of the lumbar muscles, a mass of approximately 3 cm × 2 cm was identified, although no treatment was performed considering the dense adhesion to blood vessels. The patient’s β-hCG level did not decrease during laparoscopy and hysteroscopy, but continued to increase on the first postoperative day. A lung CT examination revealed metastatic lung lesions and a full abdominal CT and pelvic MRI examination revealed a lumbar muscle mass associated with choriocarcinoma. The patient underwent six cycles of chemotherapy. After the fifth cycle of treatment, the patient’s symptoms were relieved and her β-hCG level returned to normal. Surprisingly, the lumbar muscle mass also disappeared. In this case, we did not have pathological evidence to confirm the diagnosis of choriocarcinoma. However, pathological evidence of choriocarcinoma is not a necessary condition for a final diagnosis[13]. The patient’s β-hCG levels and clinical symptoms were more important in this regard. To our knowledge, this is the first report of choriocarcinoma metastasizing to the lumbar muscle.
Choriocarcinoma misdiagnosed as an ectopic pregnancy is common. The main reason for this is because lung enhanced CT examinations are important for diagnosing the presence or absence of lung metastases. Misdiagnoses that delay the detection of metastasis is due to negative chest radiographs without lung CT at the first admission. It has been reported that if only chest radiography is performed, approximately 40% of patients with lung metastases may be missed[14]. Therefore, a craniocerebral and full abdominal MRI should be performed to understand metastatic lesions in cases such as the one presented here. This will potentially improve the diagnosis and cure rate.
When the clinical, pathological, and auxiliary imaging examinations do not match, we must carefully identify the cause, reflect on whether the diagnosis is correct, and closely follow up the patient. Choriocarcinoma is relatively uncommon in the clinic. The characteristics of choriocarcinoma are similar to those of an ectopic pregnancy, which easily leads to misdiagnosis. Therefore, in future clinical diagnoses, medical staff should understand the subtle differences between ectopic pregnancy and choriocarcinoma. Considerations should include the possibility of choriocarcinoma in order to reduce misdiagnosis. Although choriocarcinoma has a highly malignant biological behavior, it responds well to chemotherapy[15]. Chemotherapy should be administered immediately after choriocarcinoma diagnosis, which is crucial for a good prognosis. One study reported that chemotherapy could successfully treat choriocarcinoma, even in advanced stages. In the current case report, the pituitary metastasis of choriocarcinoma disappeared following several chemotherapy cycles. Notably, the lumbar muscle metastasis disappeared after conventional chemotherapy treatment.
In summary, lumbar metastasis from choriocarcinoma is extremely rare. Appropriate chemotherapy can successfully treat these metastasized tumors. In addition, the final diagnosis was not only based on imaging results, but also based on laboratory examinations and clinical symptoms.
Manuscript source: Unsolicited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Avtanski D S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Wu YXJ
| 1. | Meddeb S, Rhim MS, Zarrouk W, Bibi M, Yacoubi MT, Khairi H. Unusual gestational choriocarcinoma arising in an interstitial pregnancy. Int J Surg Case Rep. 2014;5:787-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Braun-Parvez L, Charlin E, Caillard S, Ducloux D, Wolf P, Rolle F, Golfier F, Flicoteaux H, Bergerat JP, Moulin B. Gestational choriocarcinoma transmission following multiorgan donation. Am J Transplant. 2010;10:2541-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Wan X, Li J, Xie X. Extrauterine choriocarcinoma of the greater omentum after tubal pregnancy: case report. Int J Gynecol Cancer. 2006;16:1476-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Mittal S, Aird I, Haugk B. Gestational choriocarcinoma in liver mimicking ruptured ectopic pregnancy. J Obstet Gynaecol. 2012;32:499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Maestá I, Leite FV, Michelin OC, Rogatto SR. Primary pulmonary choriocarcinoma after human chorionic gonadotropin normalization following hydatidiform mole: a report of two cases. J Reprod Med. 2010;55:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Sakumoto K, Nagai Y, Inamine M, Kanazawa K. Primary omental gestational choriocarcinoma ascertained by deoxyribonucleic acid polymorphism analysis. Gynecol Oncol. 2005;97:243-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Sekine R, Hyodo M, Kojima M, Meguro Y, Suzuki A, Yokoyama T, Lefor AT, Hirota N. Primary hepatic choriocarcinoma in a 49-year-old man: report of a case. World J Gastroenterol. 2013;19:9485-9489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Sakamoto Y, Takei Y, Fujiwara H, Machida S, Taneichi A, Suzuki M. Gestational choriocarcinoma with uterine serosal metastasis mimicking ruptured ectopic pregnancy: A case report. Oncol Lett. 2015;9:2185-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Mangla M, Singla D, Kaur H, Sharma S. Unusual clinical presentations of choriocarcinoma: A systematic review of case reports. Taiwan J Obstet Gynecol. 2017;56:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Tarney CM, Tian C, Craig ER, Crothers BA, Chan JK, Gist GD, Bateman NW, Conrads TP, Hamilton CA, Larry Maxwell G, Darcy KM. Relative Effects of Age, Race, and Stage on Mortality in Gestational Choriocarcinoma. Int J Gynecol Cancer. 2018;28:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Steigrad SJ. Epidemiology of gestational trophoblastic diseases. Best Pract Res Clin Obstet Gynaecol. 2003;17:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376:717-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 551] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 13. | Liu X, Li X, Yin L, Ding J, Jin H, Feng Y. Genistein inhibits placental choriocarcinoma cell line JAR invasion through ERβ/MTA3/Snail/E-cadherin pathway. Oncol Lett. 2011;2:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shaaban AM, Rezvani M, Haroun RR, Kennedy AM, Elsayes KM, Olpin JD, Salama ME, Foster BR, Menias CO. Gestational Trophoblastic Disease: Clinical and Imaging Features. Radiographics. 2017;37:681-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Dadlani R, Furtado SV, Ghosal N, Prasanna KV, Hegde AS. Unusual clinical and radiological presentation of metastatic choriocarcinoma to the brain and long-term remission following emergency craniotomy and adjuvant EMA-CO chemotherapy. J Cancer Res Ther. 2010;6:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |