Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4816
Peer-review started: July 21, 2020
First decision: August 7, 2020
Revised: August 11, 2020
Accepted: September 2, 2020
Article in press: September 2, 2020
Published online: October 26, 2020
Processing time: 94 Days and 3.1 Hours
The incidence of postoperative nausea and vomiting (PONV) in patients undergoing laparoscopic hysterectomy is very high compared with other surgeries, even when many prophylactic measures have been taken. However, the pathogenesis of PONV is multifactorial. Female sex, a history of motion sickness or PONV, nonsmokers, and perioperative opioid use are the most closely related factors. Among the multiple risk factors, suboptimal gastrointestinal (GI) perfusion may be attributed to some cases of PONV, and increased systemic vascular resistance (SVR) may lead to GI ischemia. The hypothesis of this research was that SVR is related to PONV.
To investigate the relationship between SVR and PONV in patients undergoing laparoscopic hysterectomy.
A total of 228 patients who underwent elective laparoscopic hysterectomy were included in this prospective observational study. SVR was monitored using a noninvasive hemodynamic monitoring system. Four indices of SVR, the baseline, mean, area under the curve (AUC), and weighted AUC, were used for analysis. The incidence and severity of nausea and vomiting were evaluated while patients were awake and throughout the intervals from 0 to 2 h, 2 to 6 h, and 6 to 24 h starting upon arrival at the post-anesthesia care unit. The associations between various SVR indices and PONV were investigated by logistic regression. P < 0.05 was considered statistically significant.
The incidence of PONV in the study was 56.14% (128/228), and PONV tended to appear within 6 h after surgery. Five variables were significant in univariate analyses, however, only SVR mean [odds ratio (OR) = 1.015, 95%CI: 1.005-1.109, P = 0.047] and duration of surgery (OR = 1.316, 95%CI: 1.003-2.030, P = 0.012) were associated with PONV after logistic regression analysis. Furthermore, patients with high SVR mean were more likely to suffer from PONV after laparoscopic hysterectomy. On average, patients who developed PONV needed more time to tolerate diet and demonstrated poorer sleep quality on the first night after surgery.
In this study, PONV was a common complication after laparoscopic hysterectomy. SVR was associated with PONV, and high SVR mean was associated with a significantly increased risk of PONV.
Core Tip: We performed a prospective observational study of the risk factors for postoperative nausea and vomiting (PONV) after laparoscopic hysterectomy in a single institution. We analyzed the relationship between systemic vascular resistance (SVR) indices and the occurrence of PONV and found that higher SVR mean was associated with increased risk of PONV after laparoscopic hysterectomy.
- Citation: Qu MD, Zhang MY, Wang GM, Wang Z, Wang X. Intraoperative systemic vascular resistance is associated with postoperative nausea and vomiting after laparoscopic hysterectomy. World J Clin Cases 2020; 8(20): 4816-4825
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4816.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4816
Postoperative nausea and vomiting (PONV) is defined as nausea, retching or vomiting that occurs within 24 h after an operation, remaining prevalent despite the utilization of various antiemetic measures. The incidence of PONV after general anesthesia is approximately 30%[1] and can be as high as 80% after gynecological laparoscopic surgery[2]. The consequences of PONV include patient discomfort, additional postoperative complications, prolonged hospitalization, and increased health care costs[3]. The pathogenesis of PONV is multifactorial, and its etiology and pathogenesis are not clear.
Suboptimal gastrointestinal (GI) perfusion may be responsible for some cases of PONV[4]. Insufficient GI perfusion leads to an increase in serotonin release in the GI tract, which induces PONV by stimulating the chemoreceptor trigger zone adjacent to the vomiting center[5-7]. However, currently, GI perfusion cannot be easily and continuously monitored in patients. Optimal systemic vascular resistance (SVR) is essential for the perfusion of any metabolically active tissue bed, such as the GI tract[8]. Increased SVR is related to suboptimal GI perfusion, and reducing SVR can significantly improve GI perfusion[9,10]. We used intraoperative SVR values to reflect GI perfusion in this study.
The aim of this prospective observational study was to observe the associations between SVR and PONV in patients undergoing laparoscopic hysterectomy in order to provide new ideas for the treatment of PONV. We chose laparoscopic hysterectomy because patients undergoing this operation demonstrate a higher incidence of PONV[2]. We excluded patients with a history of smoking and motion sickness or PONV, since these are the main factors affecting PONV[11]. Furthermore, there were risk factors for suboptimal GI perfusion and increased SVR in our study population, including surgical stress, body tilting[12], pneumoperitoneum[12] and vasopressor use[13]. The hypothesis was that SVR is related to PONV.
This was a prospective, observational, single-cohort study. The study was approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (NO. 2018-052). Both verbal and written informed consents were obtained from patients before surgery.
A total of 304 patients aged between 18 and 65 years who underwent elective laparoscopic hysterectomy (American Society of Anesthesiologists status I–II) from February 2018 to June 2019 at the Shandong Provincial Hospital participated in this study. The exclusion criteria were as follows: (1) Patient refusal; (2) Emergency surgery; (3) History of smoking; (4) Prior history of PONV or history of motion sickness; (5) Vaginal or abdominal (open) hysterectomy; (6) Concurrent GI surgery; (7) Chemotherapy or radiotherapy before surgery; (8) Antiemetic medication administered in the hospital within 24 h before surgery; (9) Intensive care unit admission after surgery; and (10) Significant cardiovascular, neurologic, psychiatric, pulmonary, or hepatic diseases.
In the operating room, following the establishment of standard monitoring including electrocardiography, pulse oximetry, and noninvasive blood pressure, continuous noninvasive arterial blood pressure measurement was established using the noninvasive hemodynamic monitoring system LiDCO (LiDCO Ltd., Cambridge, United Kingdom). Anesthesia was then induced using intravenous dexamethasone, lidocaine, sufentanil, and propofol, and a tracheal tube was placed facilitated by cisatracurium administration. Anesthesia was typically maintained by total intravenous anesthesia using an intravenous infusion of propofol and remifentanil, avoiding inhalation anesthetics. Bispectral index monitoring was used to control the depth of anesthesia to prevent intraoperative awareness. All patients received intravenous ondansetron (4 mg) for PONV prophylaxis towards the end of surgery, and a patient-controlled venous analgesia (PCVA) device was connected to the patient before entering the post-anesthesia care unit. For PCVA, we utilized sufentanil instead of butorphanol and ondansetron based on our clinical experience.
SVR was monitored using the LiDCO rapid monitor. Monitoring and data recording started before anesthesia induction and stopped at the end of surgery. The monitor was positioned at the back of the anesthesia machine, blinded to the anesthesia team.
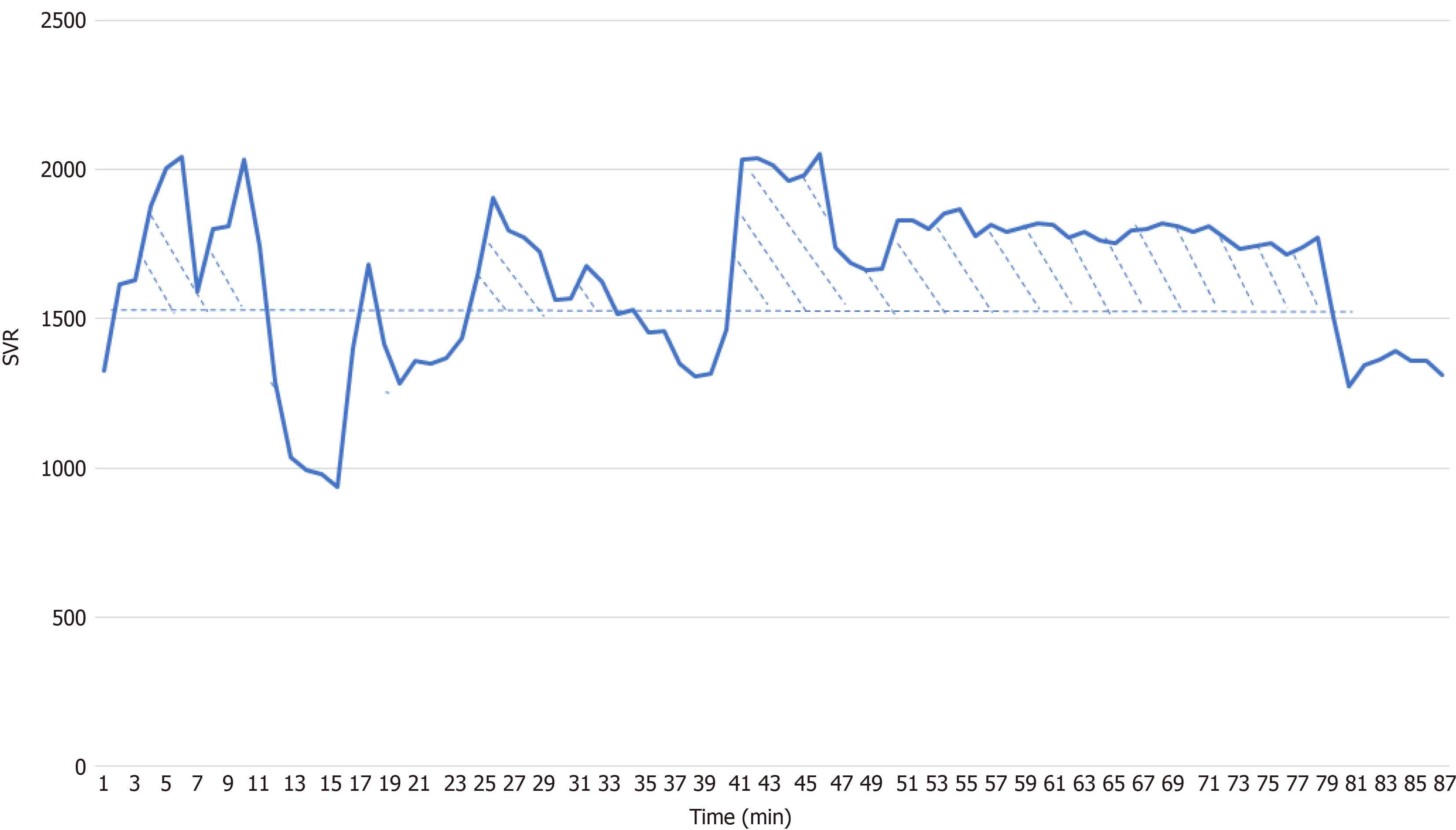
The SVR data were captured every 2 s by a research computer. The average minute values of the measurements were used for SVR index derivation. Four indices of SVR were derived, the baseline (the patient was awake and breathing room air), mean, area under the curve (AUC), and weighted AUC values. The AUC was the sum of the differences between the baseline and the data point of every minute that was higher than the baseline (Figure 1). The weighted AUC was calculated by dividing the AUC by the time of the recording.
Nausea was defined as a subjective unpleasant sensation associated with the urge to vomit. Retching was defined as the labored, spastic, and rhythmic contraction of the chest and abdominal muscles without expulsion of gastric contents. Vomiting was defined as the forceful expulsion of gastric contents from the mouth. Patients subjected to any of the signs and symptoms of nausea, retching, or vomiting within 24 h after surgery were diagnosed with PONV in this study. The incidence and severity of nausea and vomiting were evaluated while patients were awake and throughout the intervals from 0 to 2 h, 2 to 6 h, and 6 to 24 h starting upon arrival at the post-anesthesia care unit. Nausea severity was evaluated using the nausea visual analog scale.
Logistic regression requires adequate sample content. Generally, an effective sample size is 10 to 15 times the number of covariates. Sixteen covariates were included in this study, and the required sample size was at least 16 × 15 = 240. Considering the incidence of PONV, we decided to recruit 300 patients. Continuous variables are presented as the mean ± SD or median and interquartile range (P25, P75). Data were compared with the use of independent samples t-tests or Mann-Whitney U tests. Categorical variables are presented as the number of patients (percentage). Data were compared with the use of the χ2 test. We used log transformation to adjust the AUC and weighted AUC. The effect of SVR on the occurrence of PONV was assessed with the use of binary logistic regression analysis. Initially, SVR indices and perioperative variables were evaluated for univariate association with PONV. After the collinearity test, variables that were significant in univariate analyses (P < 0.1) were included in a binary logistic regression model. We chose P < 0.1 to analyze more variables in subsequent analysis. P < 0.05 was considered statistically significant. All statistical analyses were performed with the SPSS statistical package, version 24.0, Chicago, IL, United States.
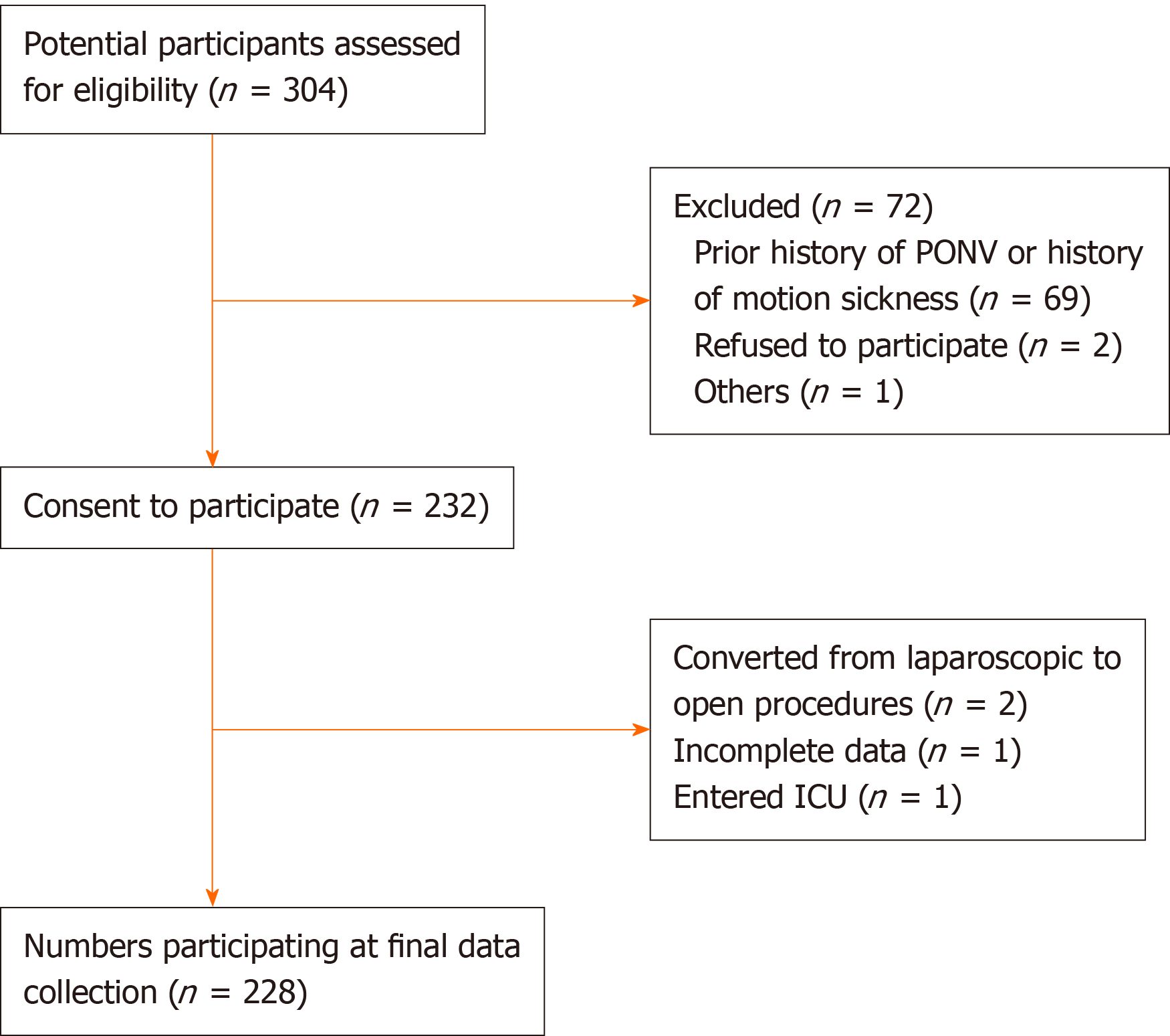
A total of 304 patients underwent elective laparoscopic hysterectomy during the study period and matched the selection criteria. Among the eligible patients, 228 gave written consent and were enrolled in the final analysis (Figure 2). The perioperative variables of all enrolled patients are listed in Table 1.
| Variables | PONV group (n = 128) | Non-PONV group (n = 100) | P value |
| Patient characteristics | |||
| Age (yr) | 50.3 ± 7.7 | 50.9 ± 6.2 | 0.672 |
| BMI (kg/m²) | 25.7 ± 3.6 | 24.6 ± 2.4 | 0.064 |
| Hb (g/L) | 121.7 ± 21.1 | 121.1 ± 20.4 | 0.902 |
| Hct (%) | 37.8 ± 5.1 | 37.5 ± 5.3 | 0.822 |
| Past medical history | |||
| Hypertension, n (%) | 22 (45.8) | 26 (54.2) | 0.250 |
| Diabetes mellitus, n (%) | 15 (53.6) | 13 (46.4) | 0.553 |
| SVR | |||
| SVR baseline (dyn*s/cm5) | 1436 ± 335 | 1272 ± 367 | 0.029 |
| SVR mean (dyn*s/cm5) | 1543 ± 386 | 1347 ± 322 | 0.050 |
| SVR AUC (dyn*s/cm5*min) | 4.53 ± 0.37 | 4.25 ± 0.47 | 0.015 |
| SVR AUC weighted (dyn*s/cm *min*min-1) | 5.25 ± 0.83 | 4.92 ± 0.97 | 0.160 |
| Anesthetic agents and drugs | |||
| Remifentanil (mg) | 1.1 ± 0.6 | 1.0 ± 0.4 | 0.406 |
| Sufentanil (μg) | 109.6 ± 20.2 | 118.4 ± 16.3 | 0.491 |
| Duration of surgery (min) | 191.1 ± 74.8 | 134.6 ± 53.8 | 0.017 |
| Intraoperative input and output | |||
| Crystalloid (mL) | 1559.6 ± 483.6 | 1519.6 ± 590.9 | 0.704 |
| Urine output (mL) | 527.5 ± 259.5 | 455.9 ± 262.2 | 0.261 |
| Estimated blood loss (mL) | 115.6 ± 97.9 | 106.2 ± 128.6 | 0.275 |
| Postoperative complications | |||
| VAS score | 3.9 ± 1.9 | 2.8 ± 1.7 | 0.414 |
| Sleep quality score | 5.7 ± 2.3 | 6.8 ± 2.2 | 0.020 |
| Time to walk (h) | 21.6 ± 7.7 | 19.6 ± 6.2 | 0.173 |
| Time to diet (h) | 11.0 (7.0, 16.3) | 7.0 (6.0, 9.4) | 0.012 |
| Length of hospital stay after surgery (h) | 109.6 ± 42.7 | 118.4 ± 58.3 | 0.379 |
A total of 128 patients developed PONV after surgery, resulting in an overall PONV rate of 56.14%. Among patients who developed PONV, in 57.03% of the cases, the first appearance of PONV occurred roughly within 6 h after surgery, a time that is consistent with previous observations[14].
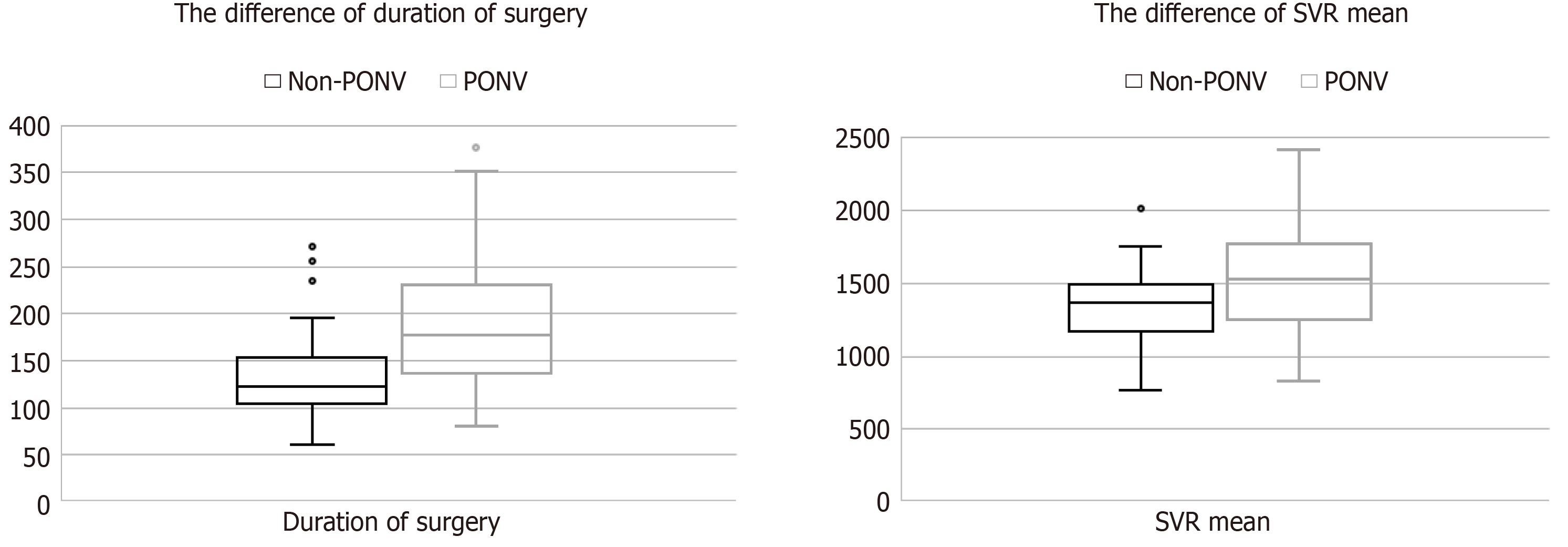
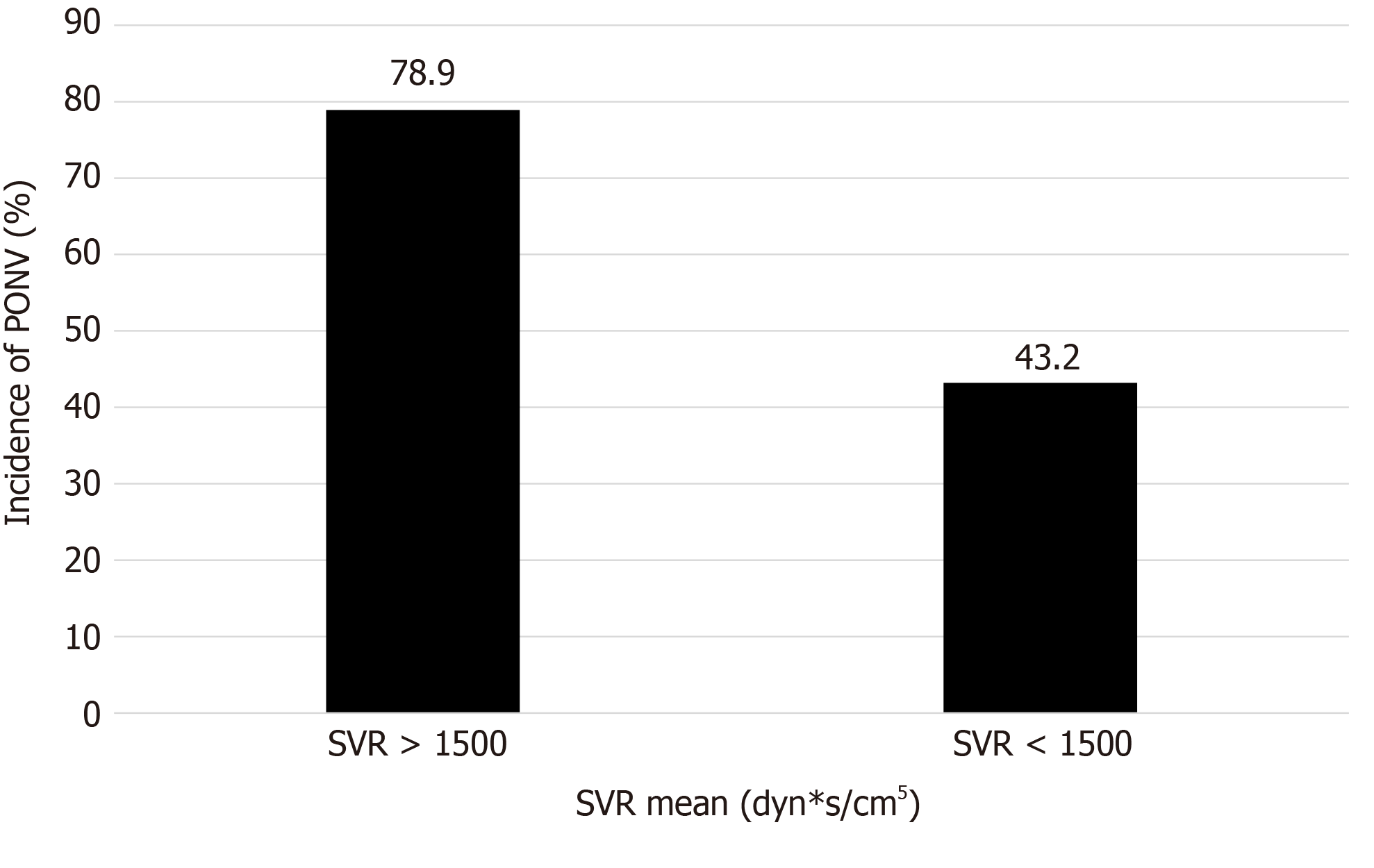
Five variables that were significant in univariate analyses (P < 0.1) were consecutively subjected to a logistic regression analysis (Table 2). As a result, duration of surgery [odds ratio (OR) = 1.316, 95%CI: 1.003-2.030, P = 0.012] and SVR mean (OR 1.015, 95%CI: 1.005-1.109, P = 0.047) were identified as independent predictors of PONV (Table 2, Figure 3). Notably, high SVR mean was associated with a significantly increased risk of PONV in this study (Figure 4).
| Variables | Univariate analysis | Binary logistic regression analysis | |
| P value | OR (95%CI) | P value | |
| Duration of surgery (min) | 0.017 | 1.316 (1.003-2.030) | 0.012 |
| SVR mean (dyn*s/cm5) | 0.050 | 1.015 (1.005-1.109) | 0.047 |
| BMI (kg/m2) | 0.064 | 0.410 | |
| SVR baseline (dyn*s/cm5) | 0.029 | 0.189 | |
| SVR AUC (dyn*s/cm5*min) | 0.015 | 0.969 | |
Comparisons between patients with and without PONV showed that the former group needed more time to tolerate diet and demonstrated poorer sleep quality on the first night postoperatively (Table 1).
There are many factors affecting PONV, including patient-, anesthesia-, and surgery-related factors. Female sex, a history of motion sickness or PONV, nonsmokers, and perioperative opioid use are the most closely related factors[15]. This prospective observational study showed that SVR was associated with PONV in patients undergoing laparoscopic hysterectomy. In addition, patients with high SVR mean were more likely to develop PONV.
Maintenance of tissue perfusion is one of the prerequisites for the proper functioning of any organ[16]. Suboptimal tissue perfusion, depending on the severity and duration, can cause organ dysfunction and increase morbidity and mortality[17]. Suboptimal GI perfusion may be responsible for some cases of PONV[4]. The GI tract is the most vulnerable to hypoperfusion of the whole body[18]. The tissue bed of the GI tract is metabolically active; however, it has minimal to no autoregulatory capacity[19] and is notoriously intolerant of even a brief period of ischemia[20]. It has been reported that one of the important mechanisms of PONV is the release of serotonin due to insufficient GI perfusion and the resulting ischemia[5-7]. Fasting before elective surgery will cause hypovolemia and GI ischemia in patients, and they are more prone to PONV. Crystal or colloid fluid for intravenous rehydration before surgery can significantly reduce the incidence of PONV[21]. Other studies have found a correlation between the occurrence of PONV and the increase in serotonin levels. Patients with PONV have higher levels of serotonin metabolites in their urine[22]. These results show the relationship between PONV, GI perfusion, and serotonin.
Furthermore, PONV is more common in patients undergoing laparoscopic surgery. Carbon dioxide pneumoperitoneum has been proposed as a potential cause[23]. Compared with traditional laparotomy, CO2 pneumoperitoneum used in laparoscopic surgery more easily causes increased intra-abdominal pressure and compresses the GI tissue mucosa, reducing blood flow[24-26]. Then, more serotonin is released, which may stimulate the chemoreceptor trigger zone through the GI vagal nerve, consequently leading to PONV[27]. In this study, after pneumoperitoneum was established, SVR increased significantly. After surgery was completed, SVR basically returned to preoperative levels (Figure 1). This shows that pneumoperitoneum during laparoscopic surgery leads to tissue hypoperfusion, which can be reflected by the increase in SVR.
According to our observations, higher SVR mean increases the risk of PONV. It is suggested that we could reduce SVR levels to relieve GI mucosal pressure during surgery, thereby reducing the incidence of PONV. SVR is an index calculated from the mean arterial pressure, right atrial pressure, and cardiac output. The normal value is generally 900-1500 dyn*s/cm5. In this study, SVR often increased under the premise of normal blood pressure. Moreover, the cardiac output decreased, which may also explain why increased SVR is related to tissue hypoperfusion.
The duration of surgery remains an independent risk factor for PONV, which is consistent with some previous studies[28]. The opioid doses administered during the perioperative period have not been shown to be related to PONV. This may be related to the fact that the patient will stop PCVA when PONV occurs. In this regard, we should pay attention to counseling patients before surgery, not to reducing the discomfort caused by PONV at the cost of pain. We also found that the visual analog scale scores of PONV patients were worse than those of non-PONV patients, which may be related to the patients’ tolerance and subjective experience. However, the occurrence of PONV was not associated with the length of hospitalization. PONV generally occurred within 24 h after surgery, and it was not the main factor that determined the time of discharge.
This study has several limitations. One major limitation is that PONV diagnosis mainly depends on the subjective symptoms of patients, thus lacking objective indicators for measurement. Another limitation is that this was a single-cohort observational study. Therefore, a causal effect of SVR on PONV could not be determined. Whether SVR monitoring-guided care can improve PONV cannot be determined by this study. Although the accuracy of the LiDCO rapid monitoring system has been shown in cardiac surgery patients, whether SVR has reference significance remains to be investigated[29].
In summary, duration of surgery and SVR mean were associated with PONV after laparoscopic hysterectomy. Patients with high SVR mean were more likely to develop PONV. Although we administered prophylactic therapy, the incidence of PONV in this study remained very high. The complexity of the pathogenesis makes the prevention of PONV more difficult, and the current lack of animal experimental models that can replace humans is also a prominent concern in the research process. We need to actively explore and constantly look for measures that can reduce the occurrence of PONV.
Postoperative nausea and vomiting (PONV) remains prevalent after general anesthesia, especially after gynecological laparoscopic surgery. The pathogenesis of PONV is multifactorial, and its etiology and pathogenesis are not clear. Suboptimal gastrointestinal (GI) perfusion may be responsible for some cases of PONV, and there are risk factors for suboptimal GI perfusion in patients undergoing laparoscopic hysterectomy. Furthermore, increased systemic vascular resistance (SVR) is related to suboptimal GI perfusion. However, there is currently no research on the relationship between SVR and PONV.
Female sex, a history of motion sickness or PONV, nonsmokers, and perioperative opioid use are the most closely related risk factors for PONV. Suboptimal GI perfusion may be attributed to some cases of PONV, and an increased SVR may lead to GI ischemia. The present study analyzed the relationship between SVR indices and PONV after laparoscopic hysterectomy, which may assist in the prevention of PONV in the future.
The aim of this prospective observational study was to observe the associations between SVR and PONV in patients undergoing laparoscopic hysterectomy. Four SVR indices, the baseline, mean, area under the curve (AUC) and weighted AUC, were assessed through logistic regression analysis. Moreover, we compared the outcomes of PONV patients and non-PONV patients.
We performed a prospective observational study of patients undergoing laparoscopic hysterectomy.
The incidence of PONV after laparoscopic hysterectomy in our institution was 56.14%, and PONV mostly occurred roughly within 6 h after surgery. SVR mean and duration of surgery were associated with PONV. High SVR mean was associated with a significantly increased risk of PONV in this study. Furthermore, patients who developed PONV needed more time to tolerate diet and demonstrated poorer sleep quality on the first night after surgery.
SVR mean and duration of surgery were associated with PONV after laparoscopic hysterectomy. Patients with high SVR mean were more likely to develop PONV.
Although we administered many prophylactic measures, the incidence of PONV remained very high, especially after gynecological laparoscopic surgery. More randomized controlled trials should be performed to explore precautions that can reduce the occurrence of PONV.
Manuscript source: Unsolicited manuscript
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Avalos-Gonzalez J, Mulvihill S, Schmidt J S-Editor: Wang JL L-Editor: Webster JR P-Editor: Xing YX
| 1. | Rüsch D, Eberhart LH, Wallenborn J, Kranke P. Nausea and vomiting after surgery under general anesthesia: an evidence-based review concerning risk assessment, prevention, and treatment. Dtsch Arztebl Int. 2010;107:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Geng ZY, Liu YF, Wang SS, Wang DX. Intra-operative dexmedetomidine reduces early postoperative nausea but not vomiting in adult patients after gynaecological laparoscopic surgery: A randomised controlled trial. Eur J Anaesthesiol. 2016;33:761-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Yang XY, Xiao J, Chen YH, Wang ZT, Wang HL, He DH, Zhang J. Dexamethasone alone vs in combination with transcutaneous electrical acupoint stimulation or tropisetron for prevention of postoperative nausea and vomiting in gynaecological patients undergoing laparoscopic surgery. Br J Anaesth. 2015;115:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Li G, Lin L, Dai F, Guo X, Meng L. Muscular tissue oxygen saturation during robotic hysterectomy and postoperative nausea and vomiting: exploring the potential therapeutic thresholds. J Clin Monit Comput. 2019;33:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Pusch F, Berger A, Wildling E, Zimpfer M, Moser M, Sam C, Krafft P. Preoperative orthostatic dysfunction is associated with an increased incidence of postoperative nausea and vomiting. Anesthesiology. 2002;96:1381-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Gan TJ, Mythen MG, Glass PS. Intraoperative gut hypoperfusion may be a risk factor for postoperative nausea and vomiting. Br J Anaesth. 1997;78:476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Ali SZ, Taguchi A, Holtmann B, Kurz A. Effect of supplemental pre-operative fluid on postoperative nausea and vomiting. Anaesthesia. 2003;58:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Driessen B, Jahr JS, Lurie F, Griffey SM, Gunther RA. Effects of haemoglobin-based oxygen carrier hemoglobin glutamer-200 (bovine) on intestinal perfusion and oxygenation in a canine hypovolaemia model. Br J Anaesth. 2001;86:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Hopster K, Wittenberg-Voges L, Kästner SBR. Xylazine infusion in isoflurane-anesthetized and ventilated healthy horses: Effects on cardiovascular parameters and intestinal perfusion. Can J Vet Res. 2017;81:249-254. [PubMed] |
| 10. | Dancker C, Hopster K, Rohn K, Kästner SB. Effects of dobutamine, dopamine, phenylephrine and noradrenaline on systemic haemodynamics and intestinal perfusion in isoflurane anaesthetised horses. Equine Vet J. 2018;50:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1297] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 12. | Joris JL, Noirot DP, Legrand MJ, Jacquet NJ, Lamy ML. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993;76:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 284] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Oldenburg WA, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 438] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 14. | Carroll NV, Miederhoff P, Cox FM, Hirsch JD. Postoperative nausea and vomiting after discharge from outpatient surgery centers. Anesth Analg. 1995;80:903-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Tateosian VS, Champagne K, Gan TJ. What is new in the battle against postoperative nausea and vomiting? Best Pract Res Clin Anaesthesiol. 2018;32:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Choi M, Shapiro AMJ, Zemp R. Tissue perfusion rate estimation with compression-based photoacoustic-ultrasound imaging (Erratum). J Biomed Opt. 2018;23:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Hasanin A, Mukhtar A, Nassar H. Perfusion indices revisited. J Intensive Care. 2017;5:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Gutierrez G, Bismar H, Dantzker DR, Silva N. Comparison of gastric intramucosal pH with measures of oxygen transport and consumption in critically ill patients. Crit Care Med. 1992;20:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 120] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Kiel JW, Riedel GL, Shepherd AP. Autoregulation of canine gastric mucosal blood flow. Gastroenterology. 1987;93:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Beuk RJ, Heineman E, Tangelder GJ, Kurvers HA, Bonke HJ, oude Egbrink MG. Effects of different durations of total warm ischemia of the gut on rat mesenteric microcirculation. J Surg Res. 1997;73:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Chaudhary S, Sethi AK, Motiani P, Adatia C. Pre-operative intravenous fluid therapy with crystalloids or colloids on post-operative nausea & vomiting. Indian J Med Res. 2008;127:577-581. [PubMed] |
| 22. | Rüsch D, Strasser C, Celik I, Lengkong M, Wulf H, Scholz J. [Vomiting after gynecologic laparoscopy and under general anesthesia is associated with changes in excretion of serotonin metabolites]. Anaesthesist. 2004;53:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Watcha MF. The cost-effective management of postoperative nausea and vomiting. Anesthesiology. 2000;92:931-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Goll V, Akça O, Greif R, Freitag H, Arkiliç CF, Scheck T, Zoeggeler A, Kurz A, Krieger G, Lenhardt R, Sessler DI. Ondansetron is no more effective than supplemental intraoperative oxygen for prevention of postoperative nausea and vomiting. Anesth Analg. 2001;92:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res. 1987;43:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 208] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Diebel LN, Dulchavsky SA, Wilson RF. Effect of increased intra-abdominal pressure on mesenteric arterial and intestinal mucosal blood flow. J Trauma. 1992;33:45-8; discussion 48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 226] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS Drugs. 2007;21:813-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Cohen MM, Duncan PG, DeBoer DP, Tweed WA. The postoperative interview: assessing risk factors for nausea and vomiting. Anesth Analg. 1994;78:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 311] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Lighthall GK, Singh S. Perioperative Maintenance of Tissue Perfusion and Cardiac Output in Cardiac Surgery Patients. Semin Cardiothorac Vasc Anesth. 2014;18:117-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |