Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4838
Peer-review started: April 10, 2020
First decision: September 14, 2020
Revised: September 14, 2020
Accepted: September 26, 2020
Article in press: September 26, 2020
Published online: October 26, 2020
Processing time: 198 Days and 19.5 Hours
Patients affected by cystic fibrosis can present with metabolic alkalosis such as Bartter’s syndrome. In this case report we want to underline this differential diagnosis and we aimed focusing on the suspect of cystic fibrosis, also in case of a negative newborn screening.
In a hot August –with a mean environmental temperature of 36 °C– an 8-mo-old female patient presented with severe dehydration complicated by hypokalemic metabolic alkalosis, in absence of fever, diarrhea and vomiting. Differential diagnosis between cystic fibrosis and tubulopathies causing metabolic alkalosis (Bartter’s Syndrome) was considered. We started intravenous rehydration with subsequent improvement of clinical conditions and serum electrolytes normalization. We diagnosed a mild form of cystic fibrosis (heterozygous mutations: G126D and F508del in the cystic fibrosis transmembrane conductance regulator gene). The trigger factor of this condition had been heat exposure.
When facing a patient with hypokalemic metabolic alkalosis, cystic fibrosis presenting with Pseudo-Bartter’s syndrome should be considered in the differential diagnosis, even if the newborn screening was negative.
Core Tip: We report a case of cystic fibrosis presenting with hypokalemic metabolic alkalosis caused by dehydration after heat exposure. We diagnosed a mild form of cystic fibrosis. In particular we wanted focusing on differential diagnosis between cystic fibrosis and Bartter’s Syndrome. We want to highlight that atypical forms of cystic fibrosis could escape to neonatal screening and prompt diagnosis is important for prognosis.
- Citation: Palladino F, Fedele MC, Casertano M, Liguori L, Esposito T, Guarino S, Miraglia del Giudice E, Marzuillo P. Dehydrated patient without clinically evident cause: A case report. World J Clin Cases 2020; 8(20): 4838-4843
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4838.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4838
Cystic fibrosis (CF) is a monogenic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene on chromosome 7. CF is complex and greatly variable in clinical expression[1]. Airways, pancreas, male genital system, intestine, liver, bone, and kidney are involved. CFTR-related disorders are conditions determined by mutations in the CFTR gene but not giving the usual CF clinical picture. They are often mild form of CF and their clinical manifestations are limited to a single district and include episodes of recurrent pancreatitis or isolated bilateral bronchiectasis. Males can manifest bilateral agenesis of the vas deferens with no digestive or respiratory involvement[2]. Both CF and CFTR-related disorders can present metabolic alkalosis, such Bartter’s syndrome (BS).
In this case report we want to underline differential diagnosis between cystic fibrosis and tubulopathies causing metabolic alkalosis (such as BS). Moreover, we aimed focusing on the possibility of suspect diagnosis of cystic fibrosis, nevertheless a negative newborn screening.
In a hot August –with a mean environmental temperature of 36 °C– an 8-mo-old female patient come to our observation because of somnolence and reduced response to stimuli.
Weight loss in the last 7 d and low-quantity micturition in the last 24 h were reported.
In the first months of life the patient presented three episodes of upper respiratory tract infections.
Neonatal screening for cystic fibrosis, hypothyroidism, and phenylketonuria were normal. She was assuming about 400 mL/d of milk. With the exception of 400 IU/d of Vitamin D, no other medications were administered.
She showed slightly dry mucous membranes, tachycardia (140 beats/min) and refill time of about 2 s. Fever was absent as such as vomiting or diarrhea.
Urinalysis did not reveal any abnormality. Serum chemistry was as follows: Venous pH 7.5, bicarbonate 35.1 mmol/L, sodium 135 mEq/L, potassium 2.6 mEq/L, chloride 86 mEq/L, creatinine 0.31 mg/dL, calcium 10.7 mg/dL, phosphorous 4.3 mg/dL, magnesium 2.2 mEq/L. Aspartate and alanine aminotransferase, γ-Glutamyl-transferase, glycaemia, bilirubin, erythrocyte sedimentation rate, C-reactive protein, procalcitonin, alkaline phosphatase and complete blood count were within normal limits. Urinary calcium/creatinine ratio was 0.02 mg/mg, fractional excretion of sodium (FeNa) 0.7%. Electrocardiography was normal.
After 36 h of treatment, clinical conditions of the patient and serum electrolytes improved. Venous pH was 7.44, HCO3- 25.5 mmol/L, Na 142 mEq/L, K 4.5 mEq/L, CL 105 mEq/L.
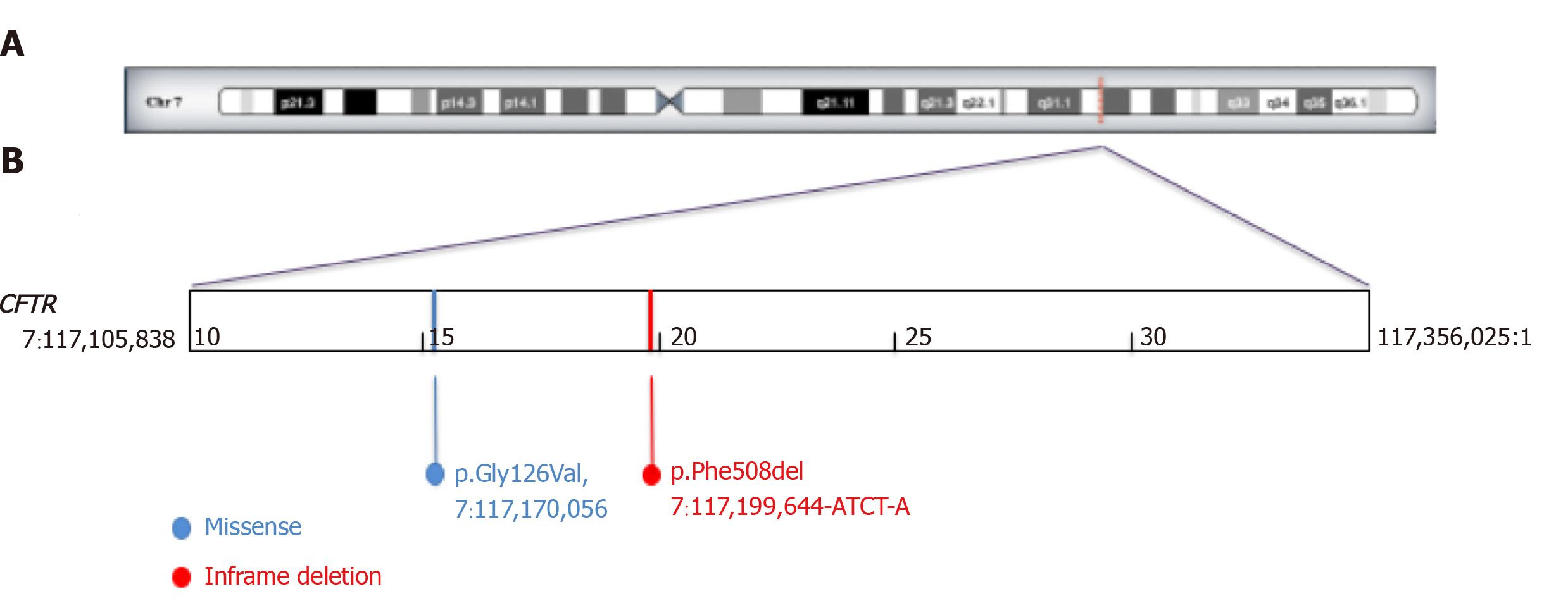
We submitted our patient to sweat test when she became well hydrated with normal acid-base and electrolyte balance. The sweat test showed NaCl in the sweat of 100 mmol/L (normal value < 60 mmol/L). High sweat NaCl values were confirmed at the following sweat test after 2 d (sweat NaCl 87 mmol/L). So, we performed genetics analysis for cystic fibrosis. Molecular diagnosis showed the following heterozygous mutations: G126D and F508del in the gene CFTR (Figure 1). This genotype has been described as cause of mild cases of cystic fibrosis or of atypical forms (better known as CFTR-related disorders)[3].
Abdomen ultrasonography was normal.
Hypokalemic metabolic alkalosis due to cystic fibrosis presenting with Pseudo-Bartter syndrome.
When patient was admitted intravenous rehydration with a solution containing NaCl 0.9% and glucose 2.5% with the addition of KCL 40 mEq/m2 per day was started. When dehydration status and hypokalemic metabolic alkalosis was resolved this infusion was stopped.
Gene mutation analysis identified two heterozygous mutations of the CFTR gene: G126D and F508del. This genotype has been described as cause of mild cases of cystic fibrosis or of atypical forms (better known as CFTR-related disorders)[3].
The variable phenotype of patient affected by CFTR-Related disorders makes very complicated the genetic counseling. A regular clinical evaluation is necessary because CF symptoms may appear later[1]. She will undergo follow up visits at 3, 6, 12 mo after the diagnosis and yearly thereafter, because the atypical form (or CFTR-related disorders) could get worse over time. Proper immunization and influenza vaccination were recommended[4].
The main causes of metabolic alkalosis are shown in the Table 1[5-8]. Our patient had no signs of gastro-intestinal losses neither assumed any drug or too much calcium and absorbable alkali. Therefore, possible causes were or skin (due to cystic fibrosis) or renal (due mainly to BS) losses. However, our patient recovered too promptly compared with a dehydrated patient with BS[9,10]. Moreover, FeNa was < 1% demonstrating extra-renal losses of sodium (Table 2)[11] and increasing the suspect of cystic fibrosis. In the cystic fibrosis, just chloride depletion (in our case through sweating) is the main cause of electrolyte abnormalities. Physical activity, fever, and heat exposure can determinate an excessive sweat production that causes an extracellular fluid volume and chloride depletion.
| Main causes of metabolic alkalosis |
| Chloride depletion syndromes |
| Gastric losses (vomiting, nasogastric tube) |
| Intestinal losses (congenital chloridrorrhea, villous adenomas) |
| Kidney losses (loop or thiazide diuretics) |
| Skin losses (cystic fibrosis) |
| Potassium depletion syndromes |
| Gastrointestinal losses (laxative abuse) |
| Kidney losses: |
| Primary hyperaldosteronism (11β-HSDH deficit, licorice, Liddle syndrome); |
| Secondary hyperaldosteronism (renovascular or malignant hypertension, hemangiopericytoma, Wilm’s tumor, Bartter and Gitelman syndrome, |
| thiazide and loop diuretics, hypercapnia quickly corrected) |
| Other causes |
| Repeated blood transfusion containing potassium citrate |
| Bone metastasis |
| Enteral nutrition with low chloride |
| Milk-alkali syndrome |
| Cystic fibrosis | Bartter’s syndrome | |
| ABE | Alkalosis | Alkalosis |
| FeNa+u | < 1% | > 1% |
| Na+pl | ↓ | ↓ |
| Cl-pl | ↓ | ↓ |
| Cl-u | ↓ | ↑ |
The extracellular fluid volume depletion leads to a release of antidiuretic hormone with subsequent sodium reduction, and activation of renin-aldosterone system with potassium reduction. Moreover, chloride depletion causes increased renal bicarbonate reabsorption. This mechanism is regulated by a chloride–bicarbonate exchanger (pendrin) located on the intercalated cells, sited in cortical collected duct. When chloride depletion occurs, HCO3- secretion is inhibited by insufficient Cl- for anion exchange. In addition, pendrin is reduced in case of potassium depletion. All these mechanisms determine hypokalemic metabolic alkalosis. According to these pathophysiological mechanisms, chloride supplementation, more than sodium and potassium supplementation, is needed to correct the metabolic alkalosis state. The presence of chloride, in fact, increases pendrin activity, with bicarbonate secretion in the lumen of collected duct[12,13].
This kind of clinical and laboratory presentation of CF (known as Pseudo-BS)[14-18], usually, involves children < 2.5 years and is the presenting clinical picture of CF. Episode of vomiting, excessive sweating, heat exposure, fever or respiratory infection could cause a Pseudo-BS, in case of an underlining CF[15,18]. In our case, with the exception of heat exposure, there were no other reasons justifying the dehydration (gastrointestinal fluid losses with diarrhea and/or vomiting, reduction of salt and fluid intake and/or absorption, excessive sweat production for physical activity or fever).
For this reason, we performed sweat test in the suspect of cystic fibrosis. Sweat test is the gold standard to diagnose classical or atypical forms of CF[19]. In fact, even with over 1000 mutations in the CFTR gene on chromosome 7 are known and it is possible to find children with cystic fibrosis which do not present identifiable gene mutations[20]. Nevertheless, some mutations could show atypical and very mild clinical manifestations[1]. In these cases, sweat test results can be intermediate or negative (2%) and so other diagnostic tests are indicated, if clinical manifestations persist (i.e., genetic CFTR tests, CFTR functional tests, such as nasal potential difference, intestinal current measurement)[21,22]. Moreover, it is possible that these patients passed the new-born screening because their sufficient pancreatic status[21-24]. So, clinicians must not exclude, a priori, the suspicious of CF in patients with 1 or more clinical manifestations of CF, despite of negative newborn screening results[25-27].
In conclusion, with this case presentation we would highlight that the Pseudo-Bartter’s syndrome could be an initial clinical presentation of cystic fibrosis, and that the heat exposure might be a trigger of this condition. When facing a Pseudo-Bartter’s syndrome, cystic fibrosis should not be excluded from differential diagnosis, even if the new-born screening was negative.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandey A, Yang L S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Castellani C, Assael BM. Cystic fibrosis: a clinical view. Cell Mol Life Sci. 2017;74:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 2. | Bombieri C, Claustres M, De Boeck K, Derichs N, Dodge J, Girodon E, Sermet I, Schwarz M, Tzetis M, Wilschanski M, Bareil C, Bilton D, Castellani C, Cuppens H, Cutting GR, Drevínek P, Farrell P, Elborn JS, Jarvi K, Kerem B, Kerem E, Knowles M, Macek M, Munck A, Radojkovic D, Seia M, Sheppard DN, Southern KW, Stuhrmann M, Tullis E, Zielenski J, Pignatti PF, Ferec C. Recommendations for the classification of diseases as CFTR-related disorders. J Cyst Fibros. 2011;10 Suppl 2:S86-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Bobadilla JL, Macek M, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Hum Mutat. 2002;19:575-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 725] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 4. | Cystic Fibrosis Foundation, Borowitz D, Parad RB, Sharp JK, Sabadosa KA, Robinson KA, Rock MJ, Farrell PM, Sontag MK, Rosenfeld M, Davis SD, Marshall BC, Accurso FJ. Cystic Fibrosis Foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome during the first two years of life and beyond. J Pediatr. 2009;155:S106-S116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Soifer JT, Kim HT. Approach to metabolic alkalosis. Emerg Med Clin North Am. 2014;32:453-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Galla JH. Metabolic alkalosis. J Am Soc Nephrol. 2000;11:369-375. [PubMed] |
| 8. | Brinkman JE, Sharma S. Physiology, Metabolic Alkalosis. [Updated 2020 Jul 26]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482291/. |
| 9. | Yalçin E, Kiper N, Doğru D, Ozçelik U, Aslan AT. Clinical features and treatment approaches in cystic fibrosis with pseudo-Bartter syndrome. Ann Trop Paediatr. 2005;25:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Fulchiero R, Seo-Mayer P. Bartter Syndrome and Gitelman Syndrome. Pediatr Clin North Am. 2019;66:121-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Ballestero Y, Hernandez MI, Rojo P, Manzanares J, Nebreda V, Carbajosa H, Infante E, Baro M. Hyponatremic dehydration as a presentation of cystic fibrosis. Pediatr Emerg Care. 2006;22:725-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol. 2012;23:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Scurati-Manzoni E, Fossali EF, Agostoni C, Riva E, Simonetti GD, Zanolari-Calderari M, Bianchetti MG, Lava SA. Electrolyte abnormalities in cystic fibrosis: systematic review of the literature. Pediatr Nephrol. 2014;29:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Kennedy JD, Dinwiddie R, Daman-Willems C, Dillon MJ, Matthew DJ. Pseudo-Bartter's syndrome in cystic fibrosis. Arch Dis Child. 1990;65:786-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Kintu B, Brightwell A. Episodic seasonal Pseudo-Bartter syndrome in cystic fibrosis. Paediatr Respir Rev. 2014;15 Suppl 1:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Vilotijević-Dautović G, Stojanović V. Pseudo-Bartter's Syndrome in Patients with Cystic Fibrosis: A Case Series and Review of the Literature. Srp Arh Celok Lek. 2015;143:748-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Faraji-Goodarzi M. Pseudo-Bartter syndrome in children with cystic fibrosis. Clin Case Rep. 2019;7:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Hizal GM, Cıkı K, Esref S, Birbilen A, Tugcu GD, Emiralioğlu N, Yalcin E, Ersoz DD, Ozcelik U, Kiper N. Clinical features of pseudo-bartter syndrome in cystic fibrosis. Eur Resp J. 2017;50:PA1343. [DOI] [Full Text] |
| 19. | Heap S. Guidelines for the Performance of the Sweat Test for the Investigation of Cystic Fibrosis in the UK 2nd Version. An Evidence Based Guideline. R College Paediatr Child Health. 2014;2:1-121. |
| 20. | Ratkiewicz M, Pastore M, McCoy KS, Thompson R, Hayes D, Sheikh SI. Role of CFTR mutation analysis in the diagnostic algorithm for cystic fibrosis. World J Pediatr. 2017;13:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, Howenstine M, McColley SA, Rock M, Rosenfeld M, Sermet-Gaudelus I, Southern KW, Marshall BC, Sosnay PR. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181S:S4-S15.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 541] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 22. | Sermet-Gaudelus I, Brouard J, Audrézet MP, Couderc Kohen L, Weiss L, Wizla N, Vrielynck S, LLerena K, Le Bourgeois M, Deneuville E, Remus N, Nguyen-Khoa T, Raynal C, Roussey M, Girodon E. Guidelines for the clinical management and follow-up of infants with inconclusive cystic fibrosis diagnosis through newborn screening. Arch Pediatr. 2017;24:e1-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Rosenfeld M, Sontag MK, Ren CL. Cystic Fibrosis Diagnosis and Newborn Screening. Pediatr Clin North Am. 2016;63:599-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Hammond KB, Abman SH, Sokol RJ, Accurso FJ. Efficacy of statewide neonatal screening for cystic fibrosis by assay of trypsinogen concentrations. N Engl J Med. 1991;325:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 110] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Padoan R, Genoni S, Moretti E, Seia M, Giunta A, Corbetta C. Genetic and clinical features of false-negative infants in a neonatal screening programme for cystic fibrosis. Acta Paediatr. 2002;91:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Dunn CT, Skrypek MM, Powers AL, Laguna TA. The need for vigilance: the case of a false-negative newborn screen for cystic fibrosis. Pediatrics. 2011;128:e446-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Collaco JM, Panny SR, Hamosh A, Mogayzel PJ. False negative cystic fibrosis newborn screen. Clin Pediatr (Phila). 2010;49:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |