Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4858
Peer-review started: August 25, 2020
First decision: September 12, 2020
Revised: September 18, 2020
Accepted: September 28, 2020
Article in press: September 28, 2020
Published online: October 26, 2020
Processing time: 62 Days and 4.8 Hours
Carotid blowout syndrome (CBS) is a rupture of the carotid artery and is mainly caused by radiation and resection of head and neck cancers or direct tumor invasion of the carotid artery wall. It is a life-threatening clinical situation. There is no established and effective mode of management of CBS. Furthermore, there is no established preceding sign or symptom; therefore, preventive efforts are not clinically meaningful.
We described two cases of CBS that occurred in patients with head and neck cancer after definitive chemoradiotherapy (CRT) using three-dimensional conformal intensity-modulated radiation therapy. Two men aged 61 and 56 years with locally advanced head and neck cancer were treated with definitive CRT. After completing CRT, both of them achieved complete remission. Subsequently, they had persistent severe pain in the oropharyngeal mucosal region and the irradiated neck despite the use of opioid analgesics and rehabilitation for relief of contracted skin. However, continuous follow-up imaging studies showed no evidence of cancer recurrence. Eleven to twelve months after completing CRT, the patients visited the emergency room complaining about massive oronasal bleeding. Angiograms showed rupture of carotid artery pseudoaneurysms on the irradiated side. Despite attempting to secure hemostasis with carotid arterial stent insertion and coil embolization, both patients died because of repeated bleeding from the pseudoaneurysms.
In patients with persistent pain in irradiated sites, clinicians should be suspicious of progressing or impending CBS, even in the three-dimensional conformal intensity-modulated radiation therapy era.
Core Tip: Even though recent endovascular interventions show a moderately successful hemostasis rate, carotid blowout syndrome (CBS) is still a life-threatening complication of radiotherapy of head and neck cancer. Here, we described CBS in two patients who achieved complete remission after definitive chemoradiotherapy but later developed persistent severe pain in the irradiated region. Clinicians should be suspicious that pain can be a sign of progressing or impending CBS, and in such cases, they should consider the rapid adoption of angiographic endovascular intervention to prevent patients from developing devastating hypovolemic shock.
- Citation: Kim M, Hong JH, Park SK, Kim SJ, Lee JH, Byun J, Ko YH. Rupture of carotid artery pseudoaneurysm in the modern era of definitive chemoradiation for head and neck cancer: Two case reports. World J Clin Cases 2020; 8(20): 4858-4865
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4858.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4858
Carotid blowout syndrome (CBS) is a rare but fatal condition because extensive bleeding leads to hypovolemic shock and sudden death. A carotid arterial wall, which has been weakened by radiotherapy or surgery due to cancer of the head and neck, cannot sustain its integrity against the patient’s blood pressure. Thus, patients who, after completion of concurrent chemoradiotherapy (CCRT), develop massive oronasal bleeding should be suspected of occurrence of CBS. However, because of the rare occurrence rate of CBS and the fact that there are no specific tools for the prediction of patients at high risk for CBS, rapid diagnosis is relatively difficult.
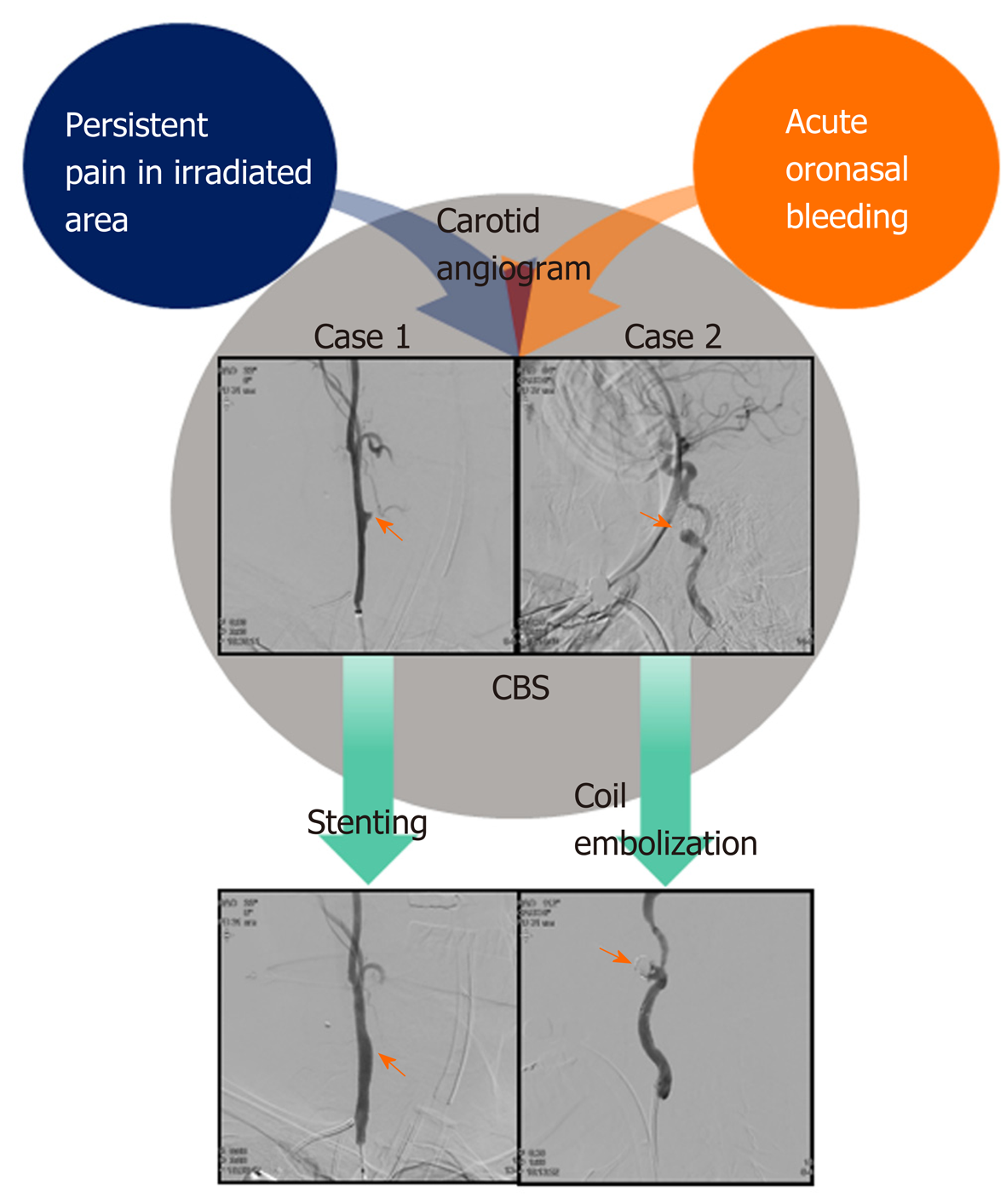
Herein, we describe two cases of massive oronasal bleeding in patients with hypopharyngeal and nasopharyngeal carcinoma who had achieved complete remission but developed severe pain in the irradiated area after completion of definitive CCRT. In both cases, the patients had persistent pain due to the progression of the carotid artery pseudoaneurysm, despite receiving opioid analgesics and interventional pain management. When patients with head and neck squanlous cell carcinoma complained of persistent unexplained severe pain in the irradiated area after completion of CCRT, clinicians should consider that the pain might be from the ongoing development of CBS (Figure 1).
Case 1: A 61-old-year man visited our emergency room (ER) complaining of extensive oral bleeding and syncope.
Case 2: A 56-year-old man visited the ER complaining of massive epistaxis.
Case 1: Thirteen months ago, he had been diagnosed with locally advanced hypopharyngeal cancer (cT1N2bM0) and had completed definitive CCRT with weekly 40 mg/m2 doses of cisplatin for 7 wk. The patient received a prescription dose of 7425 cGy in 33 fractions to the gross primary and nodal tumor, 6525 cGy to the high-risk nodal region and 4950 cGy to the low-risk nodal region. Routine surveillance conducted every 3 mo after CCRT showed complete remission; however, he developed severe pain around the right neck area. Despite the use of opioid analgesics and rehabilitation department consultation, his pain did not improve. He also had cause to visit the ER, complaining of severe pain and edema around the tongue and neck area.
Case 2: One year before, he had been diagnosed with nasopharyngeal cancer (cT2N2M0) and received definitive CCRT with weekly doses of cisplatin 40 mg/m2. Radiotherapy was delivered using a Hi-Art Tomotherapy system. The patient received a prescription dose of 7425 cGy in 33 fractions to the gross primary and nodal tumor, 6075 cGy to the high-risk nodal region and 4500 cGy to the low-risk nodal region. After the completion of CCRT, he continuously experienced severe posterior neck pain and radiating headache, resulting in a severe reduction in his quality of life. However, computed tomography and magnetic resonance imaging (MRI) follow-up scans showed no evidence of recurrence or other abnormal findings. Laryngoscopic surveillance revealed an ulcer on the posterior tongue, hard palate and posterior oropharyngeal wall. Repeated tissue biopsy of the ulcer showed inflammatory cells but no cancer cells.
Case 1: His vital sign was stable.
Case 2: In the ER, laryngoscopic evaluation failed to identify a definitive focus for the massive bleeding from the nasal cavity, and his blood pressure was 60/40 with a pulse rate > 120 bpm. The patient subsequently fell into a mental stupor.
Case 1: In the ER, enhanced computed tomography angiography of the head and neck revealed a bulging contour in the right common carotid arterial lumen. An angiogram further revealed a ruptured pseudoaneurysm (6.8 mm × 3.4 mm) of the right common carotid artery (CCA).
Case 2: Suspicious of arterial rupture, we performed an angiogram of the carotid artery and detected a pseudoaneurysm (16 mm × 8.5 mm in size) in the right internal carotid artery (ICA) with leakage of blood.
The final diagnosis made was ruptured pseudoaneurysm of the right CCA secondary to head and neck irradiation.
The final diagnosis made was ruptured pseudoaneurysm of the right ICA secondary to head and neck irradiation.
Following the insertion of a covered stent (Lifestream® balloon expandable vascular covered stent 9 × 58, Bard, Ireland) via a femoral approach, the bleeding from the carotid arterial rupture site was controlled and his vital signs stabilized. However, one day later, he suddenly experienced weakness of his left upper arm. MRI of the patient’s brain showed multifocal acute infarctions in the right cerebellum, and fronto-parieto-occipital cortices originating from a thromboembolism at the stent site. Although we commenced anticoagulation with aspirin and clopidogrel, sequelae from the weakness in his upper arm remained. After 3 d in the intensive care unit (ICU), he was discharged.
A covered stent was not suitable for this patient due to a tortuous ICA. Stent-assisted coil embolization was performed urgently to preserve ICA flow, but this did not stop his nasal bleeding. The next day, a second angiogram showed that the pseudoaneurysm was re-growing in the right ICA. We performed total occlusion of the right ICA.
Four months later, he returned to the ER complaining of a small amount of hemoptysis. Angiography revealed complete occlusion of the right CCA due to in-stent thrombosis with collateral vessels to the distal ICA. Consequently, we performed embolization with particle embolic agent (Gelfoam 355-500 µm, Boston Scientific, United States). However, during a subsequent period of intensive care unit care, the patient experienced a repeat massive episode of bleeding and died from hypovolemic shock.
An MRI of the patient’s brain subsequently revealed multifocal extensive acute infarctions in the area of the right middle cerebral artery and bilateral anterior cerebral arteries. After remaining unconscious for over 29 d, the patient died due to multi-organ failure caused by hypovolemic shock.
Pseudoaneurysmal rupture of the carotid artery (carotid blow-out syndrome, CBS) is a rare but devastating complication in patients with head and neck cancer. CBS generally occurs as a postoperative complication, or when a tumor compromises the vascular axis[1]. The overall incidence of pseudoaneurysm in the carotid artery has been reported to range from 3% to 4.5%[2].
CBS has been categorized into three severity levels[1,2]. The threatened type (type I) is characterized by carotid artery exposure without active bleeding. Impending blowouts (type II) show mild bleeding episodes that can be resolved temporarily. Type III CBS can cause death rapidly because massive bleeding can occur and may compromise the airway. It is associated with a higher re-bleeding rate than the other types. The two patients described above experienced severe pain in the irradiated area for around 1 year, despite pain management and as yet, no evidence of carotid artery bleeding. However, retrospectively, during the period when the patients complained of persistent severe pain, the pseudoaneurysm of the carotid artery might have been growing without notice. After bleeding occurred, the first patient was rapidly and successfully treated with insertion of a carotid arterial stent and he was alive, even experiencing neurological sequelae from thromboembolism of the stent. However, he died due to re-bleeding. In the second patient, bleeding was hardly controlled, and he finally expired because of sequelae of massive bleeding.
The risk factors for CBS include healing problems with wound dehiscence, cutaneous flap necrosis or pharyngocutaneous fistulas[3]. Other suggested causes include diabetes mellitus, poor nutrition, prolonged corticosteroid use, and uncontrolled hypertension[3]. Previous irradiation also increases the CBS risk seven-fold[4]. Vascular changes after radiotherapy induce premature atherosclerosis with stenosis, and adventitial fibrosis, with resulting arterial wall weakening[2]. These post-radiogenic changes might have led to the subsequent devastating pain resulting from the developing pseudoaneurysm in our patients. Actually, before bleeding occurred, both patients had complained of severe neck pain. The second patient subsequently presented with an ulcer on the posterior tongue, hard palate, and oropharyngeal posterior wall without evidence of recurrence of cancer.
CBS was a rare event during the 3-D conformal radiotherapy era and was also reported rarely after the development of new radiation techniques such as Intensity Modulated Radiation Therapy (IMRT). Theoretically, IMRT can reduce the risk of radiation-induced complications by reducing the radiation dose absorbed by adjacent organs. However, some cases of CBS have been reported following IMRT with dose escalation. For example, Kwong et al[5] reported that dose escalation with IMRT (76 Gy) for treating patients with nasopharyngeal cancer caused the development of pseudoaneurysm in 4% (2 of 50) patients[5]. Both patients presented with sudden onset of profuse bleeding around 7 mo after treatment. Similarly, our patients also received IMRT with dose escalations up to 74.25 Gy to the gross primary and nodal tumor. In cases of re-irradiation, CBS is reported more frequently, with 5.3% occurrence rate in patients receiving re-irradiation using 5 fraction stereotactic body radiation therapy[6].
Angiography is the best method for diagnosis and treatment of CBS[7]. Endovascular techniques offer an efficient alternative to the classical surgical approach, and with much lower morbidity rates. However, the reported clinical outcomes of endovascular procedures have still been devastating. Mean postprocedural survival time was 10 mo, and only 39% of patients survived to the time of the final follow-up[7]. In addition, the re-bleeding rate was 27% after treatment. With respect to adverse effects, permanent vessel occlusion results in immediately higher cerebral ischemia, and stenting induces potentially delayed complications[8,9]. There are no randomized prospective studies evaluating differences in survival outcomes between coil embolization and stenting. Emergency open surgery is not recommended due to the poor outcomes associated with local wound infection, flap necrosis, hemodynamic instability, global cerebral ischemia and consumptive coagulopathy secondary to extreme blood loss[2]. The mortality and neurovascular morbidity of CBS patients treated via an open surgical approach can be as high as 40% and 60% respectively[1,10].
Both patients described in this report manifested CBS at about one year after the completion of CRT. And during that period, they experienced persistent pain on ipsilateral neck area. The time interval between CBS and radiation was reported in the literature to vary from 1 to 20 years[11]. Characteristics of previous reports about CBS after radiotherapy are presented in Table 1. There are neither specific tools for the prediction of patients at a high risk for CBS nor tools for the prevention of massive bleeding. Besides, there have not been research evaluating the correlation between CBS occurrence and progressing pain. We suggest that clinicians should have a high index of suspicion of CBS in patients who, after completion of CCRT, develop unexplainable pain in the regions around the carotid arteries. If signs of acute bleeding are present, clinicians should establish an airway via emergency tracheotomy or oral intubation. Following airway management, clinicians must establish large-bore intravenous access to facilitate rapid volume resuscitation. Then, emergency angiography should be considered to confirm the diagnosis and decide upon the appropriate course of intervention. Within our knowledge, this is the first case report describing the relevance between persistent pain on the irradiated region and CBS occurrence in patients with head and neck cancer.
| Ref. | Year | No. of total patients | Rupture type, acute/ impending/ threatened | Radiation dose | Time to develop CBS from diagnosis (mo) | Survival time | Treatment category | Re-bleeding after treatment | Neurological sequelae (n) |
| Roh et al[10] | 2008 | 16 | Acute: 8; Impending: 7; Threatened: 7 | 78.5 Gy (46–127) | 23 mo (3-142) | 5 mo (1-21) | Endovascular intervention: 16 | 5 (31%) | 3 (19%) |
| Luo et al[12] | 2008 | 14 | (-) | 73 Gy (54-110) | 33 mo (8-70) | 21 mo (4-48) | Endovascular intervention: 14 | (-) | 3 (21%) |
| Chen et al[13] | 2015 | 87 | Acute: 34; Impending: 53 | 73 Gy (SD, 29.6) | 31 mo (SD, 27.2) | Died within 30 d: 19 (52.9%) | Endovascular intervention: 71, Surgery: 16 | 40 (46%) | 13 (15%) |
| Chang et al[14] | 2015 | 96 | Acute: 47; Ongoing: 49 | (-) | 3.5 yr (0.2–34, SD 2.8) | 10 mo (0.07–110, SD 34.1) | Endovascular intervention: 96 | 26 (27%) | 19 (20%) |
| Liang et al[15] | 2016 | 37 | Acute: 25; Impending: 9; Threatened: 3 | (-) | 478 d (246-1752) | 90-d/1-yr estimated survivals: 60.9%/36.6% | Endovascular intervention: 25; Surgery: 12 | 11 (30%) | 4 (11%) |
Pseudoaneurysmal rupture of the carotid artery (CBS) is a rare but devastating complication in patients with head and neck cancer. CBS generally occurs as a postoperative complication, or when a tumor compromises the vascular axis[1]. The overall incidence of pseudoaneurysm in the carotid artery has been reported to range from 3% to 4.5%[2].
CBS has been categorized into three severity levels[1,2]. The threatened type (type I) is characterized by carotid artery exposure without active bleeding. Impending blowouts (type II) show mild bleeding episodes that can be resolved temporarily. Type III CBS can cause death rapidly because massive bleeding can occur and may compromise the airway. It is associated with a higher re-bleeding rate than the other types. The two patients described above experienced severe pain in the irradiated area for around 1 year, despite pain management and as yet, no evidence of carotid artery bleeding. However, retrospectively, during the period when the patients complained of persistent severe pain, the pseudoaneurysm of the carotid artery might have been growing without notice. After bleeding occurred, the first patient was rapidly and successfully treated with insertion of a carotid arterial stent and he was alive, even experiencing neurological sequelae from thromboembolism of the stent. However, he died due to re-bleeding. In the second patient, bleeding was hardly controlled, and he finally expired because of sequelae of massive bleeding.
The risk factors for CBS include healing problems with wound dehiscence, cutaneous flap necrosis or pharyngocutaneous fistulas[3]. Other suggested causes include diabetes mellitus, poor nutrition, prolonged corticosteroid use, and uncontrolled hypertension[3]. Previous irradiation also increases the CBS risk seven-fold[4]. Vascular changes after radiotherapy induce premature atherosclerosis with stenosis, and adventitial fibrosis, with resulting arterial wall weakening[2]. These post-radiogenic changes might have led to the subsequent devastating pain resulting from the developing pseudoaneurysm in our patients. Actually, before bleeding occurred, both patients had complained of severe neck pain. The second patient subsequently presented with an ulcer on the posterior tongue, hard palate, and oropharyngeal posterior wall without evidence of recurrence of cancer.
CBS was a rare event during the 3-D conformal radiotherapy era and was also reported rarely after the development of new radiation techniques such as IMRT. Theoretically, IMRT can reduce the risk of radiation-induced complications by reducing the radiation dose absorbed by adjacent organs. However, some cases of CBS have been reported following IMRT with dose escalation. For example, Kwong et al[5] reported that dose escalation with IMRT (76 Gy) for treating patients with nasopharyngeal cancer caused the development of pseudoaneurysm in 4% (2 of 50) patients[5]. Both patients presented with sudden onset of profuse bleeding around 7 mo after treatment. Similarly, our patients also received IMRT with dose escalations up to 74.25 Gy to the gross primary and nodal tumor. In cases of re-irradiation, CBS is reported more frequently, with 5.3% occurrence rate in patients receiving re-irradiation using 5 fraction stereotactic body radiation therapy[6].
Angiography is the best method for diagnosis and treatment of CBS[7]. Endovascular techniques offer an efficient alternative to the classical surgical approach, and with much lower morbidity rates. However, the reported clinical outcomes of endovascular procedures have still been devastating. Mean postprocedural survival time was 10 mo, and only 39% of patients survived to the time of the final follow-up[7]. In addition, the re-bleeding rate was 27% after treatment. With respect to adverse effects, permanent vessel occlusion results in immediately higher cerebral ischemia, and stenting induces potentially delayed complications[8,9]. There are no randomized prospective studies evaluating differences in survival outcomes between coil embolization and stenting. Emergency open surgery is not recommended due to the poor outcomes associated with local wound infection, flap necrosis, hemodynamic instability, global cerebral ischemia and consumptive coagulopathy secondary to extreme blood loss[2]. The mortality and neurovascular morbidity of CBS patients treated via an open surgical approach can be as high as 40% and 60% respectively[1,10].
Both patients described in this report manifested CBS at about one year after the completion of CRT. And during that period, they experienced persistent pain on ipsilateral neck area. The time interval between CBS and radiation was reported in the literature to vary from 1 to 20 years[11]. Characteristics of previous reports about CBS after radiotherapy are presented in Table 1. There are neither specific tools for the prediction of patients at a high risk for CBS nor tools for the prevention of massive bleeding. Besides, there have not been research evaluating the correlation between CBS occurrence and progressing pain. We suggest that clinicians should have a high index of suspicion of CBS in patients who, after completion of CCRT, develop unexplainable pain in the regions around the carotid arteries. If signs of acute bleeding are present, clinicians should establish an airway via emergency tracheotomy or oral intubation. Following airway management, clinicians must establish large-bore intravenous access to facilitate rapid volume resuscitation. Then, emergency angiography should be considered to confirm the diagnosis and decide upon the appropriate course of intervention. Within our knowledge, this is the first case report describing the relevance between persistent pain on the irradiated region and CBS occurrence in patients with head and neck cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trifan A, Yang XJ S-Editor: Zhang L L-Editor: A P-Editor: Zhang YL
| 1. | Esteller E, León X, de Juan M, Quer M. Delayed carotid blow-out syndrome: a new complication of chemoradiotherapy treatment in pharyngolaryngeal carcinoma. J Laryngol Otol. 2012;126:1189-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Suárez C, Fernández-Alvarez V, Hamoir M, Mendenhall WM, Strojan P, Quer M, Silver CE, Rodrigo JP, Rinaldo A, Ferlito A. Carotid blowout syndrome: modern trends in management. Cancer Manag Res. 2018;10:5617-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 3. | Wong DJY, Donaldson C, Lai LT, Coleman A, Giddings C, Slater LA, Chandra RV. Safety and effectiveness of endovascular embolization or stent-graft reconstruction for treatment of acute carotid blowout syndrome in patients with head and neck cancer: Case series and systematic review of observational studies. Head Neck. 2018;40:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Maran AG, Amin M, Wilson JA. Radical neck dissection: a 19-year experience. J Laryngol Otol. 1989;103:760-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Kwong DL, Sham JS, Leung LH, Cheng AC, Ng WM, Kwong PW, Lui WM, Yau CC, Wu PM, Wei W, Au G. Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;64:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Gebhardt BJ, Vargo JA, Ling D, Jones B, Mohney M, Clump DA, Ohr JP, Ferris RL, Heron DE. Carotid Dosimetry and the Risk of Carotid Blowout Syndrome after Reirradiation with Head and Neck Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2018;101:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Bond KM, Brinjikji W, Murad MH, Cloft HJ, Lanzino G. Endovascular treatment of carotid blowout syndrome. J Vasc Surg. 2017;65:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Chiesa Estomba CM, Betances Reinoso FA, Osorio Velasquez A, Castro Macia O, Gonzalez Cortés MJ, Araujo Nores J. Carotid blowout syndrome in patients treated by larynx cancer. Braz J Otorhinolaryngol. 2017;83:653-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Upile T, Triaridis S, Kirkland P, Archer D, Searle A, Irving C, Rhys Evans P. The management of carotid artery rupture. Eur Arch Otorhinolaryngol. 2005;262:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Roh JL, Suh DC, Kim MR, Lee JH, Choi JW, Choi SH, Nam SY, Kim SY. Endovascular management of carotid blowout syndrome in patients with head and neck cancers. Oral Oncol. 2008;44:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Lee CW, Yang CY, Chen YF, Huang A, Wang YH, Liu HM. CT angiography findings in carotid blowout syndrome and its role as a predictor of 1-year survival. AJNR Am J Neuroradiol. 2014;35:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Luo CB, Teng MM, Chang FC, Chang CY, Guo WY. Radiation carotid blowout syndrome in nasopharyngeal carcinoma: angiographic features and endovascular management. Otolaryngol Head Neck Surg. 2008;138:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Chen YJ, Wang CP, Wang CC, Jiang RS, Lin JC, Liu SA. Carotid blowout in patients with head and neck cancer: associated factors and treatment outcomes. Head Neck. 2015;37:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Chang FC, Luo CB, Lirng JF, Lin CJ, Lee HJ, Wu CC, Hung SC, Guo WY. Endovascular Management of Post-Irradiated Carotid Blowout Syndrome. PLoS One. 2015;10:e0139821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Liang NL, Guedes BD, Duvvuri U, Singh MJ, Chaer RA, Makaroun MS, Sachdev U. Outcomes of interventions for carotid blowout syndrome in patients with head and neck cancer. J Vasc Surg. 2016;63:1525-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |