Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4883
Peer-review started: July 17, 2020
First decision: August 22, 2020
Revised: August 28, 2020
Accepted: September 16, 2020
Article in press: September 16, 2020
Published online: October 26, 2020
Processing time: 100 Days and 18.8 Hours
Target therapy is licensed by United States Food and Drug Administration on certain cancers. Both sorafenib and lenvatinib are tyrosine kinase inhibitor and indicated on radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC). Lenvatinib is more effective in cancers' control than sorafenib, but causes more nephrotoxicity than sorafenib does. This case is the second published case about the serial adaptions from lenvatinib to sorafenib for improving the proteinuria and, meanwhile, achieving the therapeutic goal.
A 56-year-old man suffered from bilateral edematous lower extremities after 1-mo prescription of lenvatinib of 20 mg/d for RAI-refractory DTC. Aside from this symptom, he also developed hypertension. His laboratory showed grade-3 proteinuria (estimated 24-h urine protein: 9993 mg), hypoalbuminemia and hypercholesterolemia. Anti-vascular endothelial growth factor (VEGF) therapy-induced nephrotic syndrome was impressed. After reduced dosage of lenvatinib of 10 mg/d and related symptomatic drugs, limited improvement was observed in both adverse effects and caner control. Under this condition, we substituted sorafenib of 400 mg/d for lenvatinib of 10 mg/d. After a 5-mo prescription, not only hypertension and peripheral edema were greatly improved, but also proteinuria was improved from grade three to grade one (estimated 24-h urine protein: 962 mg). At the same time the cancer control was achieved, judged from computed tomography and laboratory evidence [thyroglobulin (Tg) before prescription of sorafenib: 354.7 ng/mL; Tg after prescription of sorafenib: 108.9 ng/mL].
Adaption from lenvatinib to sorafenib is a feasible method to improve the anti-VEGF therapy-induced nephrotic syndrome and achieve the therapeutic goal at the same time.
Core Tip: Trials of anti-vascular endothelial growth factor (VEGF) therapy mostly focused on drugs with more potent to its receptors and with better progression-free survival, but few reports discussed about nephrotoxicity induced by anti-VEGF drugs, especially achieving improvement by regimens’ adaptions. This study is the second published case report about the successful adaption from lenvatinib, a more potent tyrosine kinase inhibitor (TKI), to sorafenib, a less potent TKI, to improve the nephrotoxicity and, meanwhile, achieve the therapeutic goal of cancer control.
- Citation: Yang CH, Chen KT, Lin YS, Hsu CY, Ou YC, Tung MC. Improvement of lenvatinib-induced nephrotic syndrome after adaptation to sorafenib in thyroid cancer: A case report. World J Clin Cases 2020; 8(20): 4883-4894
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4883.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4883
With the rapid blooms and breakthroughs in molecular biology and precision medicine, the medical community has more weapons for cancer control than ever before. In the past 10 years, drugs created based on various pharmacologic theories have been introduced and thrived. Target therapies, such as anti-vascular endothelial growth factor (VEGF) therapy, are one such weapon. Among all anti-VEGF drugs, tyrosine kinase inhibitor (TKI) is one of popular subgroups currently under study and licensed by The United States Food and Drug Administration. This drug typically targets more than one receptor. Thus, it is often described as multi-targets tyrosine kinase inhibitor (m-TKI).
Ongoing m-TKI trials primarily focus on comparing the efficacy of different drugs or combination with other treatments. Adverse effects, such as hand-foot syndrome and nephrotoxicity, are reported sporadically, and very few case reports have been released on the clinicians’ practical experiences to simultaneously improve disease conditions and maintain therapeutic effects. Adverse effects often lead to a reduced dosage or even therapy interruption. The case in this report is believed to be the second case[1] changing from lenvatinib to sorafenib on radioactive iodine (RAI)-refractory metastatic differentiated thyroid cancer (DTC) for gross proteinuria. Fortunately, both of us simultaneously achieved therapeutic goals and improved kidney conditions.
A 56-year-old man sought evaluation for progressively severe edema in the lower limbs bilaterally after lenvatinib treatment for DTC.
Approximately 1 mo after starting lenvatinib, the patient began to experience a feeling of bloating on both feet. Although his walking ability was unaffected, the uncomfortable feeling was progressively exacerbated. Occasional dysuria was also documented, but the patient denied painful or burning micturition. No other associated symptoms or issues with other organs were reported.
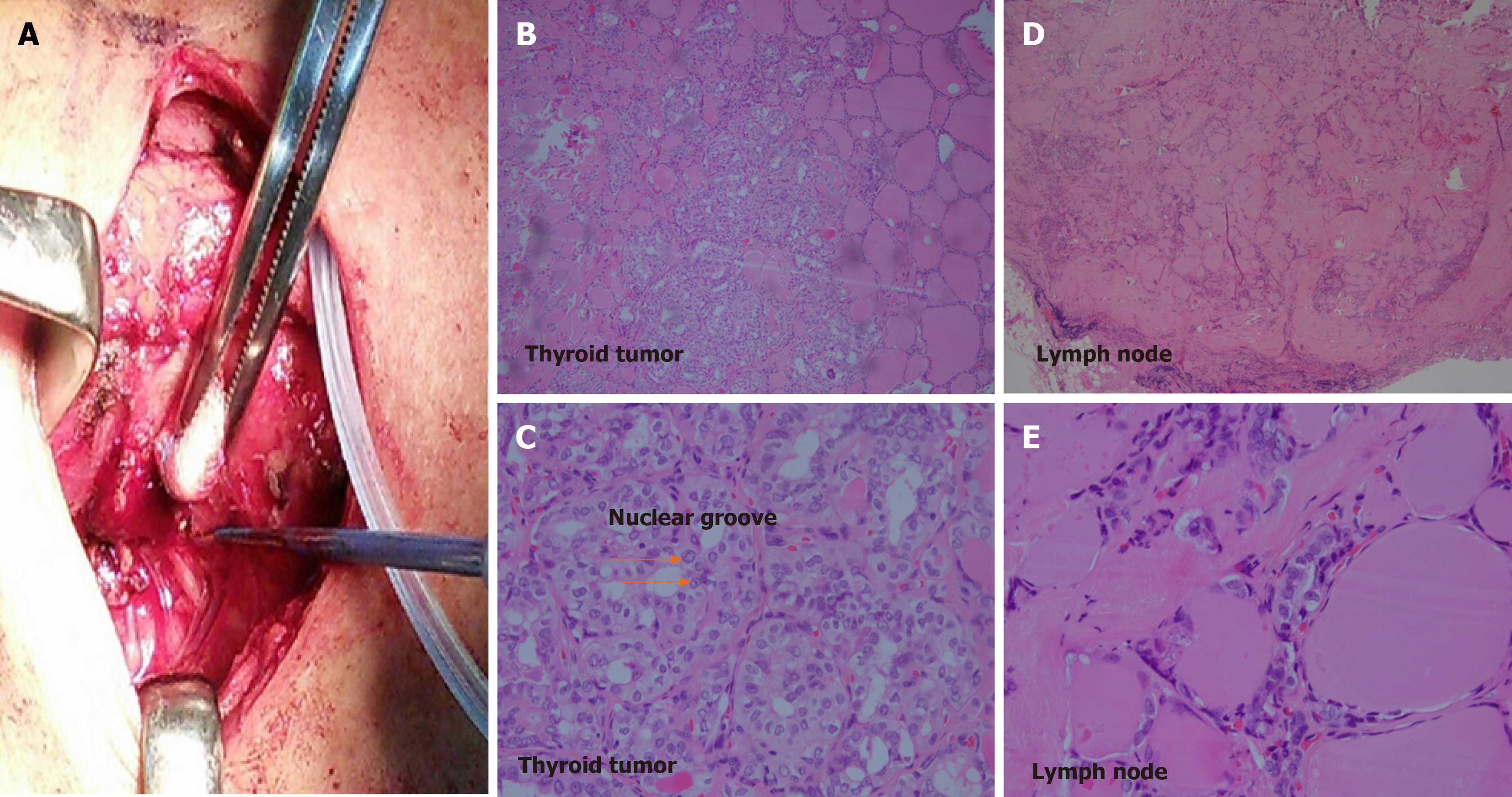
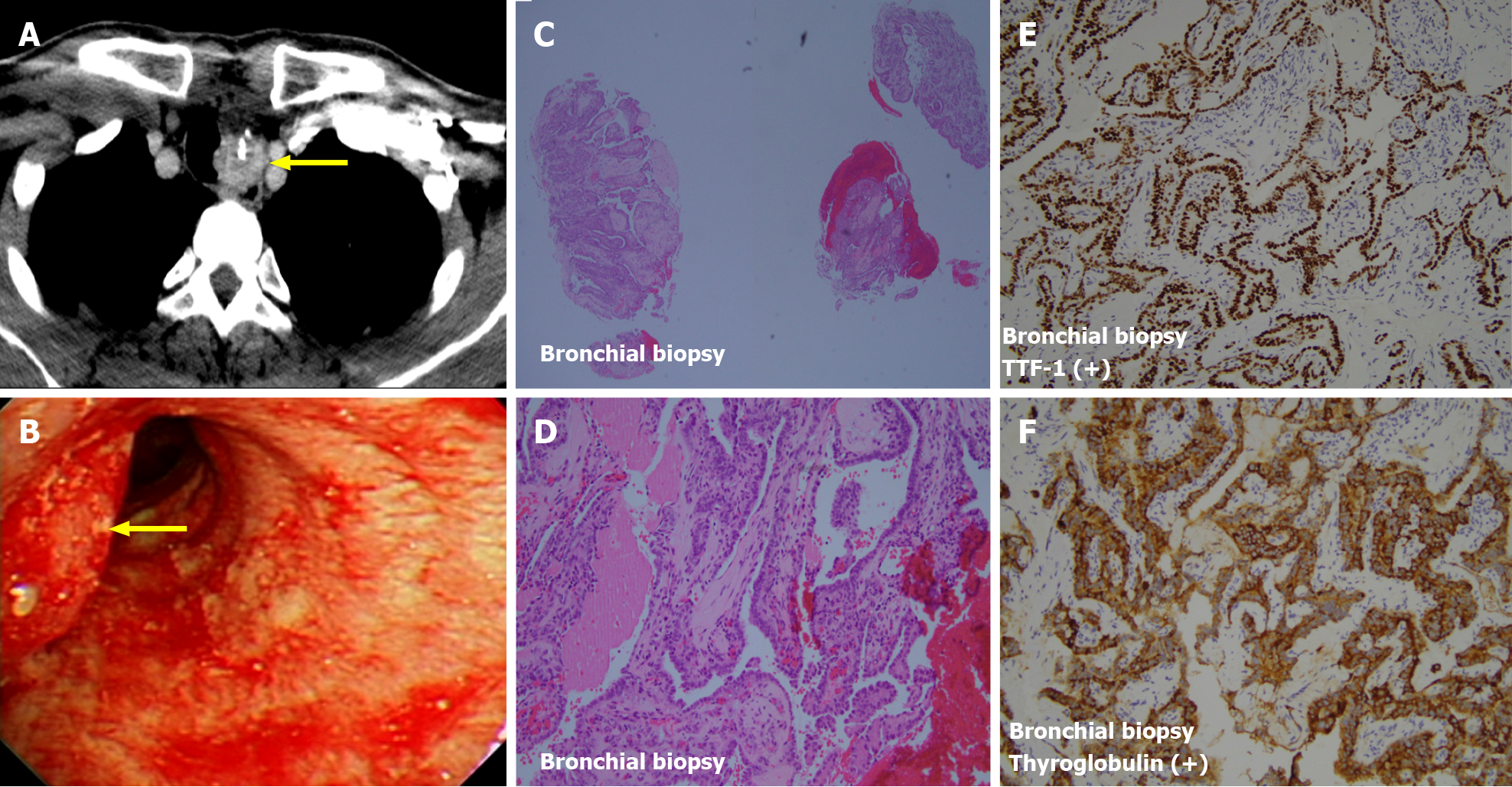
The diagnosis of papillary type DTC was made by biopsy 2 years before the current presentation, and the patient underwent bilateral total thyroidectomy. Pathologic evaluation showed papillary carcinoma staged at T1b at the left lobe and a nodular goiter at the right lobe (Figure 1). Two positive lymph nodes at level six (pre-tracheal, paratracheal, and pre-laryngeal sites) from central dissection revealed metastatic cancer (N1a). Postoperative computed tomography showed no identified distant organ involvement before beginning RAI (total dosage of 450 mCi). After completion of RAI, no other distant organ metastasis was seen on computed tomography. However, 11 mo later, the patient presented with hoarseness, difficulties swallowing, and hemoptysis. Tracheal deviation was seen on chest radiography. Based on unexplained hemoptysis and tracheal space-occupying mass, bronchoscopy was performed, and a biopsy specimen showed metastatic papillary carcinoma (Figure 2). Computed tomography was indicated and performed, and multiple metastatic nodules were seen in both lungs (Figure 3).
Thereafter, lenvatinib, one of m-TKI agents, was suggested to treat this RAI-refractory metastatic DTC. Oral lenvatinib was started at 20 mg/d. However, 1 wk after initiation, hypertension developed (155/92 mmHg), and nifedipine at 30 mg/d was started.
At the patient’s current presentation for foot evaluation, vital signs were as follows: temperature, 35.2 °C; heart rate, 77 bpm; respiratory rate, 18 breaths/min; and blood pressure, 161/96 mmHg. Oxygen saturation in room air was 98%. Moderate pitting edema grade 3+ was assessed. Neurologic examination showed normal sensory and motor functions. Chest examination revealed neither dull tone during percussion nor diminished breath sounds during auscultation. The abdomen appeared ovoid without distention, and negative shifting dullness was assessed. No knocking pain was detected on bilateral costovertebral angle examination.
The patient’s thyroglobulin (Tg) was 398 ng/mL, significantly decreased from an initial value beyond detectable limits (> 486 ng/mL) on diagnosis of RAI-refractory DTC. Anti-Tg antibody remained undetectable. Complete blood count results were as follows: white blood cell, 5500/μL; hemoglobin, 12.3 g/dL; and platelets, 202000/μL. Differential count results were neutrophils, 69.3%; lymphocytes, 22.5%; monocytes, 5.7%; eosinophils, 2.0%; basophils, 0.5%; and bands, 0%, which were all within normal range. Liver function tests (aspartate aminotransferase: 23 IU/L; alanine amino-transferase: 28 IU/L; and prothrombin time: 10.20 s) were all normal, but hypercholesterolemia (312 mg/dL) was observed. Serum albumin was tested to be hypoalbuminemia (2.4 g/dL). Total bilirubin was 0.5 mg/dL. Kidney function tests revealed glomerular hyperfiltration (creatinine, 0.56 mg/dL; blood urea nitrogen, 12 mg/dL; estimated glomerular filtration rate, 161 mg/dL). Urine analysis showed trace hematuria, but no urinary tract infection. Estimated 24-h urine protein was 9993 mg (random urine protein of 285.5 mg/dL; urine volume of 3500 mL/d).
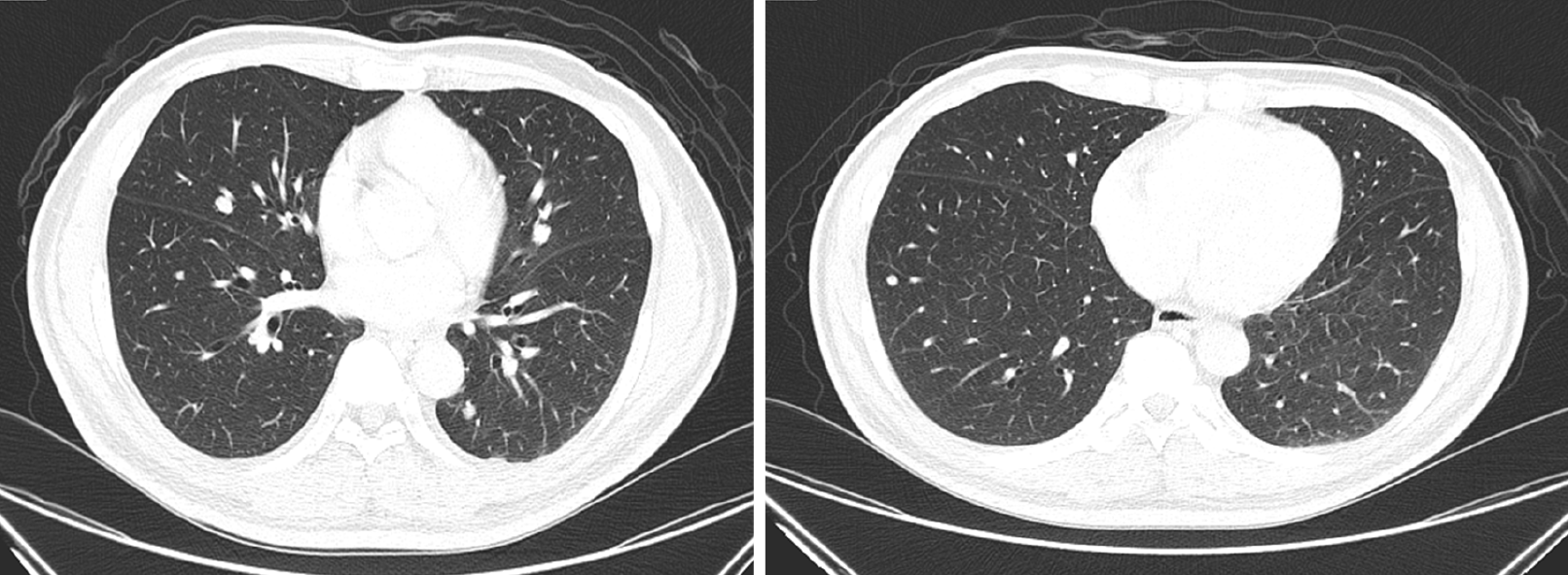
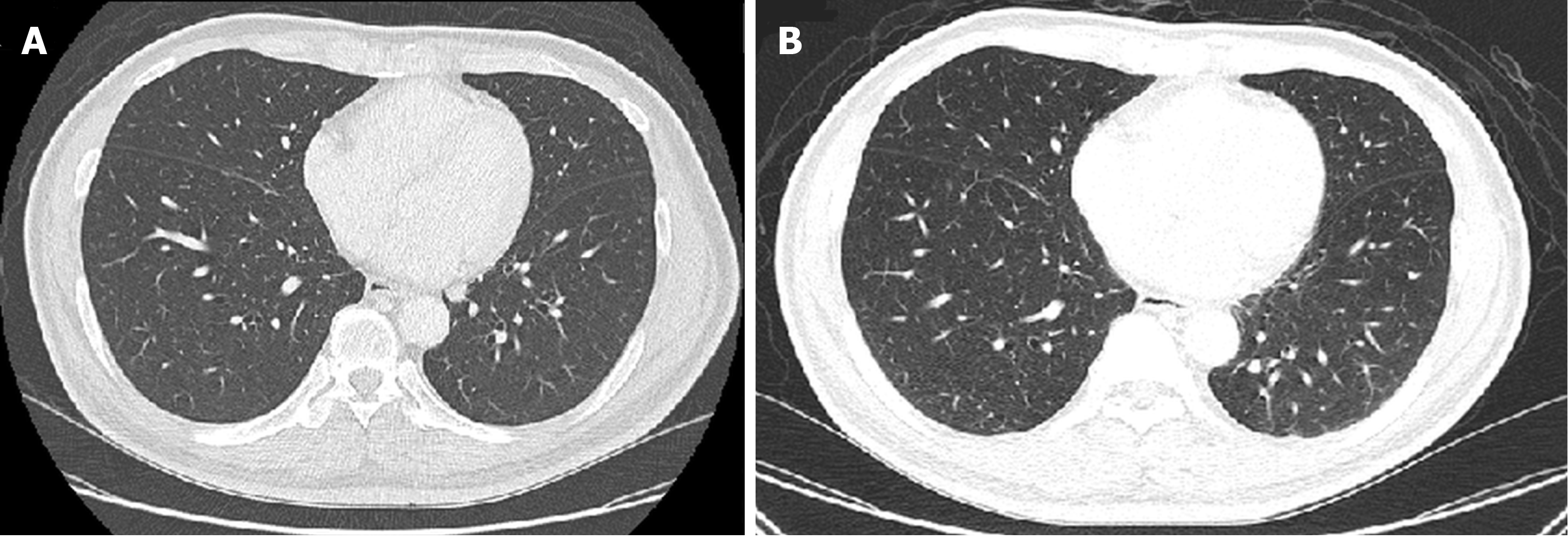
Recurrent thyroid cancer was seen at the left paratracheal space of the superior mediastinum with left tracheal wall invasion, and multiple lung metastases in both lungs were also evident on computed tomography. Limited improvement of the metastatic nodules on both lungs was observed (Figure 4). No abnormalities were seen on computed tomography for the upper and lower urinary tract.
Cystoscopy and ureteroscopy were performed, and no specific abnormalities or visible tumors were seen along the urinary tract.
Based on the studies we had done; the final diagnosis was anti-VEGF therapy-induced nephrotic syndrome.
The decision was made to decrease lenvatinib to 10 mg/d and to continue monitoring clinical symptoms and signs. In addition, oral furosemide 40 mg/d was prescribed to resolve the lower extremity edema. Collection of 24-h urine to assess urine protein was recommended, and investigation of serum Tg level by monthly interval to assess the cancer control was suggested. Computed tomography could be considered to provide an objective measurement of therapeutic response.
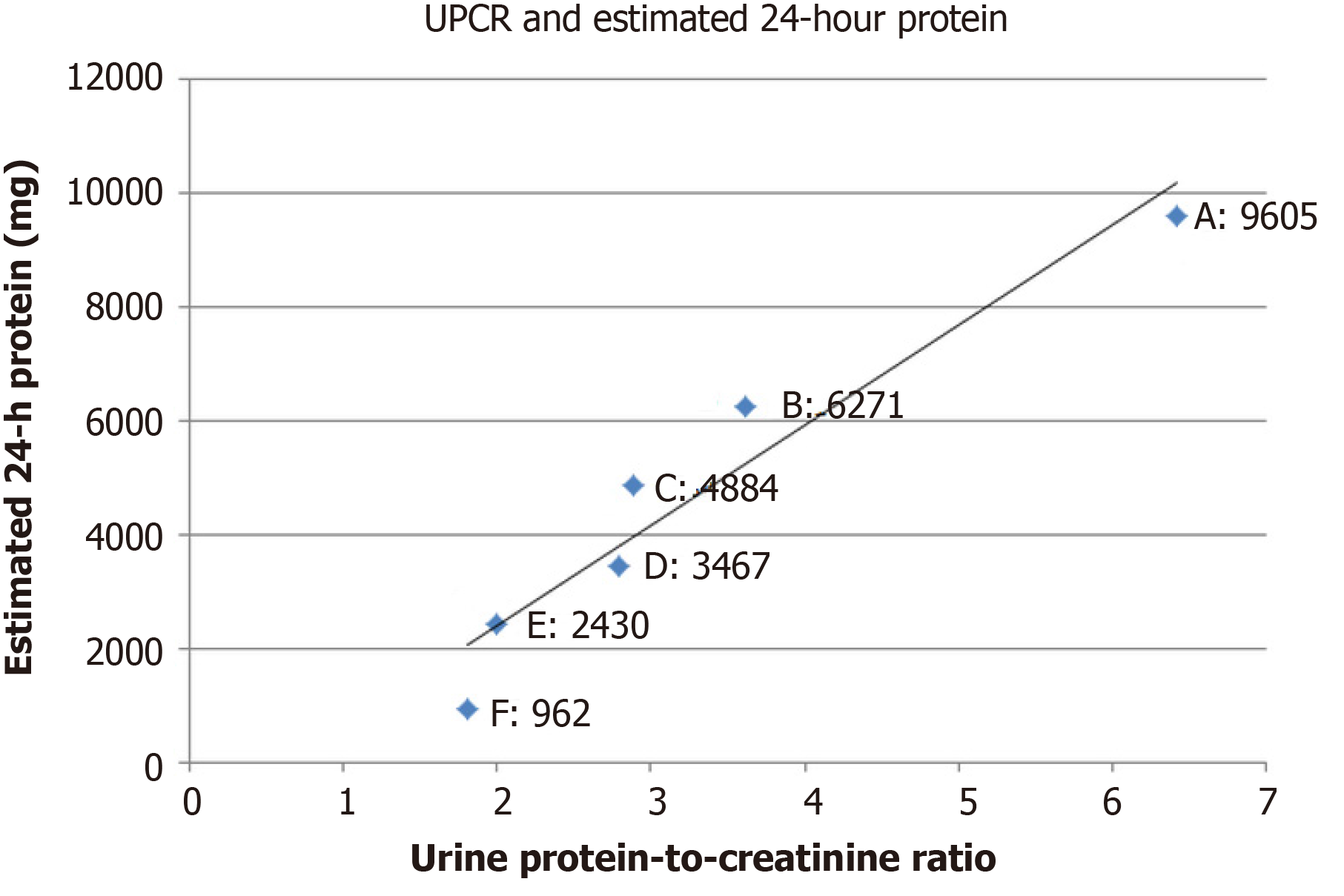
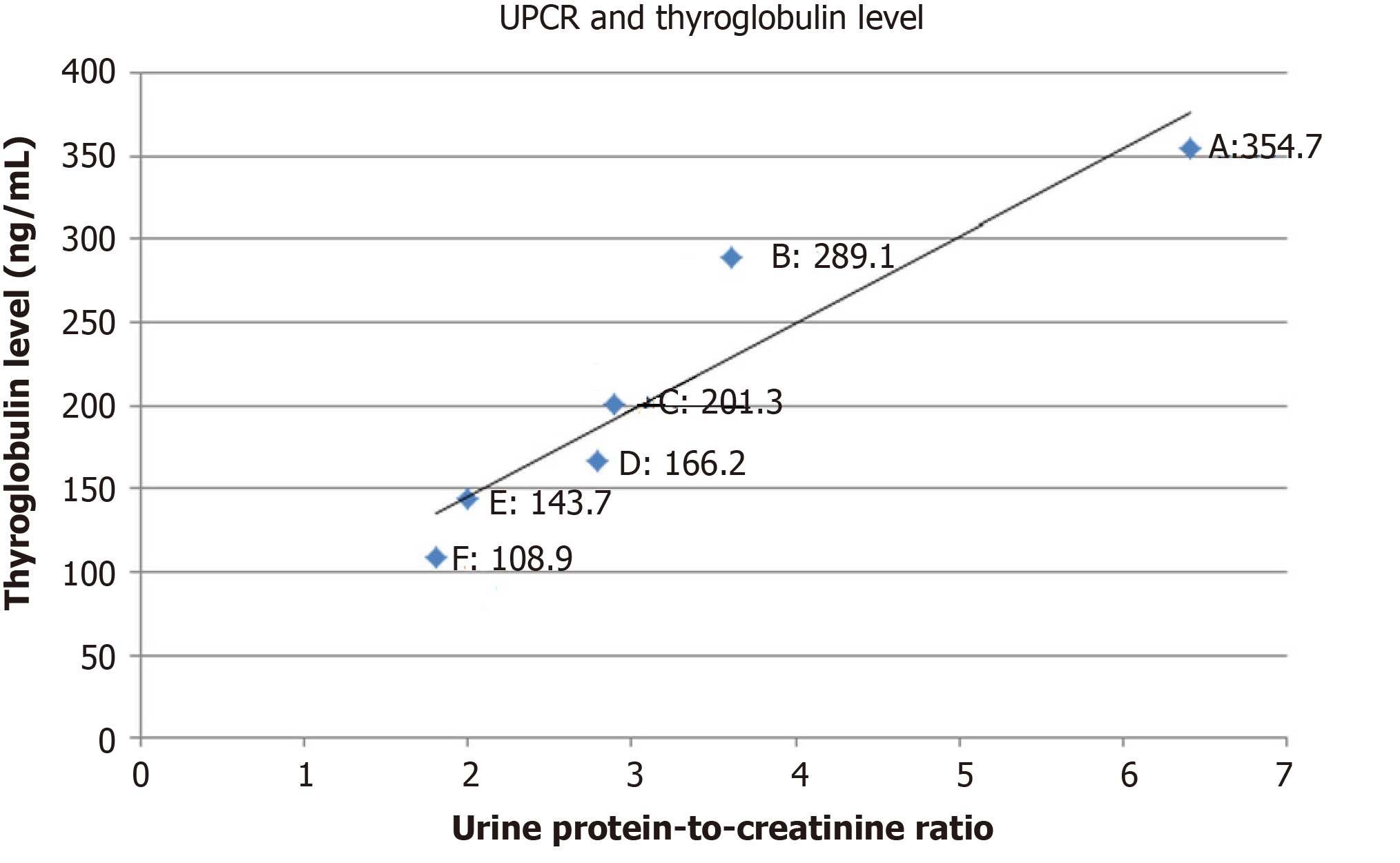
At 1-mo follow-up, bilateral lower extremities remained edematous with furosemide 40 mg/d and hypertension persisted at 158/95 mmHg. Laboratory results showed that the estimated 24-h urine protein was 9605 mg, with random urine protein of 228.7 mg/dL and urine volume of 4200 mL/d. The random urine creatinine was 35.70 mg/dL, with urine protein-to-creatinine ratio (UPCR) of 6.41. The Tg value was 354.7 ng/mL. Computed tomography demonstrated limited improvement for the progression of lung metastases (Figure 5). Based on continued clinical adverse effects and minimal improvement of disease progression, the decision was made to replace the lenvatinib at 10 mg/d with sorafenib at 400 mg/d while maintaining furosemide at 40 mg/d and to follow-up in 1 mo. Meanwhile, nifedipine of 30 mg/d was kept for sustained hypertension.
At 1-mo follow-up for sorafenib therapy, mild improvement was observed in the lower limb edema, graded as 2+. Blood pressure had decreased to 115/65 mmHg. Serum albumin was 3.0 g/dL. Random urine protein decreased to 174.2 mg/dL, with 24-h urine volume of 3600 mL, estimated 24-h urine protein of 6271 mg, and random urine creatinine of 48.20 mg/dL (UPCR, 3.61). The Tg value was 289.1 ng/mL. Based on these improvements, the sorafenib dose of 400 mg/d was maintained for 1 mo until the next follow-up. Furosemide of 40 mg/d was still kept for symptomatic control.
At 2-mo follow-up, continued improvement was observed for the lower limb edema, now assessed as grade 1+. Blood pressure was 121/69 mmHg. Serum albumin was 3.4 g/dL. Random urine protein was 132.00 mg/dL, with 24-h urine volume of 3700 mL, estimated 24-h urine protein of 4884 mg, and random urine creatinine of 45.6 mg/dL (UPCR, 2.89). The Tg value was 201.3 ng/mL. Based on these improvements, the sorafenib dose of 400 mg/d was maintained for 1 mo until the next follow-up, but the regular nifedipine of 30 mg/d was changed to pro re nata using when the systolic blood pressure was > 140 mmHg. Furosemide of 40 mg/d was kept for another month.
At 3-mo follow-up, lower limb edema had substantially improved and was assessed as grade 0-1+. The average home blood pressures were 116/61 mmHg. Serum albumin was 4.0 g/dL. Random urine protein had decreased to 96.3 mg/dL, with 24-h urine volume of 3600 mL, estimated 24-h urine protein of 3467 mg, and random urine creatinine of 34.5 mg/dL (UPCR, 2.79). The Tg value was 166.2 ng/mL. Both nifedipine and furosemide were stopped, and sorafenib at 400 mg/d was maintained for 1 mo until the next follow-up.
At 4-mo follow-up, random urine protein was 81.0 mg/dL, with 24-h urine volume of 3000 mL, estimated 24-h urine protein of 2430 mg, and random urine creatinine of 40.5 mg/dL (UPCR, 2.0). The Tg value was 143.7 ng/mL. Serum creatinine was 0.84 mg/dL.
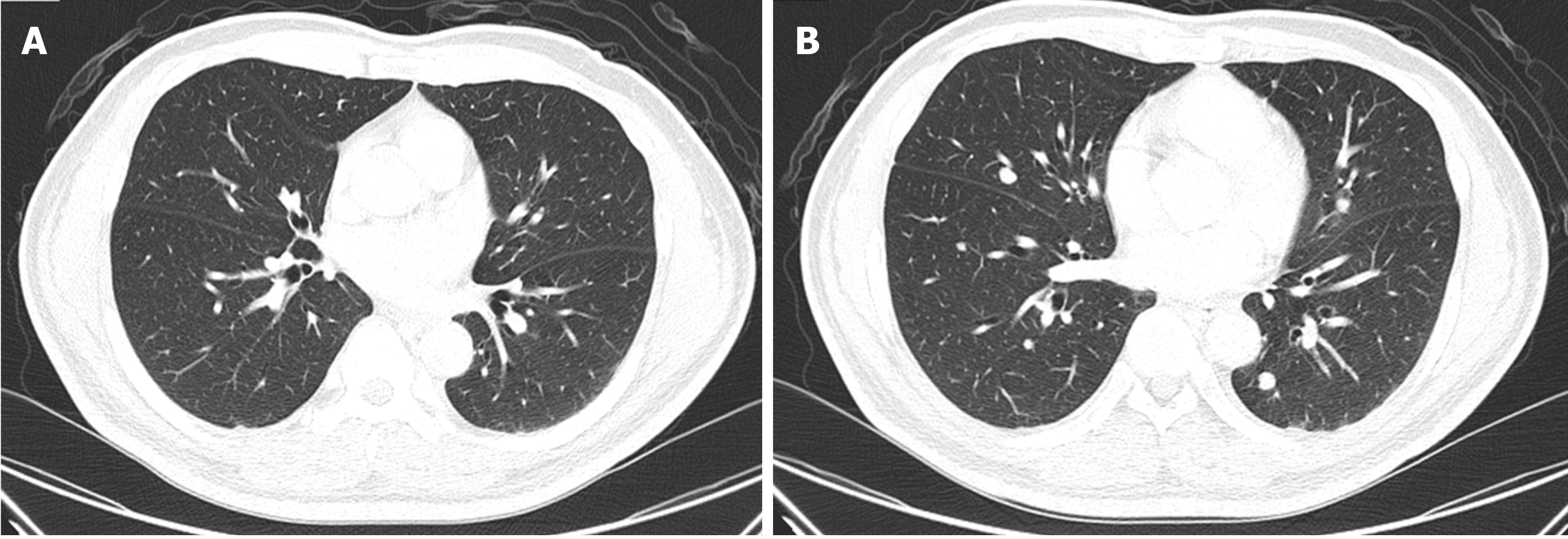
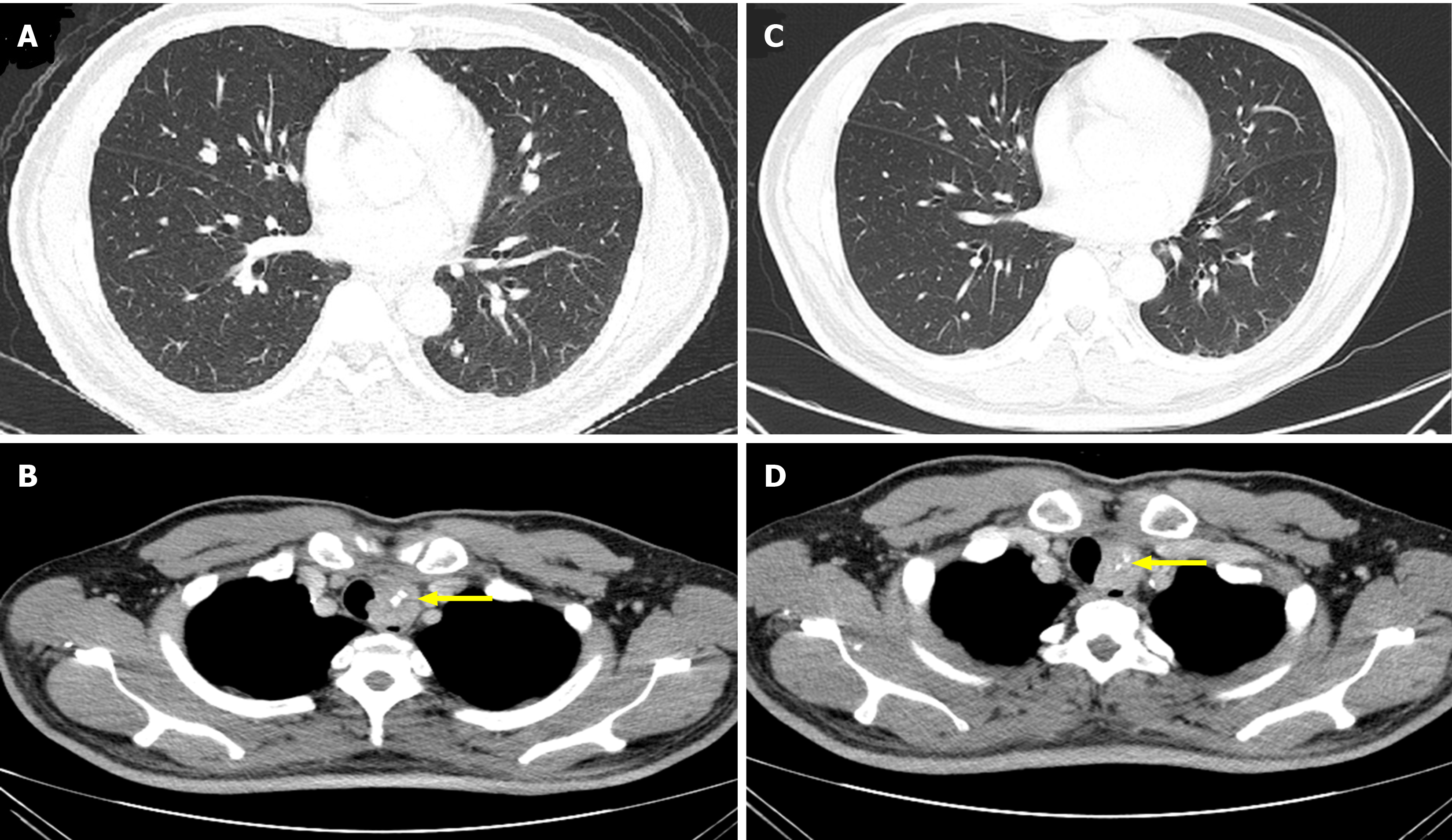
At 5-mo follow-up, random urine protein was 40.1 mg/dL, with 24-h urine volume of 2400 mL, estimated 24-h urine protein of 962 mg, and random urine creatinine of 22.15 mg/dL (UPCR, 1.81). The Tg value was 108.9 ng/mL. Computed tomography showed partial resolution of the metastatic paratracheal and lung lesions (Figure 6). The decision was made to maintain sorafenib but to taper the dosage according to the clinical response, which was surveyed by monthly serum Tg level with, or without, computed tomography every 3 mo.
In summary, after changing lenvatinib at 10 mg/d to sorafenib at 400 mg/d for 5 mo, the proteinuria was greatly improved from grade 3 to 1 (Figure 7), while the therapeutic effects were manifested by decreasing Tg levels (Figure 8), and computed tomography showed partial tumor resolution (Figure 6). Beyond these responses, hypertension and edematous symptoms could be monitored without medications.
The traditional therapies for advanced DTC are surgery, followed by postoperative RAI and thyroid-stimulating hormone suppression. The chance of distant metastasis and developing refractory RAI is estimated to be 6%[2], which represents a poor prognosis. With the understanding of molecular biology, anti-VEGF therapy has emerged as an alternative to the standard approach for advanced DTC. Among the anti-VEGF therapeutic agents, sorafenib[3] and lenvatinib[4] are indicated for progressive or metastatic RAI-refractory DTC to significantly improve the chances for progression-free survival. However, developing nephrotoxicity from both of these drugs is possible, which complicates the clinical course and considerably affects quality of life.
According to a basic understanding of biology, plasma filtration occurs at the capillaries of the glomerulus in the nephron and must travel across three layers to enter the collecting duct system, which are permeable endothelial cells, glomerular basement membrane, and podocytes’ foot process. Oxygen-hungry cells, such as cancer cells, use VEGF to construct new vessels by binding it directly to particular receptors of endothelial cells[5]. This process is called angiogenesis, and it is crucial for the proliferation and outgrowth of cancer cells. This process is the specific target of anti-VEGF therapy. However, the binding receptors exist not only in vascular endothelial cells but also in permeable endothelial cells in the glomerular filtration barrier. Anti-VEGF therapy in this setting would lead to the breakdown of endothelial fenestration and result in nephrotoxicity, such as proteinuria or nephrotic syndrome. Typically, VEGF-A is secreted by podocytes[6] and renal tubules[7] and then binds to receptors of tyrosine kinase (RTKs) on fenestrated endothelial cells or podocytes. Two common types of RTKs in kidney are VEGF receptor-1 (VEGFR-1) and VEGFR-2. After the binding of VEGF-A to RTKs, the serial downstream cascades will be provoked. The most discussed pathways include signals from Pl3 kinase/AKT pathways to endothelial nitric oxide synthase (eNOS) or mammalian target of rapamycin (m-TOR) and a signal from the RAF/MAPK/ERK pathways to B-Raf proto-oncogene regulation. Anti-VEGF therapy interrupts any parts of this transduction, causing the corresponding adverse effects. Asides from the proteinuria and nephrotic syndrome, nephrotoxicity will contribute to hypertension if any parts from Pl3 Kinase/AKT pathway to eNOS is injured. In this case, hypertension is also observed, and subsides with the improvement of nephrotic syndrome. Thus, we attribute the hypertension in this case to effects of nephrotoxicity.
Understanding that the causes of nephrotoxicity are various, a careful look at molecular expression may offer a general outline. Regulations of RelA and c-mip possess decisive roles in this process[8]. The ways that anti-VEGF therapy interrupts VEGF-A/VEGFR pathways can be roughly divided into two important times, before or after the binding of VEGF-A to VEGFR. When VEGF-A is arrested by drugs, such as antibody-like bevacizumab and ranibizumab, or is trapped, such as by aflibercept, before binding to VEGFR, increased RelA translocation into the nuclei and upregulation of transcription factor NF-κB will occur. Moreover, reduced glomerular complement factor H will result in increased complement activation. These changes will make this type of damage featuring thrombotic microangiopathy manifest as nephrotoxicity. In contrast, when anti-VEGF therapy, such as m-TKI, works after the binding of VEGF-A to VEGFR, the downstream signals will be interrupted and will result in RelA accumulation at the cytoplasm and promote c-mip expression. This interaction will lead to alterations in the cytoskeleton and will manifest as nephrotic syndrome or minimal change disease as nephrotoxicity. The role of complement factor H of this type still remains unclear. However, this rough classification is based on molecular aspects and is not absolutely compatible with clinical situations. Actually, TMA, induced by lenvatinib and leading to hemodialysis, was reported before as well[9,10]. Herein, we suggested that if proteinuria is still not improved after reduced dosage or regimens’ adaptions, renal biopsy is still inevitable to make a final confirmation of the underlying pathogenesis.
According to guideline[11], sorafenib and lenvatinib are the only two m-TKI approved by The United States Food and Drug Administration for RAI-refractory DTC. Trials on sorafenib and lenvatinib and their associated nephrotoxicity are briefly summarized in Table 1. In a phase III trial from Brose et al[3] (DECISION trial), hypertension - as one of the signs of nephrotoxicity - was not the chief reason leading to reduced dosage or discontinued therapeutic courses in patients with sorafenib. In another phase III trial by Schlumberger et al[4] (SELECT trial), nephrotoxic effects resulting from lenvatinib such as hypertension (19.9%) and proteinuria (18.8%) were the second and third most common reasons for interrupting the therapeutic course or requiring dose reduction. Even after strategies were initiated to address adverse effects, 1.1% of patients taking lenvatinib with hypertension still encounter treatment discontinuation, which is also the most common cause of treatment discontinuation. In this case report, the patient was initially treated with lenvatinib at 20 mg/d and experienced nephrotoxicity with nephrotic syndrome and severe proteinuria (grade ≥ 3), as well as hypertension. After reducing the dose of lenvatinib to half of the original and symptomatic controls, the adverse effects persisted. Based on evidence from molecular biology, sorafenib should lessen the adverse effects by being less potent to VEGFR receptors than lenvatinib. Half-maximal inhibitory concentrations (IC50) for VEGFR-1 and VEGFR-2 with sorafenib are 26 nmol/L and 90 nmol/L, respectively. In contrast, IC50 for VEGFR-1 and VEGFR-2 with lenvatinib are 22 nmol/L and 4 nmol/L, respectively[12]. Although VEGFR-1 and VEGFR-2 are both present in glomerular endothelial cells and podocytes, VEGFR-2 plays a more significant role in VEGF-A/VEGFR cascades than VEGFR-1. Furthermore, although sorafenib is less potent in VEGFR-2, it provides inhibitory effects to BRAF (IC50: 25 nmol/L)[12], a downstream target which is not inhibited by lenvatinib. Based on this evidence, it is reasonable to substitute lenvatinib with sorafenib, less potent to VEGFR-2 and inhibitory effects to BRAF, to improve nephrotoxicity and maintain the therapeutic effects. In fact, successful experience has also been previously reported[1]. In a study by Goto et al[1] , sorafenib at 800 mg/d was started directly in response to the second progression of RAI-refractory DTC after previous lenvatinib therapy. The current case report features a dynamic serial treatment switch from reduced lenvatinib to sorafenib, starting with a second lowest dosage in DECISION trial for much more severe case of proteinuria than that reported for the case from Goto et al[1]. However, despite this lower dose and worse disease conditions, for the patient in the current case report, the goals of improvement of nephrotoxicity and cancer control were still achieved.
| Trials | Study group with anti-VEGF therapy | Dosage | Median duration of follow-ups | Nephrotoxicity | Strategies during adverse effects |
| Brose et al[3] (DECISION trial, phase-3 trial) | RAI-refractory DTC; 207 patients | Sorafenib: 800 mg/d | 10.6 mo | Hypertension: 40.6%/9.7% (all grades/grade ≥ 3) | Dosage would be reduced to 600 mg/d, 400 mg/d or 200 mg/d |
| Peripheral edema: not documented | |||||
| Proteinuria: not documented | |||||
| Schlumberger et al[4] (SELECT trial, phase-3 trial) | RAI-refractory DTC; 261 patients | Lenvatinib: 24 mg/d | 17.1 mo | Hypertension: 67.8%/41.8% (all grades/grade ≥ 3) | Dosage would be reduced to 20 mg/d, 14 mg/d, or 10 mg/d |
| Peripheral edema: 11.1%/0.4% (all grades/grade ≥ 3) | |||||
| Proteinuria: 31%/10% (all grades/grade ≥ 3) | |||||
| Kudo et al[13] (REFLECT trial, phase-3 trial) | Unresectable HCC; 476 patients with lenvatinib; and 475 patients with sorafenib | 1 Lenvatinib: (dosage based on whether body weights above 60 kg or not): (1) 12 mg/d: 325 patients (68%); and (2) 8 mg/d: 153 patients (32%) | 1 Lenvatinib: 27.7 mo | 1 Lenvatinib: (1) Hypertension: 42%/23% (all grades/grade ≥ 3); (2) Peripheral edema: not documented; and (3) Proteinuria: 25%/6% (all grades/grade ≥ 3) | 1 Lenvatinib: Dosage would be reduced to 8 mg (initially with 12 mg/d) and 4 mg/d (initially with 8 mg/d), or 4 mg every other day |
| 2 Sorafenib: 800 mg/d | 2 Sorafenib: 27.2 mo | 2 Sorafenib: (1) Hypertension: 30%/14% (all grades/grade ≥ 3); (2) Peripheral edema: not documented; and (3) Proteinuria: 11%/2% (all grades/grade ≥ 3 | 2 Sorafenib: Dosage switch was implemented according to prescribing information in each region | ||
| Motzer et al[14] (Number NCT01136733, phase-2 trial) | Metastatic RCC; 51 patients with lenvatinib plus everolimus; and 52 patients with lenvatinib | 1 Lenvatinib plus everolimus: Lenvatinib of 18 mg/d plus everolimus of 5 mg/d | 1 Lenvatinib plus everolimus: 18.5 mo | 1 Lenvatinib plus everolimus: (1) Hypertension: 27%/14% (all grades/grade ≥ 3); (2) Peripheral edema: 27%/0% (all grades/grade ≥ 3); and (3) Proteinuria: 18%/4% (all grades/grade ≥ 3) | 1 Lenvatinib plus everolimus: (1) If possibly from lenvatinib: Dosage would be reduced from 18 mg/d to 14 mg/d, 10 mg/d, and 8 mg/d; and (2) If possibly from everolimus: Dosage would be reduced from 5 mg/d to 5 mg every other day. |
| 2 Lenvatinib: 24 mg/d | 2 Lenvatinib: 17.8 mo | 2 Lenvatinib: (1) Hypertension: 31%/17% (all grades/grade ≥ 3); (2) Peripheral edema: 15%/0% (all grades/grade ≥ 3); and (3) Proteinuria: 12%/19% (all grades/grade ≥ 3) | 2 Lenvatinib: Dosage would be reduced from 24 mg/d to 20 mg/d, 14 mg/d, and 10 mg/d |
There are two other occasions in which lenvatinib will be indicated, which are unresectable hepatocellular carcinoma (HCC)[13] and in conjunction with everolimus in the progression of metastatic renal cell carcinoma (RCC) after prior anti-VEGF therapy[14]. Of the four trials shown in Table 1, proteinuria occurs less often in patients with sorafenib at 800 mg/d, occurring at a rate of 2% in grade ≥ 3[11] maximally. Moreover, a lower occurrence of hypertension grade > 3 has been observed, with 9.7%[3] in patients taking sorafenib at 800 mg/d for 10.6 mo vs 41.8% with lenvatinib at 24 mg/d[4] for 17.1 mo. Although the inclusion of different patients and drug regimens cannot allow meaningful comparison between trials, it still provides an implication that sorafenib, although less potent for cancer control, can be safer in terms of nephrotoxicity than lenvatinib. None of trials with sorafenib at 800 mg/d have documented peripheral edema as an adverse effect[3,13]. These findings support the authors’ rationale that, with a less potent IC50, nephrotoxicity is less as well. Furthermore, switching from reduced lenvatinib dose to sorafenib at full or half-dose, according to a previously published case report[1] and based on the authors’ experience, is feasible for improving nephrotoxicity induced by m-TKI, such as proteinuria or nephrotic syndrome, while achieving the desired therapeutic response. Interestingly, in the trial from Motzer et al[14], lenvatinib at 18 mg plus everolimus at 5 mg, an m-TOR inhibitor, can achieve the same effect on progression-free survival as 24-mg lenvatinib but with a lower rate of proteinuria above grade three (Table 1). In other words, substituting approximate 25% dosage of lenvatinib with everolimus, an inhibitor working at more downstream, can largely improve rates of proteinuria above grade three and achieve the same purpose of treatment by lenvatinib of full dosage. More specifically, after switching 6-mg lenvatinib out of 24-mg lenvatinib to 5-mg everolimus, relative risk is 0.21 to 24-mg lenvatinib on proteinuria above grade three, and it only takes around six to seven patients needing to be treated to prevent one episode of proteinuria above grade three. From animal models of male Sprague–Dawley rats with adriamycin-induced nephrotic syndrome, Ramadan et al[15] discovered that an early, 2 d before induced nephrotic syndrome, low dosage of everolimus, 20 mg/L through drinking water, may exert a nephro-protective effect. Furthermore, the possible mechanism of nephrin/podocin protection is made on evidence of immunofluorescence.
Based on the previous description of molecular theories, the nephrotoxicity caused by m-TKI results from alterations in the cytoskeleton. Those changes are mostly reversible, and as shown herein, it is the view of these authors that renal biopsy is seldom needed during treatment. The emphasis should instead be placed on therapeutic monitoring. For monitoring of proteinuria and the treatment effects, collecting overnight urine is the most objective reference, yet burdensome. In fact, as Masaki et al[16] discovered by collecting urine samples from patients with thyroid cancer during lenvatinib therapy, by incorporating UPCR into assessment of adverse effects, unnecessary treatment interruption could be avoided. Moreover, Evans et al[17] used data from REFLECT trial[11] and concluded that, with a cutoff value 2.4 in UPCR, grades 2 and 3 proteinuria can be differentiated with a 96.9% sensitivity and 82.5% specificity, respectively, compared with 24-h urine collection. Actually this finding was also observed during monitoring of the current case study, with UPCR value of 2.79 between grades 2 and 3 proteinuria, and a meaningful correlation may be evident on linear regression analysis (Figure 7; Pearson correlation coefficient, 0.96; R2, 0.92; adjusted R2, 0.91). Furthermore, the UPCR was also observed to have correlation with treatment response (Figure 8; linear regression; Pearson correlation coefficient, 0.94; R2, 0.88; adjusted R2, 0.85).
In the experiences of using m-TKI for RAI-refractory metastatic DTC and from published case reports[1], nephrotoxicity, such as proteinuria or nephrotic syndrome, may be improved by changing drugs from lenvatinib, a more potent m-TKI, to sorafenib, a less potent m-TKI, while also maintaining therapeutic effects. From a previous trial[12] of metastatic RCC, substituting partial dose of lenvatinib with anti-VEGF drugs working at a downstream level, such as m-TOR inhibitor, may greatly reduce the nephrotoxicity rates and achieve the same therapeutic purpose. Renal biopsy is infrequently necessary to assess nephrotoxicity by m-TKI unless it is not improved after reduced dosage or regimens’ adaptions. Moreover, if one feels bothersome to collect 24-h urine for monitoring, UPCR is indeed a useful tool to assess proteinuria in monitoring with high sensitivity and specificity.
We thank Tung YW (Chief Surgeon, Tungs’ Taichung MetroHarbor Hospital, Taiwan) for helping perform the biopsy via bronchoscopy.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wankowicz Z S-Editor: Yan JP L-Editor: A P-Editor: Xing YX
| 1. | Goto H, Kiyota N, Otsuki N, Imamura Y, Chayahara N, Suto H, Nagatani Y, Toyoda M, Mukohara T, Nibu KI, Kasahara T, Ito Y, Miya A, Hirokawa M, Miyauchi A, Minami H. Successful treatment switch from lenvatinib to sorafenib in a patient with radioactive iodine-refractory differentiated thyroid cancer intolerant to lenvatinib due to severe proteinuria. Auris Nasus Larynx. 2018;45:1249-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Jin Y, Van Nostrand D, Cheng L, Liu M, Chen L. Radioiodine refractory differentiated thyroid cancer. Crit Rev Oncol Hematol. 2018;125:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Peña C, Molnár I, Schlumberger MJ; DECISION investigators. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1125] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 4. | Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1207] [Cited by in RCA: 1347] [Article Influence: 134.7] [Reference Citation Analysis (0)] |
| 5. | Goodsell DS. The molecular perspective: VEGF and angiogenesis. Stem Cells. 2003;21:118-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Eremina V, Quaggin SE. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens. 2004;13:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hähnel B, Gröne HJ, Koesters R, Kriz W. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol. 2009;175:1883-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Izzedine H, Mangier M, Ory V, Zhang SY, Sendeyo K, Bouachi K, Audard V, Péchoux C, Soria JC, Massard C, Bahleda R, Bourry E, Khayat D, Baumelou A, Lang P, Ollero M, Pawlak A, Sahali D. Expression patterns of RelA and c-mip are associated with different glomerular diseases following anti-VEGF therapy. Kidney Int. 2014;85:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Paschke L, Lincke T, Mühlberg KS, Jabs WJ, Lindner TH, Paschke R. Anti VEGF-TKI Treatment and New Renal Adverse Events Not Reported in Phase III Trials. Eur Thyroid J. 2018;7:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Hyogo Y, Kiyota N, Otsuki N, Goto S, Imamura Y, Chayahara N, Toyoda M, Nibu KI, Hyodo T, Hara S, Masuoka H, Kasahara T, Ito Y, Miya A, Hirokawa M, Miyauchi A, Minami H. Thrombotic Microangiopathy with Severe Proteinuria Induced by Lenvatinib for Radioactive Iodine-Refractory Papillary Thyroid Carcinoma. Case Rep Oncol. 2018;11:735-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Fugazzola L, Elisei R, Fuhrer D, Jarzab B, Leboulleux S, Newbold K, Smit J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur Thyroid J. 2019;8:227-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 12. | Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 13. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3818] [Article Influence: 545.4] [Reference Citation Analysis (1)] |
| 14. | Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomasek J, Kremer A, Kim HJ, Wood K, Dutcus C, Larkin J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 697] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 15. | Ramadan R, Faour D, Awad H, Khateeb E, Cohen R, Yahia A, Torgovicky R, Cohen R, Lazari D, Kawachi H, Abassi Z. Early treatment with everolimus exerts nephroprotective effect in rats with adriamycin-induced nephrotic syndrome. Nephrol Dial Transplant. 2012;27:2231-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Masaki C, Sugino K, Kobayashi S, Akaishi J, Hames KY, Tomoda C, Suzuki A, Matsuzu K, Uruno T, Ohkuwa K, Kitagawa W, Nagahama M, Ito K. Urinalysis by combination of the dipstick test and urine protein-creatinine ratio (UPCR) assessment can prevent unnecessary lenvatinib interruption in patients with thyroid cancer. Int J Clin Oncol. 2020;25:1278-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Evans TRJ, Kudo M, Finn RS, Han KH, Cheng AL, Ikeda M, Kraljevic S, Ren M, Dutcus CE, Piscaglia F, Sung MW. Correction: Urine protein:creatinine ratio vs 24-hour urine protein for proteinuria management: analysis from the phase 3 REFLECT study of lenvatinib vs sorafenib in hepatocellular carcinoma. Br J Cancer. 2019;121:625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |