Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.5007
Peer-review started: June 21, 2020
First decision: July 24, 2020
Revised: July 28, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: October 26, 2020
Processing time: 124 Days and 21.4 Hours
The totally implantable venous access port (TIVAP) is an important device in patients for injecting blood products, parenteral nutrition or antineoplastic chemotherapy. Metastatic spread at the site of the insertion of a TIVAP is extremely rare.
We report the case of 33-year-old male with advanced gastrointestinal stromal tumor (GIST) who underwent radical tumor resection after neoadjuvant imatinib therapy. However, a solitary GIST metastasis at the site of a TIVAP insertion developed during adjuvant imatinib treatment. Mutational analysis showed secondary mutation in KIT exon 13 (V564A), which is resistant to imatinib treatment. To our knowledge, this is the first case report of a patient with advanced GIST developing GIST metastasis at the site of a TIVAP insertion.
This case highlights that when a patient with advanced, high metastatic GIST requires TIVAP insertion, we should realize that there is a risk of developing tumor metastasis at the site of a TIVAP insertion.
Core Tip: In this article, we report a man with advanced gastrointestinal stromal tumor (GIST) who developed GIST metastasis at the site of a totally implantable venous access port (TIVAP) insertion. This case highlights that when a patient with advanced, high metastatic GIST requires TIVAP insertion, it should be realized that there is a risk of developing tumor metastasis at the site of the TIVAP insertion.
- Citation: Yin XN, Yin Y, Wang J, Shen CY, Chen X, Zhao Z, Cai ZL, Zhang B. Gastrointestinal stromal tumor metastasis at the site of a totally implantable venous access port insertion: A rare case report. World J Clin Cases 2020; 8(20): 5007-5012
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/5007.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.5007
A totally implantable venous access port (TIVAP) is an important device inserted in a large vein, such as the subclavian vein, and is used to inject blood products, parenteral nutrition or antineoplastic chemotherapy that would ravage a smaller peripheral vein. The use of TIVAP is associated with a series of complications including hemorrhage, hemothorax, pneumothorax, venous thrombosis, infection, and arterial puncture or cannulation[1,2]. The occurrence of tumor metastasis at the site of the insertion of a TIVAP is extremely rare. A few studies have reported patients with head and neck, thoracic or hematological malignant tumors who developed tumor seeding at the site of central venous access[3-6]. However, tumor metastases of abdominal solid tumors at the site of the insertion have not been reported thus far. To our knowledge, we report the first case of gastrointestinal stromal tumor (GIST) with metastatic tumor at the site of a TIVAP insertion.
A 35-year-old man presented with a painless mass at the right side of the chest wall.
One month prior to admission, the patient presented with a raised and painless mass in the right infraclavicular fossa.
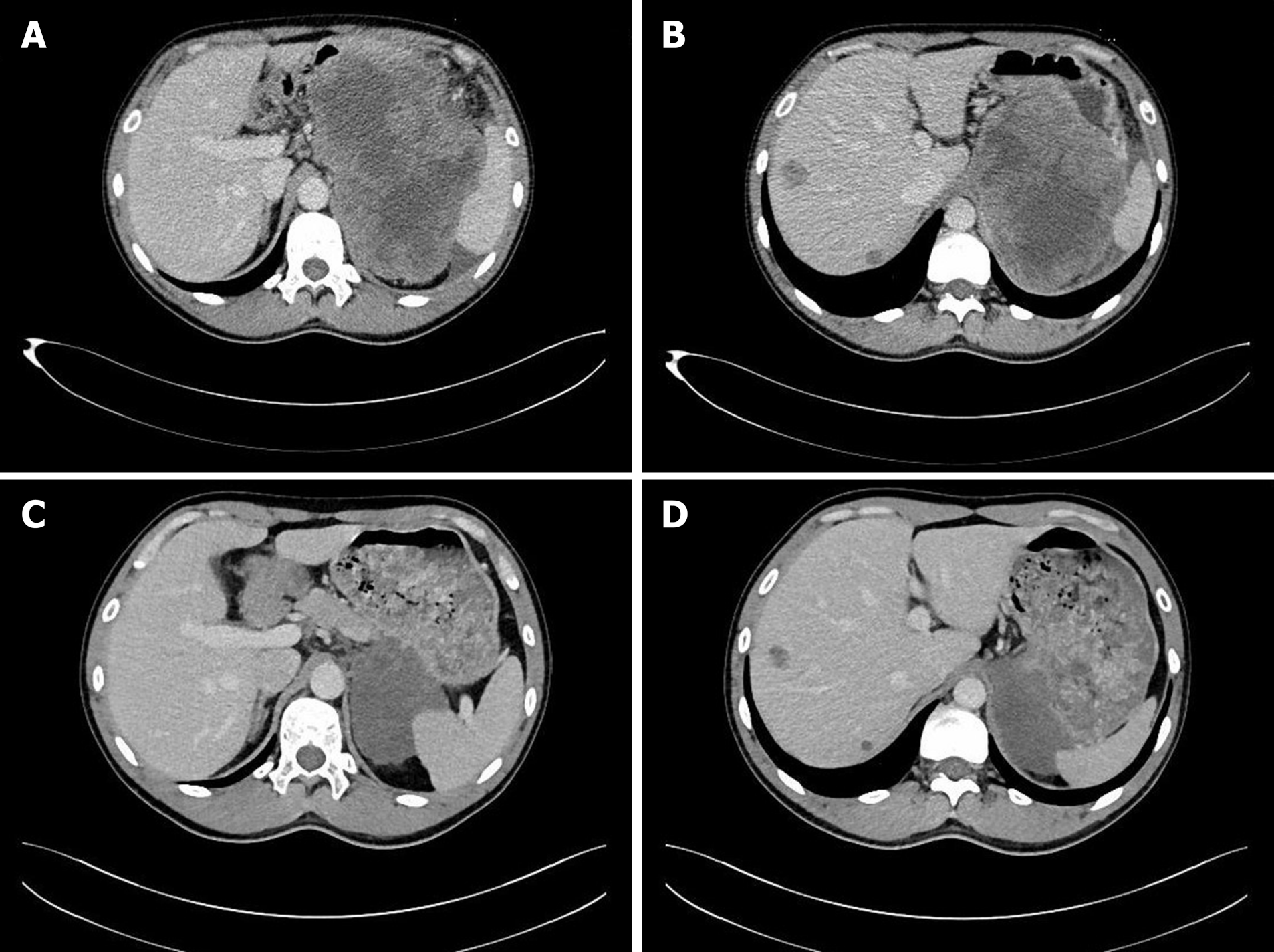
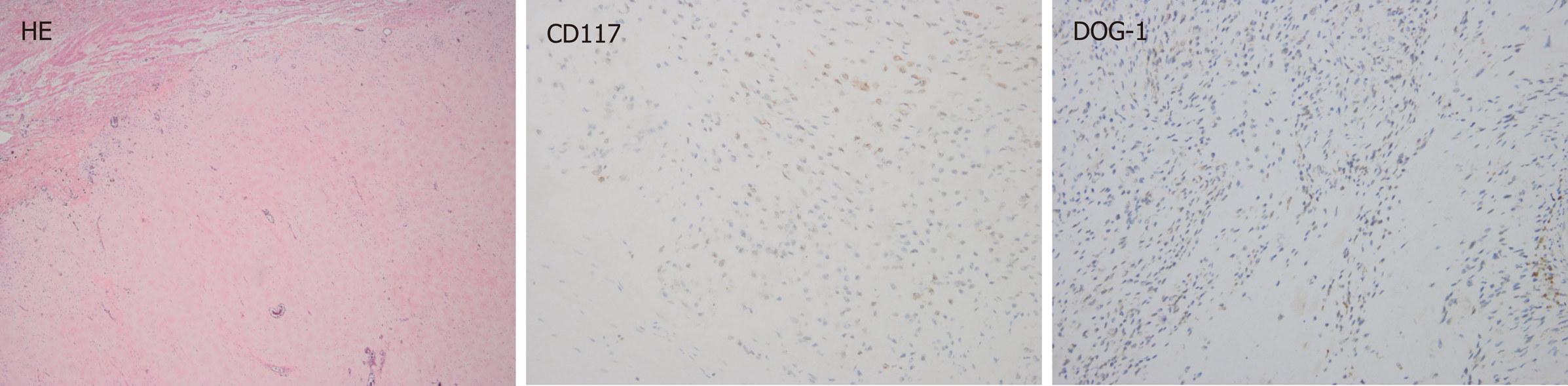
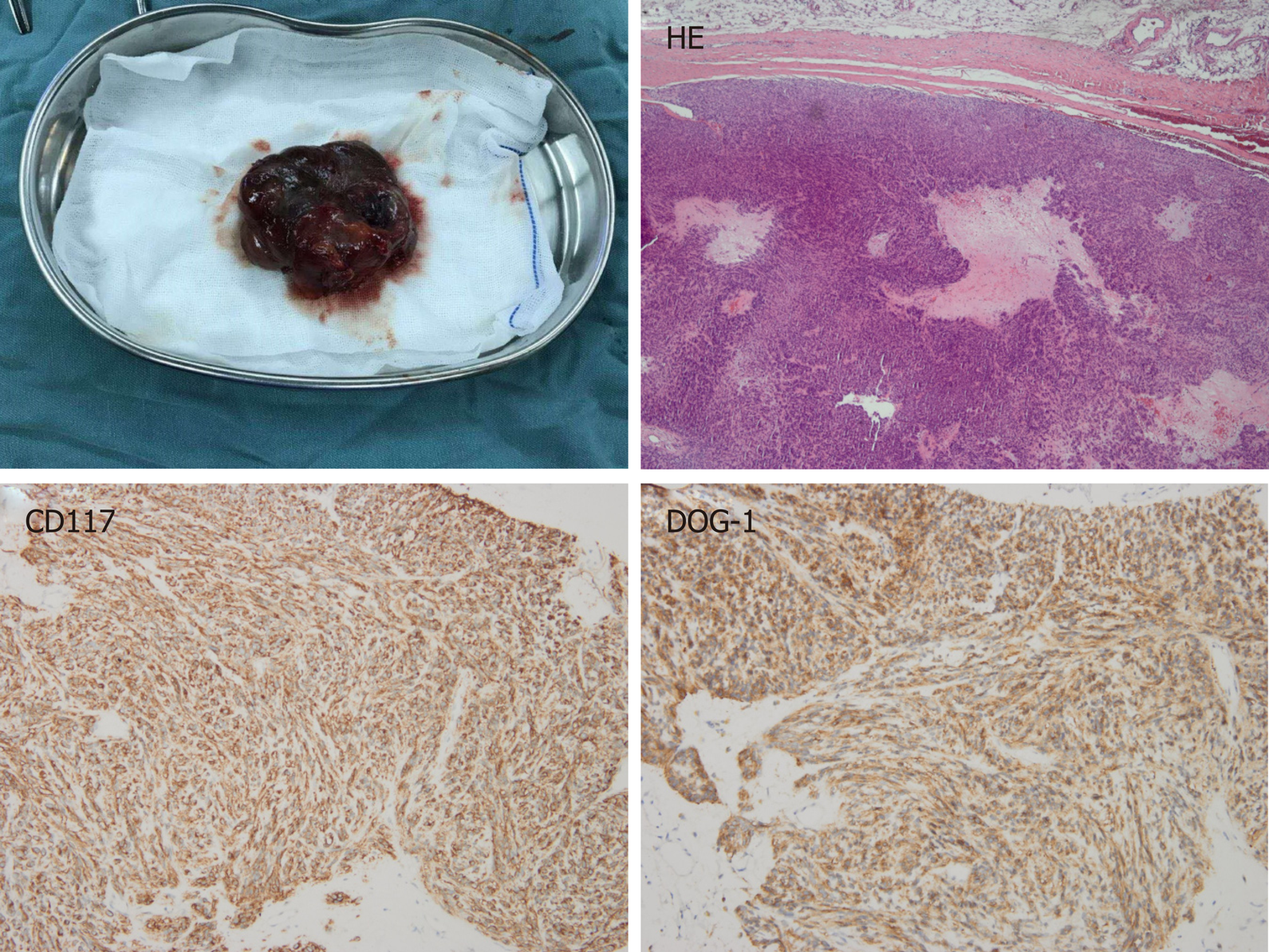
The patient was diagnosed with a gastric GIST in April 2016. A computed tomography (CT) scan showed a huge inhomogeneous soft tissue mass (approximately 18.1 cm × 11.9 cm) with central necrosis, occupying the entire left upper abdomen, along with two nodular enhancing liver lesions (the largest lesion was approximately 2.5 cm) suspicious of a metastatic malignancy in the right lobe (Figure 1A and B). In light of the pathologic diagnosis and the CT scan, the tumor was classified as an advanced GIST. The patient was administered imatinib 400 mg orally daily on a neoadjuvant basis before surgery. After 10 mo of imatinib therapy, the tumor showed a marked reduction in size (approximately 8.6 cm × 6.7 cm), tumor enhancement at arterial phase CT decreased substantially and hepatic metastatic lesions showed no enhancement (Figure 1C and D). Surgery was requested by the patient. At laparotomy, the tumor was located at the gastric fundus and invaded the nearby organs including the spleen, the tail of the pancreas and the left adrenal gland. The tumor was resected en bloc with the spleen, a portion of the stomach and the left adrenal gland, and the tail of the pancreas, and the hepatic metastatic tumors were also removed according to the findings of intraoperative ultrasonography, achieving an R0 resection. The pathological report of the resected specimen showed a tumor mass with diffuse hyalinization, fibrosis and focal hemorrhage and necrosis. Only a few spindle cells were distributed in the tumor lesion and the cells were positive for CD117 and DOG-1 (Figure 2). Unfortunately, the patient developed a gastric fistula postoperatively, and a single lumen 16 G TIVAP was inserted into the right subclavian vein via the Seldinger technique, with the aim of prolonged parenteral nutrition. Furthermore, an intestinal feeding tube was placed for enteral nutrition support. One month later, the patient was treated with total enteral nutrition and the TIVAP was removed. Two months after the operation, the gastric leak healed, oral feeding and imatinib treatment (400 mg/d) were resumed and the drains removed. The patient had no other significant past history or family history.
Physical examination revealed a raised and painless mass (approximately 6 cm × 5 cm) on the right side of the chest wall.
Except for below normal average albumin level, other laboratory examinations were normal.
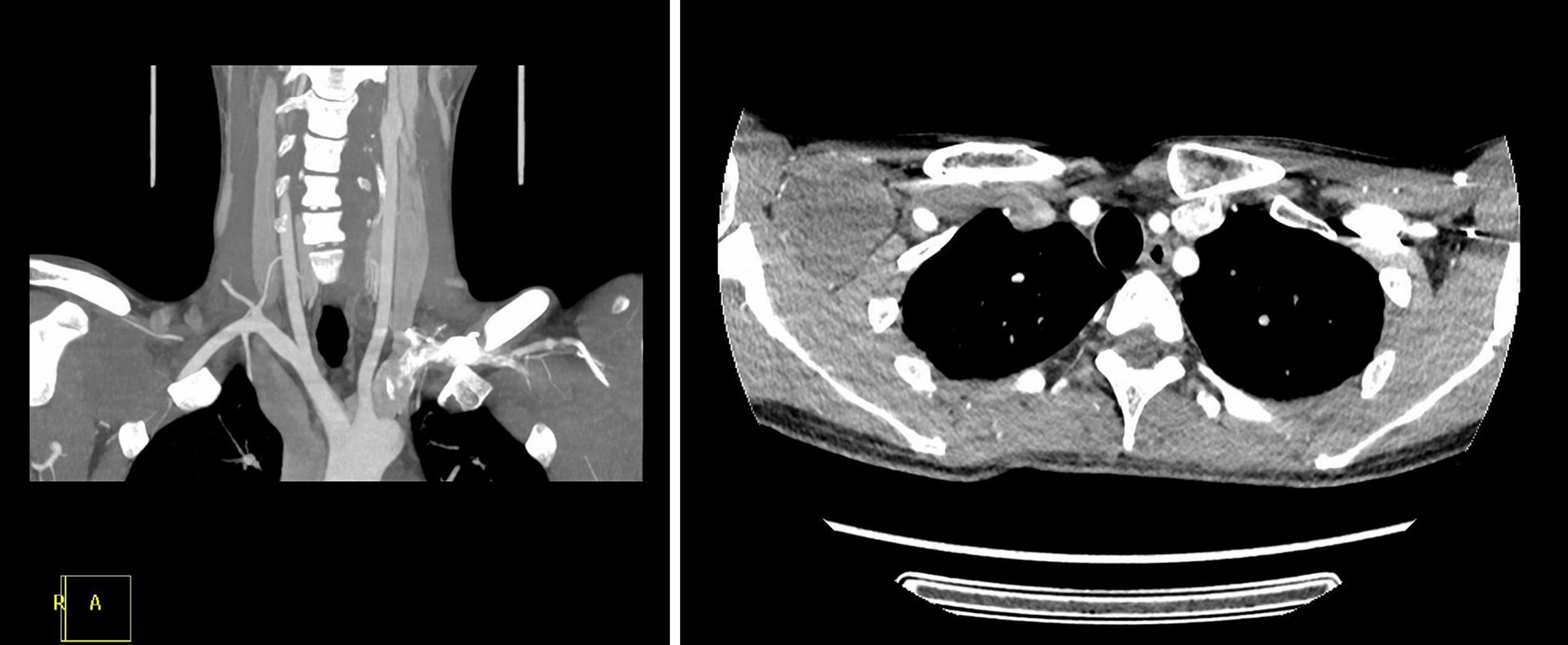
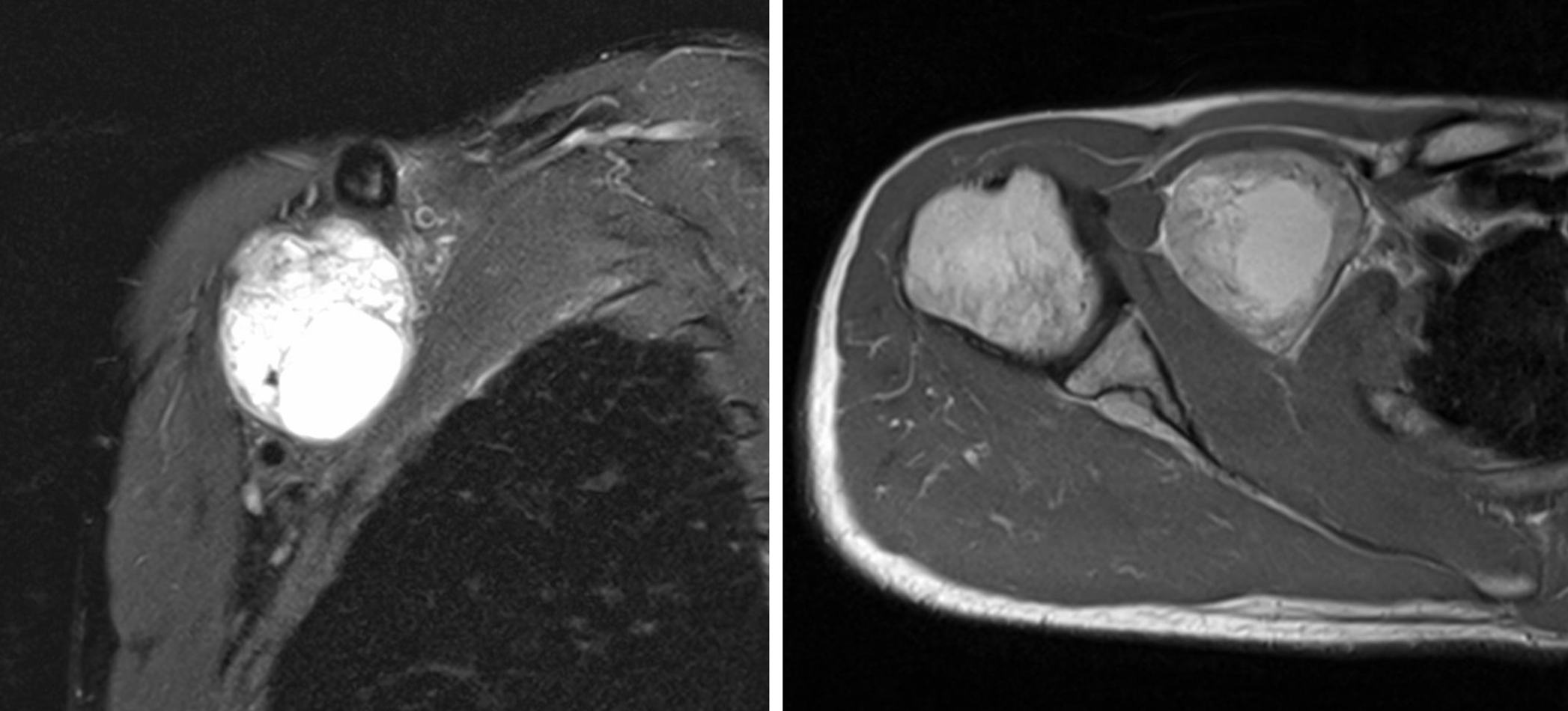
A raised mass (approximately 6 cm × 5 cm) was noted on the right side of the chest wall, which was around the site of the TIVAP insertion. A CT scan of the chest showed an inhomogeneous mass (approximately 5.7 cm × 4.6 cm) in the right infraclavicular fossa, which was supplied by the right subclavian artery and pressed the right subclavian and brachiocephalic veins (Figure 3). A magnetic resonance imaging (MRI) examination demonstrated a mixed signal mass without involvement of surrounding soft tissue and bone (Figure 4). No radiological or clinical evidence of abdominal relapse or metastasis were found on abdominal CT.
The final diagnosis of this infraclavicular mass was metastatic GIST.
Histopathology from core needle biopsy of the mass confirmed a metastatic GIST (Figure 5). Subsequently, the patient underwent radical resection of the metastatic infraclavicular GIST. Mutational analysis showed secondary mutation in KIT exon 13 (V564A), which is resistant to imatinib treatment. Switching to sunitinib treatment was suggested to the patient. The patient started sunitinib (37.5 mg/d) treatment after metastasectomy.
A postoperative CT examination showed no residual disease. The patient recovered well and has not yet shown signs of recurrence after 14 mo of clinical follow-up.
GIST is the most common mesenchymal tumor originating from the interstitial cells of Cajal in the digestive tract. GIST can occur anywhere in the gastrointestinal tract, but is most common in the stomach (65%), small intestine (25%), colon and rectum (5%-10%) and esophagus (5%)[7]. Almost all GISTs have activating mutations of KIT or PDGFRA. Metastases are usually seen in the liver and peritoneum. Lymph node and extra-abdominal metastases are rare and sites include bone, lung, skin and soft tissue[8,9]. However, tumor seeding at the site of a TIVAP implant has not been reported in the literature.
We report the case of a patient with subclavian metastasis of gastric GIST at the site of a TIVAP insertion during treatment with imatinib. After effective downsizing of an advanced gastric GIST with neoadjuvant imatinib, the patient was successfully treated with tumorectomy and continued imatinib therapy. The patient continued to do well for 23 mo until the subclavian metastatic mass at the site of the TIVAP insertion was found, and there was no radiological or clinical evidence of abdominal relapse or metastasis. In our patient, the most likely explanation for the subclavian metastatic tumor is circulating tumor cells seeding from the catheter when the subclavian vein was punctured. The trauma caused by puncture may provide an inflammatory microenvironment, promoting the implantation and growth of tumor cells.
Following a review of the literature regarding tumor metastasis at the site of central venous catheter insertion; head and neck malignancy, hematological and intrathoracic malignant tumors most commonly appear to result in tumor implants at the site of TIVAP insertion and the most common histology is squamous cell carcinoma. Compared to limited stage tumors and well differentiated tumors, aggressive and advanced tumors and poor differentiated tumors are more likely associated with tumor seeding at the site of insertion[3,10-13]. Furthermore, the puncture method or technique are also correlated with tumor spread. Previous studies reported that, for TIVAP insertion, the Seldinger technique is more likely to cause tumor seeding when compared with direct cephalic vein cut down, and multiple punctures may also increase the risk of tumor spread[5].
In summary, it should be realized that there is a risk of developing tumor metastasis at the site of insertion when a patient with advanced, high metastatic GIST requires a TIVAP insertion.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manuel-Vazquez A S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Zhang YL
| 1. | Yamaguchi RS, Noritomi DT, Degaspare NV, Muñoz GOC, Porto APM, Costa SF, Ranzani OT. Peripherally inserted central catheters are associated with lower risk of bloodstream infection compared with central venous catheters in paediatric intensive care patients: a propensity-adjusted analysis. Intensive Care Med. 2017;43:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1496] [Cited by in RCA: 1429] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 3. | Warner B, Cliff S, Shah G, Stern S. Leukaemia cutis at the site of a Groshong line insertion in a patient with acute myeloid leukaemia. Br J Haematol. 2014;164:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Crook JL, Breathnach OS, Cruser D, Johnson BE. Lung carcinoma metastasis at the site of central venous access. Chest. 1998;114:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Lee CH, Day AS, Hwang TZ. Metastasis over implantable venous access ports. Head Neck. 2013;35:E314-E316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Mangla A, Agarwal N, Mullane MR. Metastatic spread from squamous cell carcinoma of the hypopharynx to the totally implantable venous access port insertion site: Case report and review of literature. Head Neck. 2017;39:E118-E122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 8. | Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 325] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 9. | Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Ellrichmann M, Sergeev P, Bethge J, Arlt A, Topalidis T, Ambrosch P, Wiltfang J, Fritscher-Ravens A. Prospective evaluation of malignant cell seeding after percutaneous endoscopic gastrostomy in patients with oropharyngeal/esophageal cancers. Endoscopy. 2013;45:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Nisa L, Khanfir K, Giger R. Recurrence due to neoplastic seeding in head and neck cancer: report of two cases and review of the literature. Tumori. 2013;99:e144-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Cappell MS. Risk factors and risk reduction of malignant seeding of the percutaneous endoscopic gastrostomy track from pharyngoesophageal malignancy: a review of all 44 known reported cases. Am J Gastroenterol. 2007;102:1307-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Su HH, Chu ST, Hou YY, Chang KP, Chen CJ. Spindle cell carcinoma of the oral cavity and oropharynx: factors affecting outcome. J Chin Med Assoc. 2006;69:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |