Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4826
Peer-review started: May 16, 2020
First decision: July 29, 2020
Revised: August 9, 2020
Accepted: September 3, 2020
Article in press: September 3, 2020
Published online: October 26, 2020
Processing time: 162 Days and 20.3 Hours
Underwater endoscopic mucosal resection (UEMR) of colorectal lesions is emerging as an alternative method to conventional endoscopic mucosal resection (EMR); however, it is still controversial whether there is a difference in the effectiveness between UEMR and EMR.
To evaluate the effectiveness and safety of UEMR in the treatment of colorectal polyps.
Clinical studies comparing the effectiveness or safety of UEMR in the treatment of colorectal polyps were searched in medical databases, including PubMed, Embase, Cochrane Library, CNKI, and Wanfang Data, monographs, theses, and papers presented at conferences. Statistical analyses were performed using Revman 5.3 software.
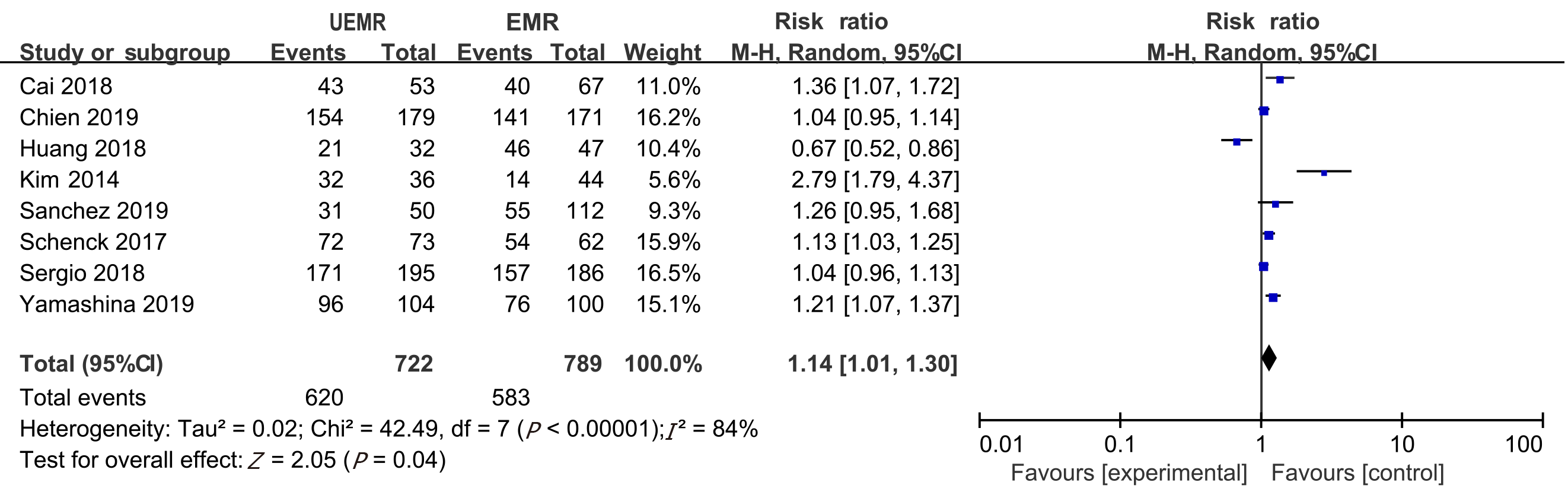
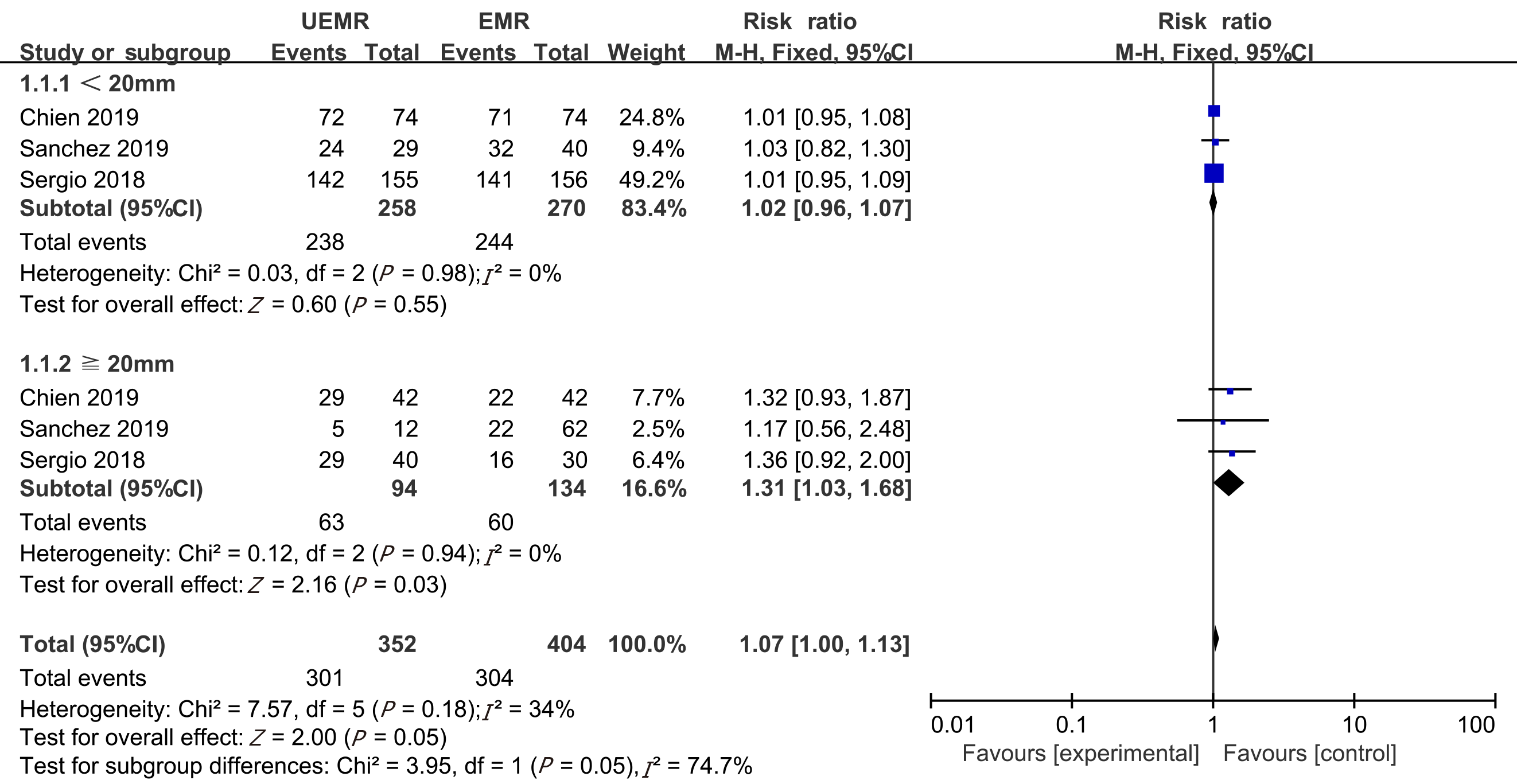
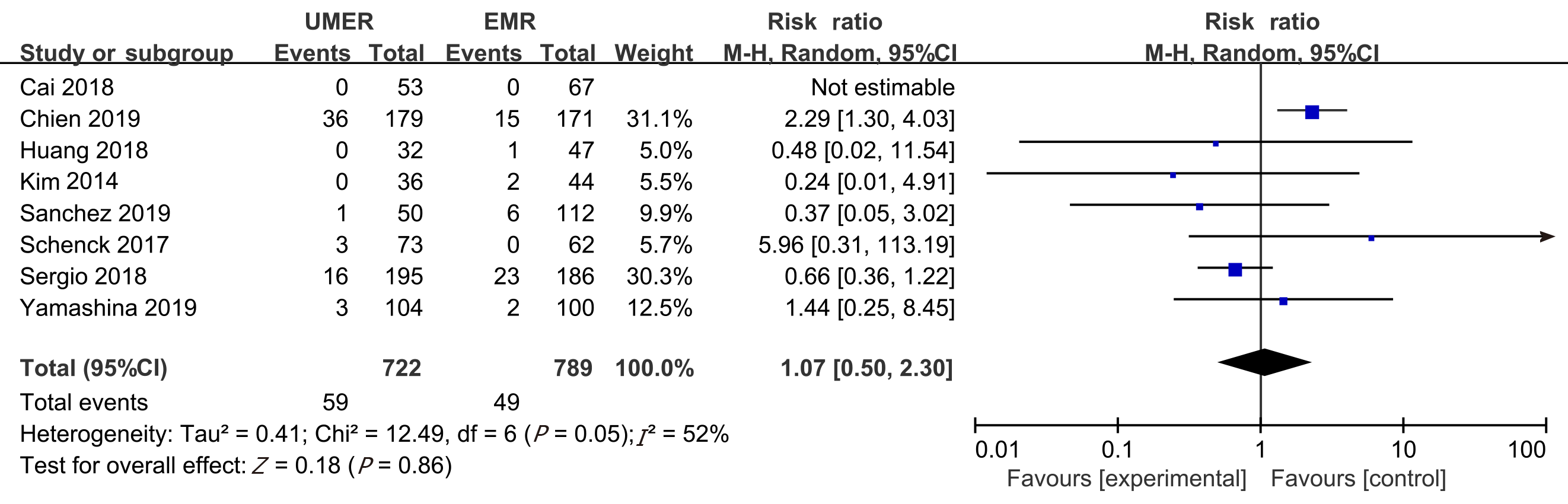
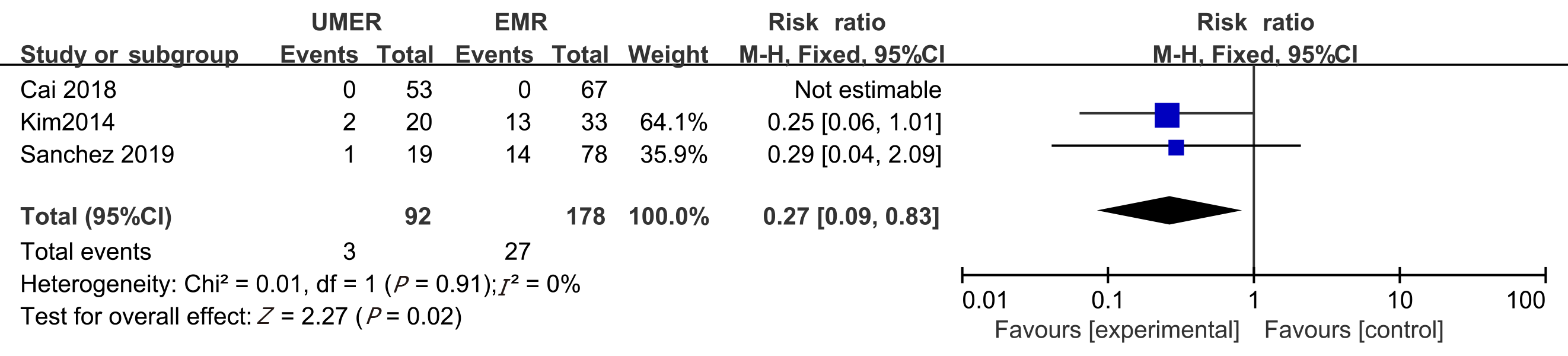
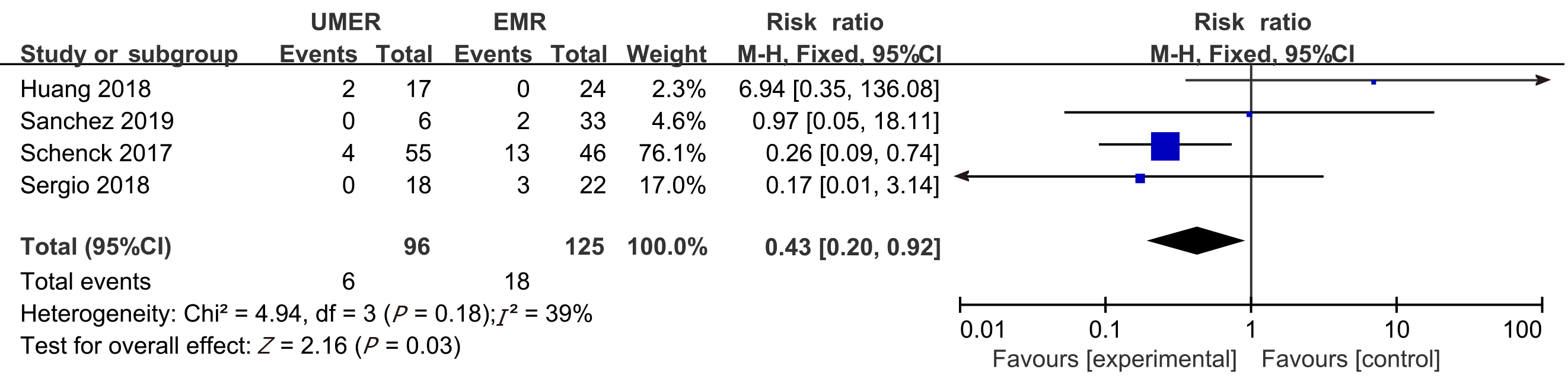
Seven non-randomized controlled trials and one randomized controlled trial met the inclusion criteria. In total, 1382 patients (1511 polyps) were included in the study, including 722 who received UEMR and 789 who received EMR. In the UEMR and EMR groups, the en bloc resection rates were 85.87% and 73.89%, respectively, with a relative risk (RR) value of 1.14 (95% confidence interval [CI]: 1.01-1.30; P < 0.05). In the sub-group analysis, the en bloc resection rate showed no statistically significant difference between the EMR and UEMR groups for polyps less than 20 mm in diameter. However, a statistically significant difference was found between the EMR and UEMR groups for polyps equal to or greater than 20 mm in diameter. The post-endoscopic resection recurrence rates at 3-6 mo of the UEMR and EMR groups were 3.26% and 15.17%, respectively, with an RR value of 0.27 (95%CI: 0.09-0.83; P < 0.05). The post-endoscopic resection recurrence rates of UEMR and EMR at 12 mo were 6.25% and 14.40%, respectively, with an RR value of 0.43 (95%CI: 0.20-0.92; P < 0.05). Additionally, the incidence of adverse events was 8.17% and 6.21%, respectively, with an RR value of 1.07 (95%CI: 0.50-2.30; P > 0.05).
UEMR is an effective technique for colorectal polyps and appears to have some advantages over EMR, particularly with regard to some treatment outcomes.
Core Tip: In this work, we aimed to evaluate the effectiveness and safety of underwater endoscopic mucosal resection in the treatment of colorectal polyps. This is the first meta-analysis comparing the effectiveness and safety of underwater vs conventional endoscopic mucosal resection for colorectal polyps.
- Citation: Ni DQ, Lu YP, Liu XQ, Gao LY, Huang X. Underwater vs conventional endoscopic mucosal resection in treatment of colorectal polyps: A meta-analysis. World J Clin Cases 2020; 8(20): 4826-4837
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4826.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4826
Colorectal cancer (CRC) is a common tumour type worldwide, ranking fifth in incidence among malignant tumours and with an increasing mortality rate[1]. Colorectal polyps, a family history of cancer, and smoking are risk factors for the development of CRC, and approximately 75% of CRC tumours develop through the "adenoma–carcinoma" pathway[2]. If not resected in time, the tumours are highly prone to becoming cancerous. Thus, early detection and treatment are important for preventing CRC. Previously, surgery was regarded as the preferred method to treat colorectal polyps, but it has disadvantages such as significant trauma and a long duration of recovery. Presently, endoscopic techniques are often used instead of surgery[3]. Endoscopic high-frequency electrocoagulation knife resection, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection[4,5] are common endoscopic treatments that are widely used in clinical practice. EMR applies to colorectal polyps less than 20 mm in diameter[6]. The en bloc resection rate and recurrence rate after EMR were 70% and 17%, respectively[7,8]. In 2012, Binmoeller et al[9] first proposed underwater endoscopic mucosal resection (UEMR), in which water was injected into the intestine instead of gas, thereby avoiding submucosal injection. Recent studies have compared the efficacy and safety of conventional EMR and UEMR in the treatment of colorectal polyps. Cadoni et al[10] concluded that UEMR showed better efficacy through controlled studies, and Rodríguez Sánchez et al[11] found no significant difference between EMR and UEMR in terms of effectiveness and safety. In the present study, we collected all clinical trials related to the treatment of colorectal polyps by UEMR to objectively and impartially evaluate the efficacy and safety of UEMR in treating colorectal polyps.
The inclusion criteria were as follows: (1) Study design: Case-controlled trials or randomized controlled trial (RCTs); (2) Subjects: Male or female patients of any age; (3) Interventions: Patients in the trial group had undergone UEMR to resect colorectal polyps, while those in the control group had undergone EMR; and (4) Outcome indexes: Patients with one or more of the following indexes: En bloc resection rate, incidence of adverse events, and post-endoscopic resection recurrence rate at 3-6 mo and at 12 mo. The en bloc resection rate was defined as resection in one piece without fragmentation, along with or extrinsic to the diathermic markings placed around the perimeter of the lesion before resection without a remnant lesion[12].
The exclusion criteria were as follows: (1) Animal experiments; (2) Duplicate articles; (3) Articles with incomplete data; and (4) Articles with ambiguous outcome indexes.
An in-depth literature search was performed using the English keywords “endoscopic mucosal resection, EMR, and UEMR”, as well as using the Chinese keywords “underwater endoscopic mucosal resection”. The computerized databases PubMed, Embase, Cochrane Library, CNKI, and Wanfang Data, monographs, theses, and papers presented at conferences were searched to identify all eligible studies published before September 2019. We also searched the related references in the retrieved studies to avoid missing trials. Articles were independently assessed by two investigators according to the inclusion and exclusion criteria. The articles were initially screened according to the title and abstract to exclude irrelevant studies. Subsequently, the full content of each article was read to identify potentially eligible articles for inclusion in the review. Controversial papers were discussed by all authors, and a consensus was reached.
Methodological index for non-randomized studies[13] was used to assess the quality of the seven included non-randomized controlled trials. The assessment criteria included the following: A clearly stated aim, inclusion of consecutive patients, prospective collection of data, endpoints appropriate to the aim of the study, unbiased assessment of the study endpoint, follow-up period appropriate to the aim of the study, loss to follow-up rate less than 5%, prospective calculation of the study size, an adequate control group, contemporary groups, baseline equivalence of groups, and adequate statistical analyses. The items were scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). According to the score, the quality assessment was divided into three grades: 0–8 indicated low quality, 9–16 indicated middle quality, and 17–24 indicated high quality.
Criteria recommended in the Cochrane system review manual (5.1.0 version)[14] were used to assess the quality of the remaining RCT articles. The assessment criteria included the following: (1) Whether the randomized method was correct; (2) Whether allocation concealment was adopted; (3) Whether a blinded method was applied; (4) Whether the data were complete; (5) Whether the results were selectively reported; and (6) Whether other factors were affecting the truism. Based on these criteria, the quality was categorized into levels A, B, and C. Level A referred to a low bias, indicating that the article completely accorded with the above criteria and had a minimum possibility of bias. Level B referred to an intermediate bias, indicating that the article partially accorded with one or more of the above criteria and had an intermediate possibility of bias. Level C referred to a serious bias, indicating that the article did not comply with one or more of the above criteria and had a high possibility of bias.
The evaluation of the quality of the studies was made by two independent reviewers. If there was any difference in score, it was resolved by consensus.
After the ineligible studies were excluded, a meta-analysis was performed to evaluate the sensitivity and specificity, where good stability implied insignificant changes and poor stability implied significant changes in the results.
The meta-analysis was performed using Revman5.3 software. The data with I2 ≥ 50% were considered heterogeneous and were analysed using the random-effects model; those with I2 < 50% were considered homogeneous and were analysed using the fixed-effects model. Additionally, the relative risk (RR) and 95% confidence interval (CI) were calculated in the meta-analyses of the en bloc resection rate, incidence of adverse events, post-endoscopic resection recurrence rate at 3-6 mo, and post-endoscopic resection recurrence rate at 12 mo.
Funnel plots were constructed using the RR values of the en bloc resection rate, incidence of adverse events, post-endoscopic resection recurrence rate at 3-6 mo, and post-endoscopic resection recurrence rate at 12 mo enrolled in the meta-analysis as the abscissa and the SE [log(RR)] as the longitudinal coordinate. Their symmetry was observed to evaluate the impact of publication bias.
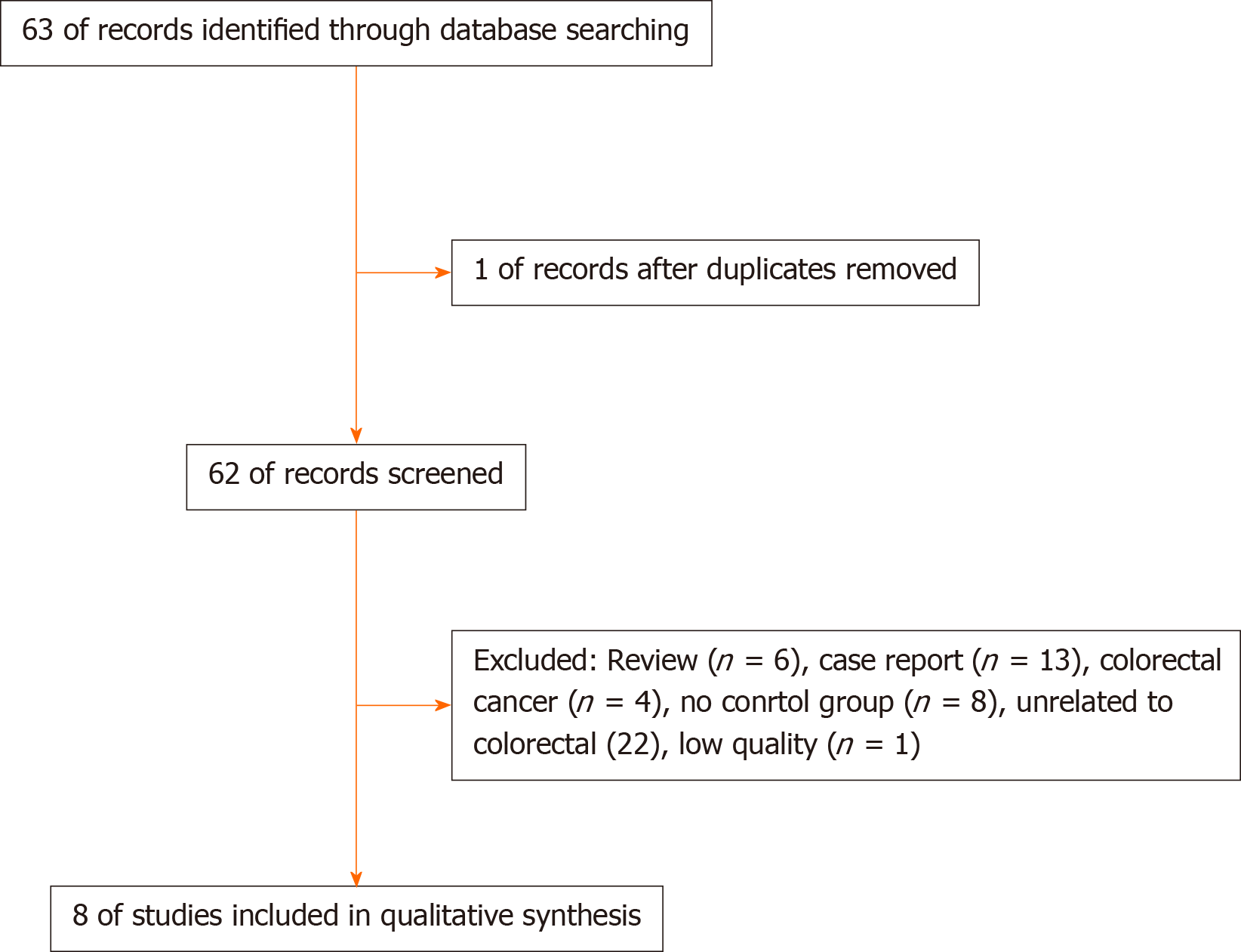
Sixty-three articles were initially collected. After reading the titles and abstracts, 54 articles were excluded due to ineligibility, and one article was excluded due to duplicate experimentation. Finally, seven non-randomized controlled trials and one RCT were included in the analysis[10-11,15-20]. The specific retrieval process is shown in Figure 1, and the characteristics of the enrolled articles are listed in Table 1. There were a total of 1382 patients with 1511 polyps, including 22 patients who received UEMR and 789 who received EMR.
| Ref. | No. Case | Age | Mean size (mm) | En bloc | Procedure time (min) | Adverse events | Recurrence rate in 3-6 mo | Recurrence rate in 12 mo | Score/Quality assessment | Paper type |
| Underwater endoscopic mucosal resection/conventional endoscopic mucosal resection | ||||||||||
| Rodríguez Sánchez et al[11] | 50/112 | 66.25 ± 10.53 | 20.78/30.38 | 31/55 | 26.14/9.82 | 1/6 | (1/19)/(14/78) | (0/6)/(2/33) | 17 | Case-control trial |
| Cadoni et al[10] | 195/186 | 64.7/65.2 | Not reported | 171/157 | No reported | 16/23 | Not reported | (0/18)/(3/22) | 15 | Case-control trial |
| Schenck et al[15] | 73/62 | 62.8/62.3 | 25.4/21.9 | 72/54 | Not reported | 3/0 | Not reported | (4/55)/(13/46) | 15 | Case-control trial |
| Yamashina et al[16] | 104/100 | 70/68 | 14/13.5 | 96/76 | 2.75/2.92 | 3/2 | Not reported | Not reported | B | RCT |
| Kim et al[17] | 36/44 | 68.3/69.2 | 18.5/16.9 | 32/14 | Not reported | 0/2 | (2/20)/(13/33) | Not reported | 13 | Case-control trial |
| Cai et al[18] | 53/67 | Not reported | Not reported | 43/40 | Not reported | 0/0 | (1/56)/(2/67) | Not reported | 17 | Case-control trial |
| Chien et al[19] | 179/171 | 63.4/65.4 | 15.8/18.0 | 154/141 | 10.2/9.7 | 36/15 | Not reported | Not reported | 16 | Case-control trial |
| Huang et al[20] | 32/47 | 66.54/61.47 | 14.2/16.4 | 21/46 | 7.34/17.82 | 0/1 | Not reported | (2/17)/(0/24) | 15 | Case-control trial |
The enrolled RCT study adopted a random method with complete data. Blindness, allocation concealment, selective reporting bias, and factors affecting the truism were not reported. According to the Cochrane system review manual (5.1.0 version), the RCT was assessed as level B study.
Seven non-randomized controlled trials had a clearly stated aim, an adequate control group, prospective collection of data, and a follow-up period appropriate to the aim of the study. The endpoints appropriately reflected the aim of the study. The above five items were scored at 2 points each. No blinding was used, and no sample size was estimated. Therefore, those two items for all the studies were scored at 0 points. Loss of the follow-up rate was more than 5%, and this item was scored at 0 points. Some studies could not be assessed in terms of the continuity of the included patients or in terms of whether the two groups were contemporary, whether they had baseline equivalence, or whether adequate statistical analyses were reported; thus, these items were all scored at 1 point. According to the non-randomized controlled trial methodological index for non-randomized studies evaluation, the average score of the seven non-randomized trials was 15.4.
Therefore, the comprehensive quality assessment of the eight clinical trials included showed that they were of “moderate quality”.
En bloc resection rate: The en bloc resection rate was reported in all the eight articles. In the UEMR and EMR groups, the en bloc resection rates were 85.87% and 73.89%, respectively. The meta-analysis revealed heterogeneity between the two groups in terms of the en bloc resection rate. Therefore, a combination analysis was performed using the random-effects model. The RR value was 1.14 (95%CI: 1.01-1.30; P = 0.04; Figure 2).
Considering the size of the polyps, we performed a sub-group analysis of the studies conducted by Chien et al[19], Rodríguez Sánchez et al[11], and Cadoni et al[10]. The meta-analysis revealed that the en bloc resection rates were 92.25% and 90.37% in the UEMR and EMR groups, respectively, for polyps with a diameter less than 20 mm. The RR was 1.02 (95%CI: 0.96-1.07; P = 0.55), with no statistical significance. However, the en bloc resection rates were 67.02% and 44.78% in the UEMR and EMR groups, respectively, for polyps with a diameter equal to or greater than 20 mm. The RR was 1.31 (95%CI: 1.03-1.68; P = 0.03), which was statistically significant (Figure 3).
Incidence of adverse events: All the eight articles reported the incidence of adverse events, which was 8.17% and 6.21% in the UEMR and control groups, respectively. The meta-analysis showed heterogeneity between the study groups. Hence, the random-effects model was used for the combination analysis, which showed an RR value of 1.07 (95%CI: 0.50-2.30; P = 0.86; Figure 4).
Post-endoscopic resection recurrence rate at 3-6 mo: Among the eight articles, the post-endoscopic resection recurrence rate at 3-6 mo was reported in three articles, which was 3.26% and 15.17% in the UEMR and control groups, respectively. The meta-analysis revealed no heterogeneity between the study groups. Hence, the fixed-effects model was used for the combination analysis. The RR value was 0.27 (95%CI: 0.09-0.83, P = 0.02; Figure 5).
Post-endoscopic resection recurrence rate at 12 mo: Among the eight articles, the post-endoscopic resection recurrence rate at 12 mo was reported in four articles — 6.25% and 14.40% in the UEMR and control groups, respectively. The meta-analysis revealed no heterogeneity between the study groups. Therefore, a combination analysis was performed using the fixed-effects model. The RR value was 0.43 (95%CI: 0.20-0.92; P = 0.03; Figure 6).
The meta-analysis after excluding the articles one by one showed no significant changes in the sensitivity and specificity, indicating good stability of the enrolled studies.
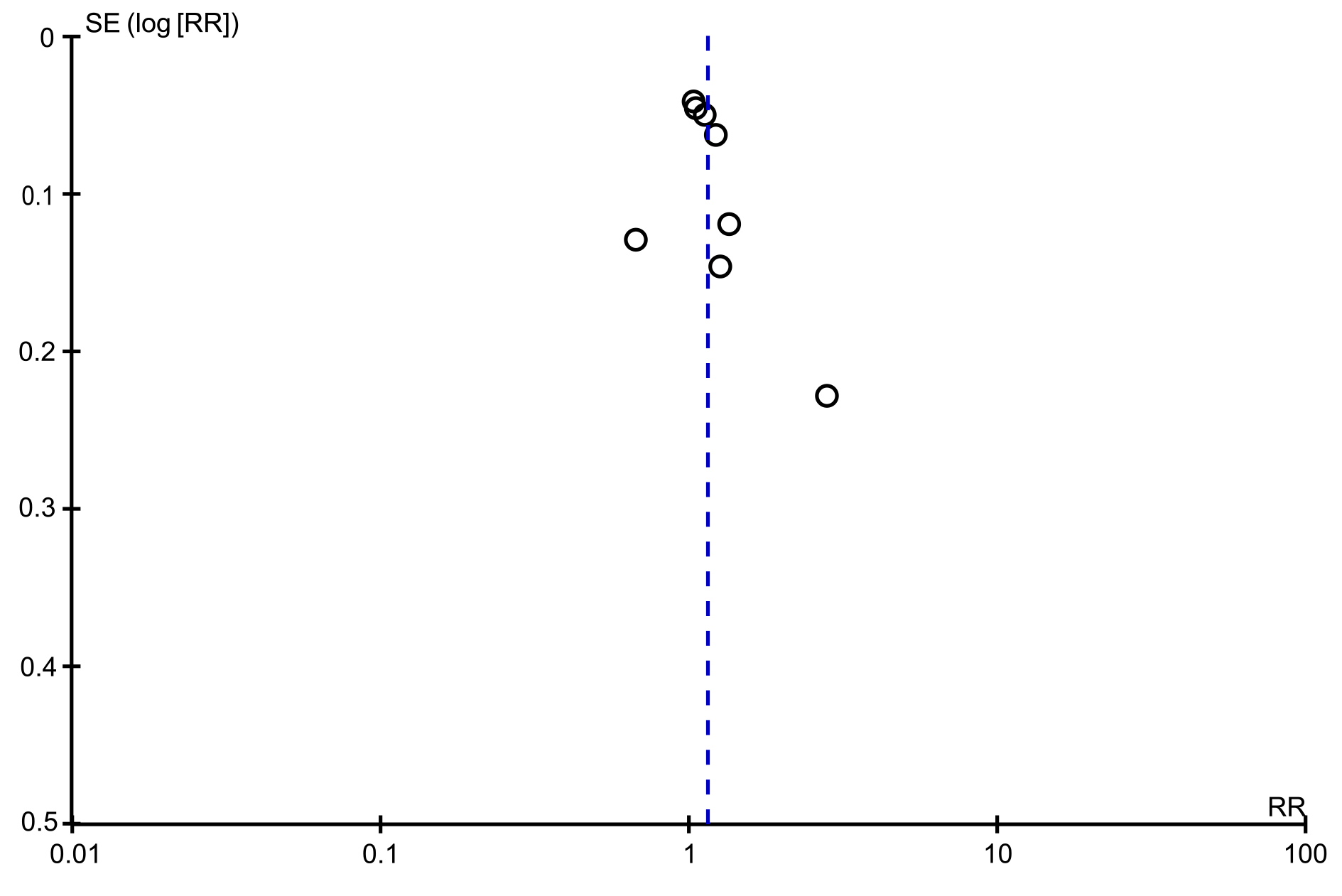
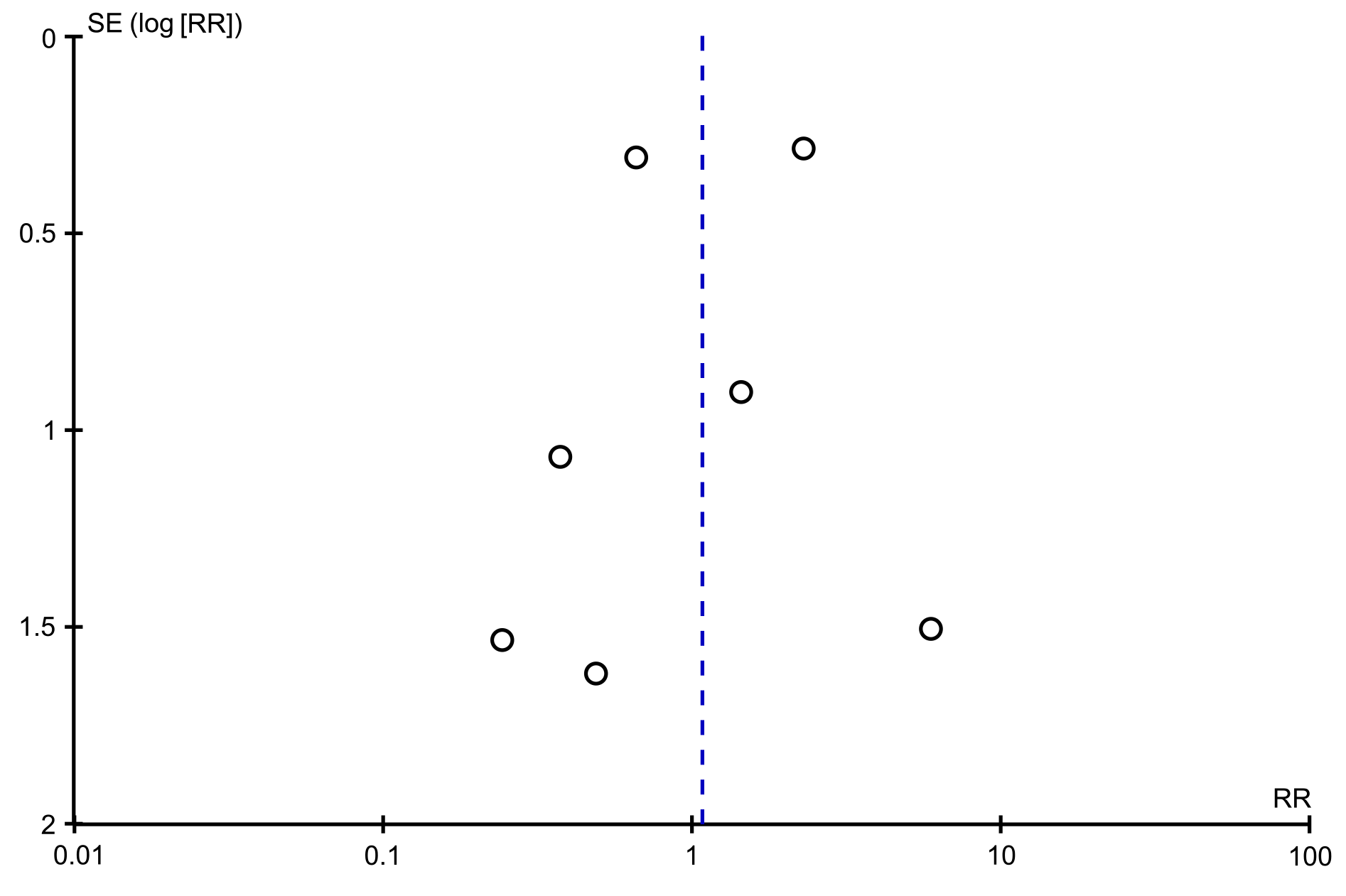
The funnel plots of the incidence of adverse events, post-endoscopic resection recurrence rate at 3-6 mo, and post-endoscopic resection recurrence rate at 12 mo were symmetrical, suggesting no publication bias. The funnel plot of the en bloc resection rate was asymmetrical, suggesting possible publication bias (Figures 7 and 8).
EMR has been an efficient treatment for colorectal polyps for several decades[21,22]. Conventional EMR is applicable for polyps less than 20 mm in diameter[6] and improves the resection rate through injecting fluid to the mucosa and elevating the polyps. However, local lesion residues and polyp recurrence might still occur, as well as adverse events such as bleeding and perforation[23]. The rates of delayed bleeding and perforation post-endoscopic resection are 1.15%-1.7% and 0.58%-0.8%, respectively[24-26]. Conventional EMR, due to the infusion of gas into the intestinal lumen, could flatten the polyps and cause thinning of the intestinal wall, leading to iatrogenic damage such as perforation. Additionally, liquid injection under the mucosa increases the tissue tension and flattens the originally flat polyps relative to the surrounding tissue. Submucosal injection might also have a risk of extraintestinal injection, which could lead to complications such as abdominal infection[27]. Moreover, EMR requires experienced skill to carry out steep mucosal elevation quickly without dispersing the solution. Therefore, identifying a more effective method to resect colorectal polyps and reduce its recurrence is crucial.
In 2012, Binmoeller et al[9], inspired by EUS, proposed UEMR that avoided submucosal injection of the intestine. Polyps could be identified through the colonoscope by rinsing the surface of the polyps and their surroundings. After exhausting the gas in the intestinal lumen, water was injected into the intestinal lumen so that the polyps were floating and then excised. Since then, some clinical studies have reported cases of successful treatment of colorectal polyps using UEMR[28-32]. Based on the domestic and international literature, we believed that polyps suitable for EMR are also suitable for UEMR. For flat polyps with a diameter of 20 mm or more, segmentation can be performed[33,34]. Some studies have suggested that UEMR is indicated for duodenal polyps and recurrent and residual lesions of colorectal polyps. The en bloc resection rate and endoscopic complete resection rate are higher for UEMR than for conventional EMR[35]. The major risk of EMR is bleeding[36], while only one case of perforation with UEMR has been reported[37]. In recent years, this technology has been extended to underwater endoscopic submucosal dissection[38,39] and underwater peroral endoscopic myotomy[40]. A multi-centre prospective study by Rodríguez Sánchez et al[11] found that the en bloc resection rates in the UEMR and EMR groups were 62% and 49% (P < 0.05), respectively, and a multi-centre randomized controlled trial by Yamashina et al[16] found that the en bloc resection rates of UEMR and EMR were 89% and 75% (P < 0.05), respectively. The efficacy of UEMR and EMR remains controversial and might be related to the size, shape, and pedicle of the polyps. Therefore, we conducted this meta-analysis to compare the efficacy and safety of UEMR and EMR in the treatment of colorectal polyps.
Presently, few clinical studies are available on the treatment of colorectal polyps by UEMR, and to the best of our knowledge, there are no on-going RCTs. Eight clinical studies were included in our study, and one was a RCT. The meta-analysis revealed that compared with the EMR group, the en bloc resection rate was improved and the procedure time was shortened in the UEMR group, while the incidence of adverse events was not significantly different. Additionally, sub-group analysis showed that the en bloc resection rate of UEMR was higher than that of EMR for polyps with a diameter less than 20 mm, though there was no significant difference. However, for polyps with a diameter equal to or greater than 20 mm, the en bloc resection rate of UEMR was significantly higher than that of EMR. According to our results, we believe that UEMR is preferred for polyps, especially for those equal to or larger than 20 mm. This could be because the water has a floating effect on the mucous membrane with polyps, and the polyps are then elevated from the muscularis propria, thereby avoiding submucosal injection[41]. Polyps soaked in water could amplify the mucosal structure due to optical effects. The meta-analysis showed that the post-endoscopic resection recurrence rate decreased at 3-6 mo and at 12 mo. This is because UEMR increased the en bloc resection rate. The en bloc resection is an effective technique to evaluate the margins of the resected lesion. Furthermore, en bloc resection has been shown to decrease the risk of post-endoscopic resection recurrence related to incomplete removal[42]. Therefore, based on this study, there appears to be some advantages with UEMR compared to EMR, particularly with regard to some treatment outcomes.
This study has some limitations that should be mentioned: (1) Only a limited number of studies were included, and only one study was a RCT. Some studies were of questionable quality, and study heterogeneity was noted. In non-randomized controlled trials, endoscopic physicians chose different treatments according to different conditions, and there was obvious selection bias. Therefore, the effectiveness and safety of UEMR need to be further confirmed by additional RCTs; (2) Sub-group analyses of different types of colorectal polyps and those based on histology were not performed. Therefore, the effectiveness and safety of UEMR and EMR for different sizes and types of colorectal polyps could not be compared; and (3) All measurements in this study were subject to the clinical experience of the endoscopists.
In summary, UEMR appears to be an effective technique for colorectal polyps. In addition, the adverse event and post-endoscopic resection recurrence rates are acceptable. Based on these results, this technique can be regarded as a therapeutic option for the resection of colorectal polyps.
It remains a matter of debate whether there is a difference in the effectiveness and safety between underwater endoscopic mucosal resection (UEMR) and endoscopic mucosal resection (EMR).
By comparing the effectiveness and safety between UEMR and EMR, we wanted to search a more effective treatment for colorectal polys.
Through this meta-analysis, we evaluated the effectiveness and safety of UEMR in the treatment of colorectal polyps.
The clinical studies that compared the effectiveness or safety of UMER in the treatment of colorectal polyps were searched in databases.
Compared with the EMR group, the en bloc resection rate was improved and the procedure time was shortened in the UEMR group, while the incidence of adverse events was not significantly different. What's more, for polyps with a diameter equal to or greater than 20 mm, the en bloc resection rate of UEMR was higher than that of EMR.
UEMR is an effective technique for colorectal polyps and appears to have some advantages over EMR, particularly with regard to some treatment outcomes.
We will carry out more studies on the safety and efficacy of UEMR in treatment of colorectal polys to confirm the findings of the present study. And through these studies, we hope that the technique of UMER could be widely used in clinical practice.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de-León-Rendón J, Schietroma M, Wijaya R S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13210] [Article Influence: 1467.8] [Reference Citation Analysis (3)] |
| 2. | Burnett-Hartman AN, Passarelli MN, Adams SV, Upton MP, Zhu LC, Potter JD, Newcomb PA. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177:625-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 3. | Church JM. Experience in the endoscopic management of large colonic polyps. ANZ J Surg. 2003;73:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr Opin Gastroenterol. 2017;33:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 6. | Yokota T, Sugihara K, Yoshida S. Endoscopic mucosal resection for colorectal neoplastic lesions. Dis Colon Rectum. 1994;37:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Khashab M, Eid E, Rusche M, Rex DK. Incidence and predictors of "late" recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009;70:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 10. | Cadoni S, Liggi M, Gallittu P, Mura D, Fuccio L, Koo M, Ishaq S. Underwater endoscopic colorectal polyp resection: Feasibility in everyday clinical practice. United European Gastroenterol J. 2018;6:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Rodríguez Sánchez J, Uchima Koecklin H, González López L, Cuatrecasas M, de la Santa Belda E, Olivencia Palomar P, Sánchez García C, Sánchez Alonso M, Muñoz Rodríguez JR, Gómez Romero FJ, López Viedma B, Agarrabeitia AB, Olmedo Camacho J, Albéniz Arbizu E. Short and long-term outcomes of underwater EMR compared to the traditional procedure in the real clinical practice. Rev Esp Enferm Dig. 2019;111:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD, Garcia-Kennedy R. Attempted underwater en bloc resection for large (2-4 cm) colorectal laterally spreading tumors (with video). Gastrointest Endosc. 2015;81:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5630] [Article Influence: 255.9] [Reference Citation Analysis (0)] |
| 14. | Higgins Jp T, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Database of Systematic Reviews. 2008;S38. [DOI] [Full Text] |
| 15. | Schenck RJ, Jahann DA, Patrie JT, Stelow EB, Cox DG, Uppal DS, Sauer BG, Shami VM, Strand DS, Wang AY. Underwater endoscopic mucosal resection is associated with fewer recurrences and earlier curative resections compared to conventional endoscopic mucosal resection for large colorectal polyps. Surg Endosc. 2017;31:4174-4183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Yamashina T, Uedo N, Akasaka T, Iwatsubo T, Nakatani Y, Akamatsu T, Kawamura T, Takeuchi Y, Fujii S, Kusaka T, Shimokawa T. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology. 2019;157:451-461.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 17. | Kim HG, Thosani N, Banerjee S, Chen A, Friedland S. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc. 2014;80:1094-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Cai Y, Wang H, Bai QX, Yu JF. Water immersion-assisted endoscopic en bloc resection for colorectal sessile tumors. Zhonghua Xiaohua Neijing Zazhi. 2018;35:482-485. [DOI] [Full Text] |
| 19. | Chien HC, Uedo N, Hsieh PH. Comparison of underwater and conventional endoscopic mucosal resection for removing sessile colorectal polyps: a propensity-score matched cohort study. Endosc Int Open. 2019;7:E1528-E1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Huang R, Li F, Ji D, Xiao Z, Zhou J, Sun T, Xiang P, Bao Z. Comparative Analysis of Curative Effect of Conventional Endoscopic Mucosal Resection, Underwater Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection in Treatment of Colorectal Lesion: A Retrospective Case-control Study. Wei Chang Bing Xue Zazhi. 2018;23:23-27. [DOI] [Full Text] |
| 21. | Deyhle P, Largiadèr F, Jenny S, Fumagalli I. A Method for Endoscopic Electroresection of Sessile Colonic Polyps. Endoscopy. 1973;5:38-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Yamashina T, Tumura T, Maruo T, Matsumae T, Yoshida H, Tanke G, Taki M, Fukuhara M, Kimura Y, Sakamoto A, Henmi S, Sawai Y, Saito S, Nishijima N, Nasu A, Komekado H, Asada M, Kita R, Kimura T, Osaki Y. Underwater endoscopic mucosal resection: a new endoscopic method for resection of rectal neuroendocrine tumor grade 1 (carcinoid) ≤ 10 mm in diameter. Endosc Int Open. 2018;6:E111-E114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Tadepalli US, Feihel D, Miller KM, Itzkowitz SH, Freedman JS, Kornacki S, Cohen LB, Bamji ND, Bodian CA, Aisenberg J. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video). Gastrointest Endosc. 2011;74:1360-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Watabe H, Yamaji Y, Okamoto M, Kondo S, Ohta M, Ikenoue T, Kato J, Togo G, Matsumura M, Yoshida H, Kawabe T, Omata M. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc. 2006;64:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisasbe T, Matsuda T, Ishikawa H, Sugihara K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 27. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishihara S, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Boku N, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol. 2015;20:207-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 490] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 28. | Rodrigues JP, Pinho R, Sousa M, Silva JC, Gomes C, Carvalho J. Underwater endoscopic mucosal resection of a laterally spreading tumor overlying a previous endoscopic carbon tattoo. Endoscopy. 2018;50:E231-E232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Takeuchi Y, Tonai Y, Ikeda K. Underwater endoscopic mucosal resection for a superficial polyp located at the anastomosis after surgical colectomy. Dig Endosc. 2017;29 Suppl 2:67-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Siau K, Ishaq S, Cadoni S, Kuwai T, Yusuf A, Suzuki N. Feasibility and outcomes of underwater endoscopic mucosal resection for ≥ 10 mm colorectal polyps. Surg Endosc. 2018;32:2656-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Kono M, Takeuchi Y, Higashino K, Uedo N, Ishihara R. Circumferential ileocecal valve removal for a colonic polyp using underwater endoscopic mucosal resection. Endoscopy. 2020;52:E7-E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Rodríguez Sánchez J, Úbeda Muñoz M, de la Santa Belda E, Olivencia Palomar P, Olmedo Camacho J. Underwater hybrid endoscopic submucosal dissection in a rectal polyp: a case report of a new application of "underwater endoscopy". Rev Esp Enferm Dig. 2018;110:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Wang AY, Flynn MM, Patrie JT, Cox DG, Bleibel W, Mann JA, Sauer BG, Shami VM. Underwater endoscopic mucosal resection of colorectal neoplasia is easily learned, efficacious, and safe. Surg Endosc. 2014;28:1348-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Uedo N, Nemeth A, Johansson GW, Toth E, Thorlacius H. Underwater endoscopic mucosal resection of large colorectal lesions. Endoscopy. 2015;47:172-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Shichijo S, Takeuchi Y, Uedo N, Ishihara R. Management of local recurrence after endoscopic resection of neoplastic colonic polyps. World J Gastrointest Endosc. 2018;10:378-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P, Rex DK. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 285] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 37. | Ponugoti PL, Rex DK. Perforation during underwater EMR. Gastrointest Endosc. 2016;84:543-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Yoshii S, Hayashi Y, Matsui T, Aoi K, Tsujii Y, Iijima H, Takehara T. "Underwater" endoscopic submucosal dissection: a novel technique for complete resection of a rectal neuroendocrine tumor. Endoscopy. 2016;48 Suppl 1 UCTN:E67-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Akasaka T, Takeuchi Y, Uedo N, Ishihara R, Iishi H. "Underwater" endoscopic submucosal dissection for superficial esophageal neoplasms. Gastrointest Endosc. 2017;85:251-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Binmoeller KF, Bhat YM. Underwater peroral endoscopic myotomy. Gastrointest Endosc. 2016;83:454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Cammarota G, Cesaro P, Cazzato A, Cianci R, Fedeli P, Ojetti V, Certo M, Sparano L, Giovannini S, Larocca LM, Vecchio FM, Gasbarrini G. The water immersion technique is easy to learn for routine use during EGD for duodenal villous evaluation: a single-center 2-year experience. J Clin Gastroenterol. 2009;43:244-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Asge Technology Committee, Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kwon R, Mamula P, Rodriguez S, Shah RJ, Wong Kee Song LM, Tierney WM. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |