Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.5019
Peer-review started: July 1, 2020
First decision: July 24, 2020
Revised: August 1, 2020
Accepted: August 29, 2020
Article in press: August 29, 2020
Published online: October 26, 2020
Processing time: 117 Days and 6.4 Hours
Plastic bronchitis (PB) frequently occurs in children after the surgical repair of congenital cardiac defects or in the presence of inflammatory or allergic diseases of the lung. Accurate epidemiological data of this condition are still lacking.
A 5-year-old boy, with a clear medical history, presented to our hospital with persistent cough and pneumonia with segmental atelectasis on chest computerized tomography. He showed no significant improvement after 1 wk of amoxicillin-clavulanate potassium treatment. Bronchial casts were extracted using flexible bronchoscopy. Pathological examination of the dendritic cast confirmed the diagnosis of type I PB. Botrytis cinerea was detected by next-generation sequencing of the bronchoalveolar lavage fluid. After the removal of the airway obstruction and fluconazole treatment, the patient recovered and was discharged 14 d after admission without the recurrence of cough.
Botrytis cinerea pneumonia should be considered in children with PB who still have prolonged cough and atelectasis after a regular course of antibiotic therapy. Flexible bronchoscopy and etiological examination should be performed in a timely manner to determine the diagnosis, clear the airway obstruction, and target etiological treatment.
Core Tip: Plastic bronchitis (PB) frequently occurs in children as a postoperative complication of congenital heart disease or in pulmonary inflammation or pulmonary allergic disease. Here, we report a case of pediatric PB secondary to Botrytis cinerea pneumonia. This case highlights that PB associated with Botrytis cinerea pneumonia should be considered in patients who still have prolonged cough and atelectasis after a regular course of antibiotic therapy. Flexible bronchoscopy and etiological examination should be performed in a timely manner to determine the diagnosis, clear the airway obstruction, and target etiological treatment.
- Citation: Liu YR, Ai T. Plastic bronchitis associated with Botrytis cinerea infection in a child: A case report. World J Clin Cases 2020; 8(20): 5019-5024
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/5019.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.5019
Plastic bronchitis (PB), which has been described many times, is characterized by the formation of bronchial casts in the airways for undefined etiology, resulting in bronchial obstruction, ventilation and gas transfer dysfunction, and respiratory and circulatory failure[1]. Although some reports have described PB in pediatric patients, such as PB with Mycoplasma pneumoniae infection, influenza virus infection, adenovirus infection, asthma, and lung transplantation[2-6], reports of PB with Botrytis cinerea infection in humans are scarce in the literature. Botrytis cinerea is considered an endophytic fungus that can cause disease in many fruit, flower, and leafy vegetable crops; it has no apparent host specificity and can infect more than 1000 plant species[7]. Botrytis cinerea is known to actively promote plant susceptibility by employing a variety of virulence factors[8]. To our knowledge, Botrytis cinerea has potential risks affecting human health[9]. We herein report a pediatric case with Botrytis cinerea infection associated with PB, and summarize its clinical characteristics, diagnosis, and treatment, in the hope of providing new ideas for the etiological diagnosis and treatment of PB.
A 5-year-old boy was hospitalized for persistent cough for 4 mo.
The patient’s parents reported the occurrence of recurrent cough over the past 4 mo, without hectic fever, expectoration, hemoptysis, night sweats, weight loss, or wheezing. Initially, the patient was diagnosed with cough-variant asthma in the outpatient department because of his impulse oscillometry examination result, which showed that the response frequency was 22.26 Hz before inhalation and decreased by 8.09% after inhalation. The intradermal allergen tests were positive for dust mites (++) and mycetes (++). He was treated with inhaled corticosteroid therapy for 3 mo, but his cough did not improve significantly.
The patient had a clear medical history, without allergies, eczema, or repeated wheezing. He had already received vaccines for bacillus Calmette–Guérin, hepatitis B, diphtheria, pertussis, tetanus, polio, measles, and epidemic encephalitis B.
The patient lived with his parents since birth and was oriented to his parents. There was no history of psychological stress or substance abuse. There was no family history of hereditary disorders.
The patient had a body temperature of 36.2 °C, heart rate of 95 beats/min, respiratory rate of 27 breaths/min, blood pressure of 92/63 mmHg, and SpO2 level of 98%. He was conscious, able to answer age-appropriate questions accurately, breathing slightly faster, and without circumoral cyanosis. He had bilateral grade 2 tonsils without exudate, no nasal discharge, and no conjunctivitis. His skin was warm and moist, without any lesions. The breathing sounds were rough in both lungs without moist rales or wheezes. No arrhythmia, enlargement of the liver and spleen, or palpable abdominal lump were observed. Physical examination of the nervous system showed no obvious abnormality. No signs of abuse were observed.
The complete blood cell count test showed all components to be within the normal range, with the exception of increased eosinophils (10.5%). The erythrocyte sedimentation rate and blood biochemistry parameters (including analyses of arterial blood gas, electrolytes, liver enzymes, creatinine, myocardial enzyme, and lactic acid) were normal. Serological testing for pathogens gave negative results for hepatitis viruses A, B, and C, herpes simplex virus, cytomegalovirus, rubella virus, tuberculosis, Toxoplasma gondii, human immunodeficiency virus, varicella-zoster virus, Treponema pallidum, respiratory syncytial virus, coxsackievirus, Legionella pneumophila, Mycoplasma pneumoniae, adenovirus, influenza viruses A and B, parainfluenza virus,and Epstein–Barr virus. Blood autoantibodies and tuberculin skin tests showed negative results. Tests for humoral immunity showed normal results.
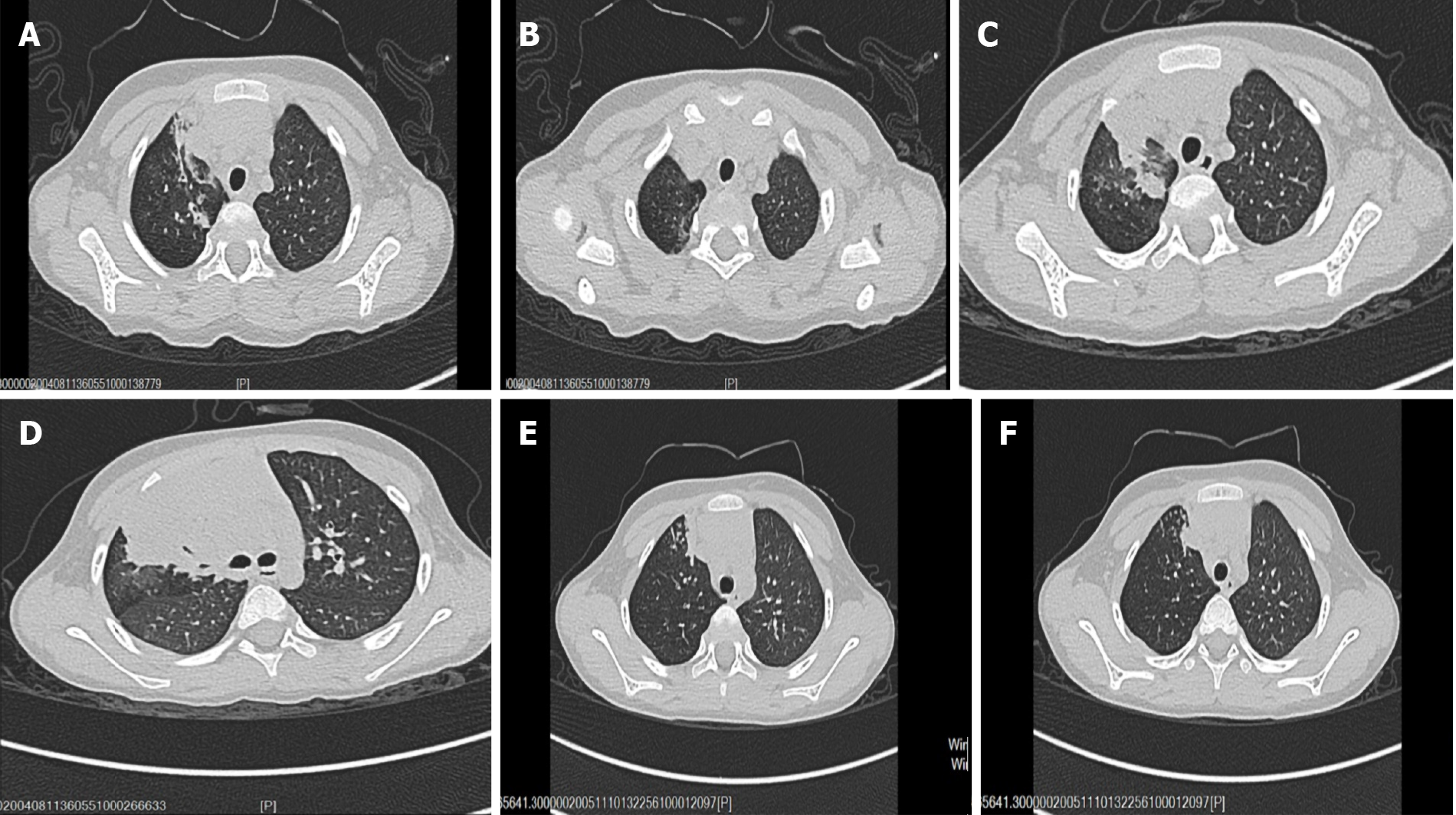
Chest computerized tomography (CT) showed right upper lobe pneumonia with segmental atelectasis (Figure 1A and B). Abdominal ultrasonography findings were normal. Electrocardiogram results were normal. Accordingly, our first clinical consideration was lobar pneumonia, and treatment with amoxicillin-clavulanate potassium (iv 30 mg/kg once every 8 h) was initiated after sputum culture and blood culture were completed.
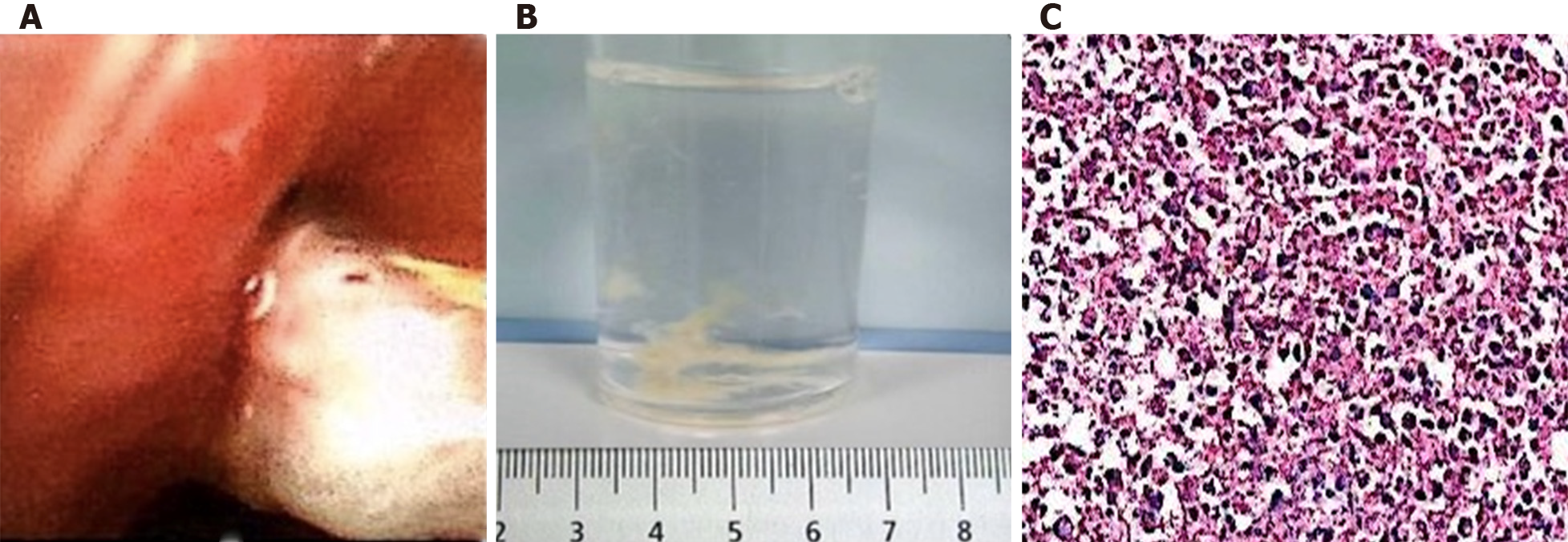
During hospitalization, the patient developed expectoration and had no significant improvement in cough symptoms. One week after treatment, although blood culture and sputum culture tests showed negative results, repeat chest CT results showed that the inflammatory lesions in the right upper lung were expanded and new lesions appeared in the right lower lung (Figure 1C and D). The patient was evaluated further by flexible bronchoscopy, histopathology examination, and next-generation sequencing of the bronchoalveolar lavage fluid. Serological tests for schistosomiasis, lung flukes, cysticercosis, liver flukes, metacercaria, Trichinella spiralis, glucan (G test), and galactomannan (GM test) were performed. The results of flexible bronchoscopy showed bronchial casts and endobronchial inflammation (Figure 2A and B), next-generation sequencing of the bronchoalveolar lavage fluid showed that it was positive for Botrytis cinerea, the serological G test was positive, the serological tests for parasites and the GM test were negative, and histopathology examination revealed an eosinophilic abscess (Figure 2C). Therefore, the patient was diagnosed with plastic bronchitis associated with Botrytis cinerea pneumonia, and the subsequent treatment consisted of fluconazole and symptomatic and supportive treatment (detailed below). Following treatment, the patient showed substantial recovery, his cough and expectoration almost disappeared, and he was discharged home.
The final diagnosis of the patient was plastic bronchitis associated with Botrytis cinerea pneumonia.
Initially, we diagnosed the patient with lobar pneumonia and treated him with amoxicillin-clavulanate potassium (iv 30 mg/kg once every 8 h). Then, we revised the diagnosis to plastic bronchitis associated with Botrytis cinerea pneumonia according to the results of flexible bronchoscopy and next-generation sequencing of the bronchoalveolar lavage fluid in addition to his increased eosinophils, G test results, and pathological examination results. The patient was given fluconazole (po 6 mg/kg per day, 7 d), and symptomatic and supportive treatment was continued from admission until he was discharged home. After discharge, he continued to take fluconazole orally for a week (po 6 mg/kg per day, 7 d).
The patient was followed for 2 wk. At the time of the writing of this case report, his cough and expectoration had disappeared, his breathing was smooth, his breathing sounds were clear in both lungs, and repeat chest CT results showed that the inflammatory lesions were diminished and the atelectasis was partially restored (Figure 1E and F).
Most reports of BC have described it in association with PB. The clinical manifestations of PB are varied, including cough, shortness of breath, wheezing, severe respiratory or circulatory failure, and multiple organ dysfunction[3,10]. Even though CT scan may yield a finger-in-glove pattern or atelectasis, the detection of bronchial dendritic casts by flexible bronchoscopy is the gold standard for the diagnosis of PB. Cases of PB are classified into two types: Type I is caused by inflammatory disease and consists mainly of inflammatory cells and fibrin, while Type II (acellular) occurs only in patients with congenital heart disease and is mainly composed of mucin with little or without infiltration of cells[11]. Histopathological examination of our patient's specimen revealed fibrinoid and necrotic tissue with extensive acute inflammatory cell infiltration, and eventually, a diagnosis of type I PB was rendered. The definitive etiologies of PB are unknown, and most cases have been reported as a complication of congenital heart defect repair in children. New causes of PB have recently been identified, such as Mycoplasma pneumoniae infection, influenza virus infection, adenovirus infection, asthma, and lung transplantation[2-6]. Interestingly, this patient's complete blood cell count test indicated eosinophilia rather than neutrophilia, and his histopathology examination revealed an eosinophilic abscess. On the basis of the results of the G test and next-generation sequencing of the bronchoalveolar lavage fluid and effective fluconazole treatment, Botrytis cinerea infection should be considered.
To the best of our knowledge, Botrytis cinerea is considered an endophytic fungus that can colonize the plant and exhibit facultative pathogenic behavior[7]. It was demonstrated that Botrytis cinerea deploys sRNAs and effector proteins to inhibit the premature death and immune response of host cells, which enables the fungus to establish and accumulate biomass inside the host prior to the necrotrophic phase[12]. A recent study found that Botrytis cinerea could be detected in the brain tissue of Alzheimer's patients by next-generation sequencing[9], suggesting that it has a potential effect on human health. In our case, this patient had a history of 3 mo of inhaled corticosteroid therapy, which may be a risk factor for infection. In addition, his intradermal allergen test was positive for mycetes, and we hypothesized that in addition to infectious factors, Botrytis cinerea may also induce allergic inflammatory reactions, which can be confirmed by his eosinophil elevation in his complete blood cell count and eosinophilic abscess in histopathology examination. Using flexible bronchoscopy to remove the casts and bronchoalveolar lavage are vital for treating PB[5]. Other treatments for PB have mainly focused on percutaneous thoracic intervention after the Fontan procedure[13]. In our case, the patient received fluconazole antifungal therapy in addition to the removal of the casts. Thus, in our opinion, in addition to timely removal of casts, etiological treatment is the key to improving the prognosis.
PB should be considered in children with prolonged cough and atelectasis. Botrytis cinerea pneumonia should be considered as a differential diagnosis in children with PB who still have prolonged cough and atelectasis after a regular course of antibiotic therapy. Flexible bronchoscopy should be performed as early as possible to confirm the diagnosis, determine the etiology, remove the obstruction, and target etiological treatment.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mastoraki A S-Editor: Huang P L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Hasan RA, Black C, Reddy R. Plastic bronchitis in children. Fetal Pediatr Pathol. 2012;31:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Lu S, Liu J, Cai Z, Shuai J, Huang K, Cao L. Bronchial casts associated with Mycoplasma pneumoniae pneumonia in children. J Int Med Res. 2020;48:300060520911263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Ding XF, Zhong LL, Zhang B, Lin L, Huang H, Liang M. [Clinical features and pathogens of plastic bronchitis in children: an analysis of 9 cases]. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:729-733. [PubMed] |
| 4. | Zhang FZ, Qin L, Yuan JX, Tang LF. Plastic bronchitis due to adenoviral infection: a case report. BMC Pediatr. 2020;20:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Soyer T, Yalcin Ş, Emiralioğlu N, Yilmaz EA, Soyer O, Orhan D, Doğru D, Sekerel BE, Tanyel FC. Use of serial rigid bronchoscopy in the treatment of plastic bronchitis in children. J Pediatr Surg. 2016;51: :1640-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Eberlein M, Parekh K, Hansdottir S, Keech J, Klesney-Tait J. Plastic bronchitis complicating primary graft dysfunction after lung transplantation. Ann Thorac Surg. 2014;98:1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | van Kan JA, Shaw MW, Grant-Downton RT. Botrytis species: relentless necrotrophic thugs or endophytes gone rogue? Mol Plant Pathol. 2014;15:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Petrasch S, Silva CJ, Mesquida-Pesci SD, Gallegos K, van den Abeele C, Papin V, Fernandez-Acero FJ, Knapp SJ, Blanco-Ulate B. Infection Strategies Deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifer as a Function of Tomato Fruit Ripening Stage. Front Plant Sci. 2019;10:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Alonso R, Pisa D, Aguado B, Carrasco L. Identification of Fungal Species in Brain Tissue from Alzheimer's Disease by Next-Generation Sequencing. J Alzheimers Dis. 2017;58:55-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Jasinovic T, Kozak FK, Moxham JP, Chilvers M, Wensley D, Seear M, Campbell A, Ludemann JP. Casting a look at pediatric plastic bronchitis. Int J Pediatr Otorhinolaryngol. 2015;79:1658-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Seear M, Hui H, Magee F, Bohn D, Cutz E. Bronchial casts in children: a proposed classification based on nine cases and a review of the literature. Am J Respir Crit Care Med. 1997;155:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Veloso J, van Kan JAL. Many Shades of Grey in Botrytis-Host Plant Interactions. Trends Plant Sci. 2018;23:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | DePopas EM, Veress LA, Ahmed F, Rausch CM, Annam A, Gupta R. Percutaneous thoracic duct intervention to treat plastic bronchitis related to Fontan palliation. Pediatr Pulmonol. 2017;52:E97-E101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |