Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4966
Peer-review started: May 5, 2020
First decision: May 15, 2020
Revised: May 26, 2020
Accepted: August 26, 2020
Article in press: August 26, 2020
Published online: October 26, 2020
Processing time: 172 Days and 19.2 Hours
Langerhans cell histiocytosis (LCH) is a rare condition wherein Langerhans cells proliferate abnormally, adversely impacting organs including lymph nodes, bones, skin, lungs, and pituitary gland. The LCH disease course varies widely among patients from a self-limiting condition to one that progresses rapidly and culminates in death. It is uncommon for multisystem LCH to be observed in adults. Herein we describe a woman suffering from multi-system LCH involvement.
A 37-year old Chinese woman was admitted to the hospital in June 2019 suffering from dyspnea that had progressed over the course of 5 years. Her medical history included: central diabetes insipidus (DI) that had been treated via radiotherapy, desmopressin acetate, and bromocriptine; bilateral pneumothorax with two surgeries having been performed to remove bullae; and autoimmune hepatitis that had been unsuccessfully treated using a combination of methylprednisolone and mycophenolate mofetil. A chest computed tomography (CT) scan revealed the presence of multiple pulmonary cysts of varying sizes. We re-analyzed right pulmonary bullae samples that had been removed in 2014, performed a systematic 18F-FDG PET/CT analysis, and convened a multidisciplinary medical team to diagnose and treat this patient. As a result, we were able to eventually diagnose this patient with LCH that was not associated with BRAF-V600E mutations.
We hope to emphasize the importance of systemic evaluation and of cooperation between multidisciplinary physicians with the goal of improving awareness and detection of this orphan disease.
Core Tip: Herein we describe the case of a young woman suffering from Langerhans cell histiocytosis (LCH) that affected organs including the lungs, liver, rib, pituitary gland, bilateral parotid gland, and bilateral submandibular glands. There have been very few previous reports of LCH with parotid gland and submandibular gland involvement. LCH is under- or misdiagnosed as a consequence of its variable and nonspecific presentation. Such misdiagnosis was evident in the patient described herein, and there was an extended period of time between initial symptom presentation and LCH diagnosis. This is a typical case of multi-system involvement LCH, and through this report we hope to help raise awareness of early LCH.
- Citation: Wang BB, Ye JR, Li YL, Jin Y, Chen ZW, Li JM, Li YP. Multisystem involvement Langerhans cell histiocytosis in an adult: A case report. World J Clin Cases 2020; 8(20): 4966-4974
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4966.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4966
The key observation associated with Langerhans cell histiocytosis (LCH) is the infiltration of certain tissues by abnormal numbers of Langerhans-like cells that are CD1a+/CD207 (Langerin)+ and that often manifest as granulomas. Unlike traditional skin-derived Langerhans cells, when analyzed at the mRNA level these LCH cells have been shown to be derived from myeloid dendritic cells (mDCs)[1]. The oncogenic BRAF-V600E mutation has also been shown to be frequently associated with LCH, offering further insight into the pathogenesis of this disease[2]. In the revised 2016 Histiocyte Society classification system, LCH has been designated as a form of inflammatory myeloid neoplasm[3].
LCH can impact any organ, with bone, skin, lungs, pituitary gland, and lymph nodes being the most commonly affected tissues[4]. In the present article, we describe the case of a 37-year-old woman suffering from LCH that affected organs including the lungs, liver, rib, pituitary gland, bilateral parotid gland, and bilateral submandibular glands. The goal of this case report is to increase awareness of this orphan disease so that physicians are better equipped to diagnose and treat it in its early stages.
A 37-year-old female was admitted to the First Affiliated Hospital of Wenzhou Medical University in June 2019 suffering from severe exertional dyspnea that had progressed over the preceding 5 years. Oxygen inhalation was sufficient to relieve these symptoms.
The patient had suffered from exertional dyspnea for 5 years. Pneumothorax had also occurred twice within 5 years, with two surgeries having been performed to remove bullae.
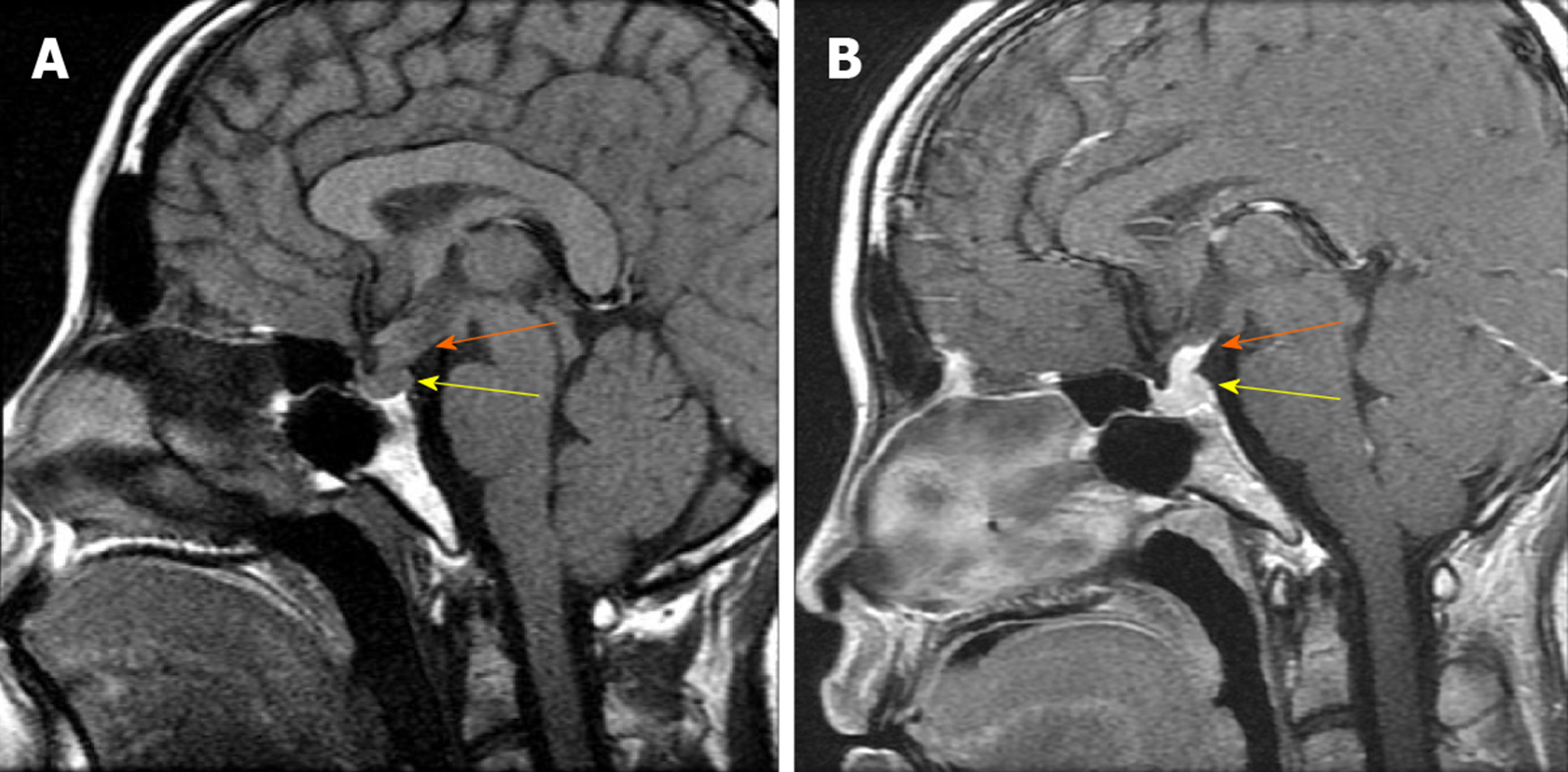
This patient had been diagnosed with central diabetes insipidus (DI) in May 2007 associated with polydipsia and polyuria, and had been treated via radiotherapy, desmopressin acetate 300 mg/d, and bromocriptine mesilate 0.625 mg/d. The patient also had elevated levels of prolactin (PRL 941 mIU/L; normal range: 70.81-566.46 mIU/L). Magnetic resonance imaging (MRI) of the sella region detected posterior pituitary and pituitary stalk lesions (Figure 1).
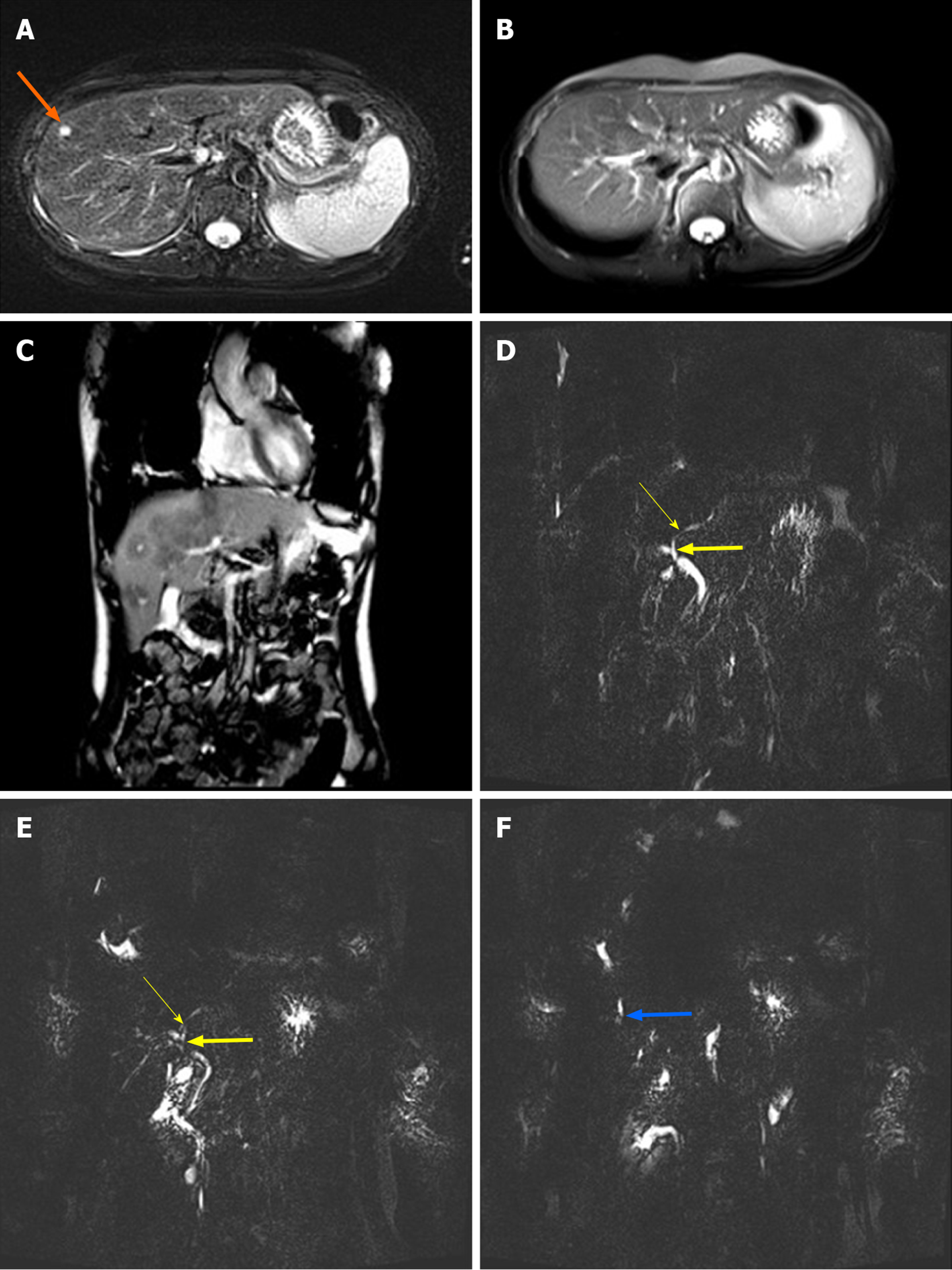
In June 2014, biochemical analysis revealed the presence of cholestasis (alkaline phosphatase, ALP 729 U/L; gamma-glutamyl transferase, γ-GT 319 U/L), as well as elevated levels of transaminases. This patient had been diagnosed with autoimmune hepatitis that had been unsuccessfully treated with a combination of methylprednisolone 4-8 mg/d and mycophenolate mofetil 1000 mg/d. The patient was also found to have elevated bilirubin levels in February 2015 (total bilirubin 58 μmol/L, direct bilirubin 45 μmol/L). An MRI analysis of the liver revealed the presence of hypointense lesions, while magnetic resonance cholangiopancreatography (MRCP) revealed local stenosis of the hepatic duct (Figure 2). An ultrasound-directed liver biopsy was performed in April 2019, with subsequent histological examination suggesting the presence of chronic hepatitis.
Physical examination upon admission detected no positive signs.
Blood gas analysis with 35% oxygen concentration revealed a PaO2 of 91.4 mmHg (normal range: 80-100 mmHg), a SaO2 of 97.4% (normal range: 91.9%-99.0%), and a P(A-a)O2 of 105.2 mmHg (normal range: 5.0-30.0 mmHg).
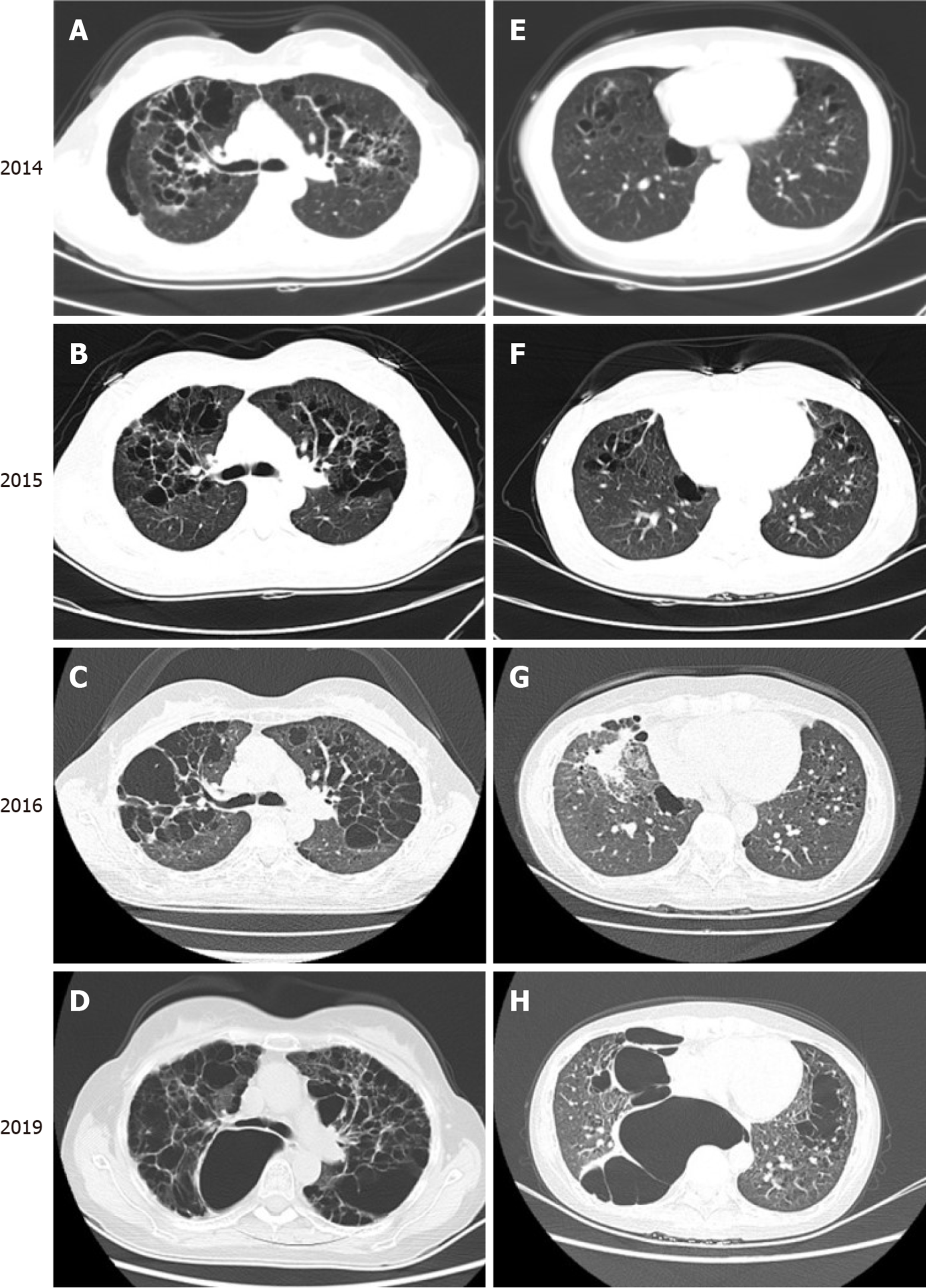
A computed tomography (CT) scan revealed the presence of cysts in both lungs. The patient exhibited an enveloping pneumothorax in the lower lobe of the right lung which may be associated with the prior pulmonary bullae resection and pleurodesis that had been conducted in 2014. During the previous 5 years, cysts in the upper and middle lungs had grown significantly, and lower lung involvement had begun to manifest (Figure 3).
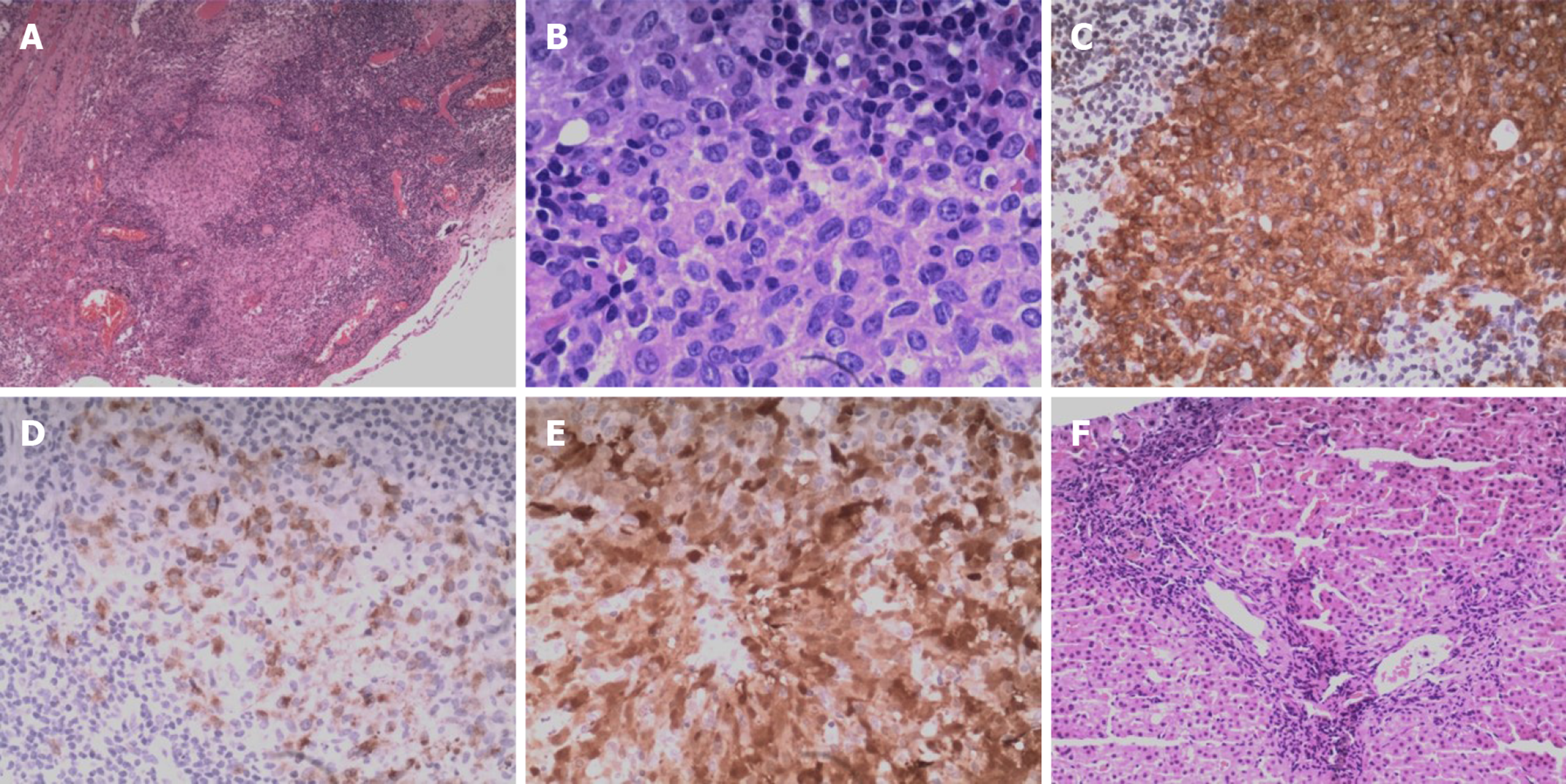
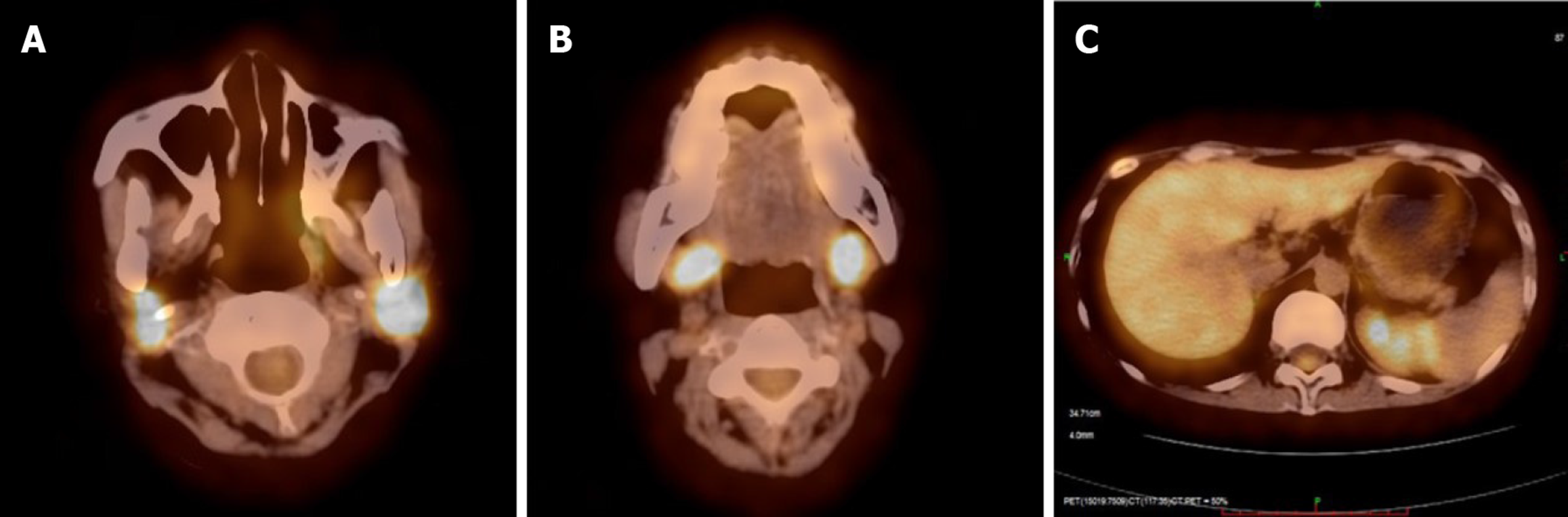
As a part of this diagnostic effort, we re-examined right pulmonary bullae sections that had been removed in 2014 and liver biopsy samples that had been collected in 2019. Nodular clusters of histiocytes were detectable upon histologic assessment of the bullae samples from this patient, as was evidence of fibrosis and inflammation. These cells exhibited a pronounced cytoplasm as well as mild cytologic atypia, slight nuclear enlargement, and the presence of nuclear grooves. We also observed scattered eosinophils in certain areas in these samples. Langerhans-like cells were found to be present based on positive staining for CD1a, CD207 (Langerin), and S-100. Based on these results, we diagnosed the patient with LCH. In the liver biopsy samples, we did not detect neoplastic Langerhans cells, but this may be a consequence of the sustained course of chronic fibrosis (Figure 4). We next conducted a systemic 18F-FDG PET/CT evaluation as a means of more reliably detecting LCH lesions[5]. This approach revealed the presence of abnormal FDG uptake in the bilateral parotid gland, bilateral submandibular gland, the liver, and the rib (Figure 5). These lesions were considered to be associated with LCH involvement, and following radiotherapy changes were detected in the pituitary gland. As a result, we were able to ultimately diagnose this patient with LCH.
The patient was treated with methylprednisolone 8 mg/d, mycophenolate mofetil 1000 mg/d, and ursodeoxycholic acid 750 mg/d. The poor condition of the patient’s lungs, however, precluded further chemotherapeutic treatment.
The patient was followed up in our outpatient department for an extended period of time. She developed a spinal compression fracture one year after diagnosis and a spinal fixation is now in preparation.
While most commonly detected in children, an estimated 1-2 per million cases of adult-onset LCH are detected annually, although the condition is believed to be underdiagnosed[6]. LCH can affect either a single-system (SS-LCH) with multifocal or unifocal involvement, or it can affect multiple systems within an individual (MS-LCH)[7]. As such, this patient was diagnosed with a fulminant form of MS-LCH. BRAF is a RAF family serine/threonine kinase that is a key mitogen-activated protein kinase (MAPK) signaling pathway regulator which leads to the activation of numerous transcription factors necessary for the growth and proliferation of cells. Genomic analyses have revealed that 35/61 (57%) of archived LCH specimens harbor oncogenic BRAF V600E mutations in a study conducted by Gayane Badalian-Very[2], and 13/26 pulmonary LCH specimens (50%) were similarly found to exhibit such mutations in a study conducted by Samia Mourah[8]. In the present case, we sought to detect such mutations in the lung lesion and peripheral blood mononuclear cell (PBMC) samples. However, no such oncogenic mutations were detected. This may be because the patient was negative for these mutations, or it may be a result of DNA degradation in these old samples.
In many cases, LCH is under- or misdiagnosed as a consequence of its variable and nonspecific presentation. Such misdiagnosis was evident in the patient described herein, and there was an extended period of time between initial symptom presentation and LCH diagnosis. Multidisciplinary cooperation was essential for the early diagnosis of LCH in the present patient. Initially, endocrinologists diagnosed this patient with DI, which is an early and common endocrine manifestation that appears in 20%-30% of patients[6,9]. Patients diagnosed with DI often exhibit anterior pituitary hormonal deficiencies that may only manifest several years following initial LCH diagnosis[10]. The most common deficiencies are those of growth hormone and gonadotropin, while 20% of patients exhibit hyperprolactinemia and deficiencies of thyroid-stimulating hormone (TSH) or corticotropin are rare[11,12]. It is important that LCH be strongly considered in all patients that develop DI with or without pituitary hormone deficiency. Secondly, the most common symptoms of LCH observed by respiratory specialists include dyspnea and pneumothorax, as in the present patient. Pulmonary LCH manifestations are most often observed in heavy smokers, and affected patients typically exhibit multiple cysts and nodules in the upper and middle lungs upon high-resolution CT (HRCT) examination[13]. Early during pulmonary LCH, Langerhans cells characteristically accumulate in the form of loose granulomas that destroy bronchiolar walls[14]. As this disease progresses, these cells become less abundant and instead form clusters wherein they are surrounded by inflammatory cell types such as lymphocytes, eosinophils, macrophages, and limited numbers of neutrophils[14]. As the disease becomes advanced, these lesions may then be replaced by either stellar fibrotic scars or cystic cavities surrounded by a fibrous ring, producing a honeycomb-like appearance when they become confluent[14]. The present case of pulmonary LCH in a non-smoker is very rare, and the differential diagnosis for such a condition includes pulmonary lymphangioleiomyomatosis (LAM), lymphoid interstitial pneumonia (LIP), and Birt-Hogg-Dubé syndrome (BHD)[15]. Thirdly, for gastroenterologists, liver involvement is typically associated with more disseminated disease and a poorer prognosis[16]. Early LCH liver involvement is restricted to Langerhans cell infiltration into the liver parenchyma, whereas during later stages chronic fibrotic sequelae often manifest around bile ducts with minimal evidence of hepatic histiocytic infiltration[17]. MCRP images of LCH patients typically reveal findings including multiple stenotic and segmental dilatations of the central and peripheral bile ducts[18]. In the present case we detected only a few histiocytes in liver biopsy specimens, likely because this biopsy was performed when the disease was relatively advanced, suggesting an unfavorable prognosis for this patient. It is possible to distinguish between primary and LCH sclerosing cholangitis based upon predominant localization in the small- and mid-sized biliary ducts, as well as immunohistochemical staining of CD1a-positive cells in the bile ducts[19,20]. It is also important to differentiate between LCH and conditions including drug-induced hepatic injury, autoimmune hepatitis, and IgG4 sclerosing cholangitis. The patient in the present report would have received an earlier diagnosis and treatment had she undergone multidisciplinary consultation at an earlier time point.
The standard of care for LCH diagnosed patients is a combination of vinblastine and prednisone that has been shown to improve overall survival despite frequent instances of treatment failure[21]. Steroids can be employed in an effort to slow or arrest pulmonary LCH progression, and in smokers it is essential that smoking be terminated immediately[7]. In patients where these frontline treatments fail, salvage treatment using cladribine and cytarabine or via allogeneic hematopoietic-cell transplantation can in some cases be curative[22-24]. Early trials of adults with BRAF V600E–positive LCH have suggested that MAPK-pathway inhibition using vemurafenib may be a promising treatment strategy[25]. Further study of the safety and efficacy of this approach, however, is still required. But the extent to which LCH can be treated is heavily dependent upon its severity and degree of progression at the time of diagnosis.
LCH remains a difficult disease to diagnose and treat in adults. However, the presence of disease manifestations in the lungs, liver, and endocrine system can, together with cooperation between multidisciplinary clinical teams, improve awareness of this orphan disease so as to lead to its more accurate and rapid diagnosis. Although this case had been misdiagnosed for many years, her data demonstrate the presentation of LCH in multiple systems in a comprehensive manner, with such presentation having the potential to offer more useful information for early diagnosis of this disease.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kai K, Kamimura K S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Liu JH
| 1. | Allen CE, Li L, Peters TL, Leung HC, Yu A, Man TK, Gurusiddappa S, Phillips MT, Hicks MJ, Gaikwad A, Merad M, McClain KL. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, Kuo FC, Ligon AH, Stevenson KE, Kehoe SM, Garraway LA, Hahn WC, Meyerson M, Fleming MD, Rollins BJ. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 857] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 3. | Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, Requena-Caballero L, Jordan MB, Abdel-Wahab O, Allen CE, Charlotte F, Diamond EL, Egeler RM, Fischer A, Herrera JG, Henter JI, Janku F, Merad M, Picarsic J, Rodriguez-Galindo C, Rollins BJ, Tazi A, Vassallo R, Weiss LM; Histiocyte Society. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 971] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 4. | Allen CE, Merad M, McClain KL. Langerhans-Cell Histiocytosis. N Engl J Med. 2018;379:856-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 5. | Albano D, Bosio G, Giubbini R, Bertagna F. Role of 18F-FDG PET/CT in patients affected by Langerhans cell histiocytosis. Jpn J Radiol. 2017;35:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Aricò M, Girschikofsky M, Généreau T, Klersy C, McClain K, Grois N, Emile JF, Lukina E, De Juli E, Danesino C. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39:2341-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Kobayashi M, Tojo A. Langerhans cell histiocytosis in adults: Advances in pathophysiology and treatment. Cancer Sci. 2018;109:3707-3713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Mourah S, How-Kit A, Meignin V, Gossot D, Lorillon G, Bugnet E, Mauger F, Lebbe C, Chevret S, Tost J, Tazi A. Recurrent NRAS mutations in pulmonary Langerhans cell histiocytosis. Eur Respir J. 2016;47:1785-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Makras P, Alexandraki KI, Chrousos GP, Grossman AB, Kaltsas GA. Endocrine manifestations in Langerhans cell histiocytosis. Trends Endocrinol Metab. 2007;18:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | García Gallo MS, Martínez MP, Abalovich MS, Gutiérrez S, Guitelman MA. Endocrine manifestations of Langerhans cell histiocytosis diagnosed in adults. Pituitary. 2010;13:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kurtulmus N, Mert M, Tanakol R, Yarman S. The pituitary gland in patients with Langerhans cell histiocytosis: a clinical and radiological evaluation. Endocrine. 2015;48:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Kaltsas GA, Powles TB, Evanson J, Plowman PN, Drinkwater JE, Jenkins PJ, Monson JP, Besser GM, Grossman AB. Hypothalamo-pituitary abnormalities in adult patients with langerhans cell histiocytosis: clinical, endocrinological, and radiological features and response to treatment. J Clin Endocrinol Metab. 2000;85:1370-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Vassallo R, Ryu JH. Smoking-related interstitial lung diseases. Clin Chest Med. 2012;33:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Tazi A. Adult pulmonary Langerhans' cell histiocytosis. Eur Respir J. 2006;27:1272-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med. 2013;7:316-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | A multicentre retrospective survey of Langerhans' cell histiocytosis: 348 cases observed between 1983 and 1993. The French Langerhans' Cell Histiocytosis Study Group. Arch Dis Child. 1996;75:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 229] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Abdallah M, Généreau T, Donadieu J, Emile JF, Chazouillères O, Gaujoux-Viala C, Cabane J. Langerhans' cell histiocytosis of the liver in adults. Clin Res Hepatol Gastroenterol. 2011;35:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Shi Y, Qiao Z, Xia C, Gong Y, Yang H, Li G, Pa M. Hepatic involvement of Langerhans cell histiocytosis in children--imaging findings of computed tomography, magnetic resonance imaging and magnetic resonance cholangiopancreatography. Pediatr Radiol. 2014;44:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Favara BE. Histopathology of the liver in histiocytosis syndromes. Pediatr Pathol Lab Med. 1996;16:413-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Kim M, Lyu C, Jin Y, Yoo H. Langerhans' cell histiocytosis as a cause of periportal abnormal signal intensity on MRI. Abdom Imaging. 1999;24:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Gadner H, Minkov M, Grois N, Pötschger U, Thiem E, Aricò M, Astigarraga I, Braier J, Donadieu J, Henter JI, Janka-Schaub G, McClain KL, Weitzman S, Windebank K, Ladisch S; Histiocyte Society. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006-5014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 22. | Donadieu J, Bernard F, van Noesel M, Barkaoui M, Bardet O, Mura R, Arico M, Piguet C, Gandemer V, Armari Alla C, Clausen N, Jeziorski E, Lambilliote A, Weitzman S, Henter JI, Van Den Bos C; Salvage Group of the Histiocyte Society. Cladribine and cytarabine in refractory multisystem Langerhans cell histiocytosis: results of an international phase 2 study. Blood. 2015;126:1415-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Bernard F, Thomas C, Bertrand Y, Munzer M, Landman Parker J, Ouache M, Colin VM, Perel Y, Chastagner P, Vermylen C, Donadieu J. Multi-centre pilot study of 2-chlorodeoxyadenosine and cytosine arabinoside combined chemotherapy in refractory Langerhans cell histiocytosis with haematological dysfunction. Eur J Cancer. 2005;41:2682-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Veys PA, Nanduri V, Baker KS, He W, Bandini G, Biondi A, Dalissier A, Davis JH, Eames GM, Egeler RM, Filipovich AH, Fischer A, Jürgens H, Krance R, Lanino E, Leung WH, Matthes S, Michel G, Orchard PJ, Pieczonka A, Ringdén O, Schlegel PG, Sirvent A, Vettenranta K, Eapen M. Haematopoietic stem cell transplantation for refractory Langerhans cell histiocytosis: outcome by intensity of conditioning. Br J Haematol. 2015;169:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque A, Gervais R, Elez-Fernandez ME, Italiano A, Hofheinz RD, Hidalgo M, Chan E, Schuler M, Lasserre SF, Makrutzki M, Sirzen F, Veronese ML, Tabernero J, Baselga J. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1220] [Cited by in RCA: 1377] [Article Influence: 137.7] [Reference Citation Analysis (0)] |