Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4726
Peer-review started: July 4, 2020
First decision: July 24, 2020
Revised: August 5, 2020
Accepted: September 3, 2020
Article in press: September 3, 2020
Published online: October 26, 2020
Processing time: 114 Days and 2.8 Hours
The outbreak of coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. COVID-19 is clinically categorized into mild, moderate, severe, and critical illness. Acute kidney injury is an independent risk factor for poor prognosis in patients with. Serum cystatin C (sCys C) is considered a more sensitive biomarker for early renal insufficiency than conventional indicators of renal function. Early detection of risk factors that affect the prognosis of severe and critically ill patients while using active and effective treatment measures is very important and can effectively reduce the potential mortality rate.
To determine the predictive value of sCys C for the prognosis of patients with COVID-19.
The clinical data of 101 severe and critically ill patients with COVID-19 at a designated hospital in Wuhan, Hubei Province, China were analyzed retrospectively. According to the clinical outcome, the patients were divided into a discharge group (64 cases) and a death group (37 cases). The general information, underlying diseases, and laboratory examination indexes of the two groups were compared. Multivariate Cox regression was used to explore the relationship between sCys C and prognosis. The receiver operating characteristic (ROC) curve was used to demonstrate the sensitivity and specificity of sCys C and its optimal cut-off value for predicting death.
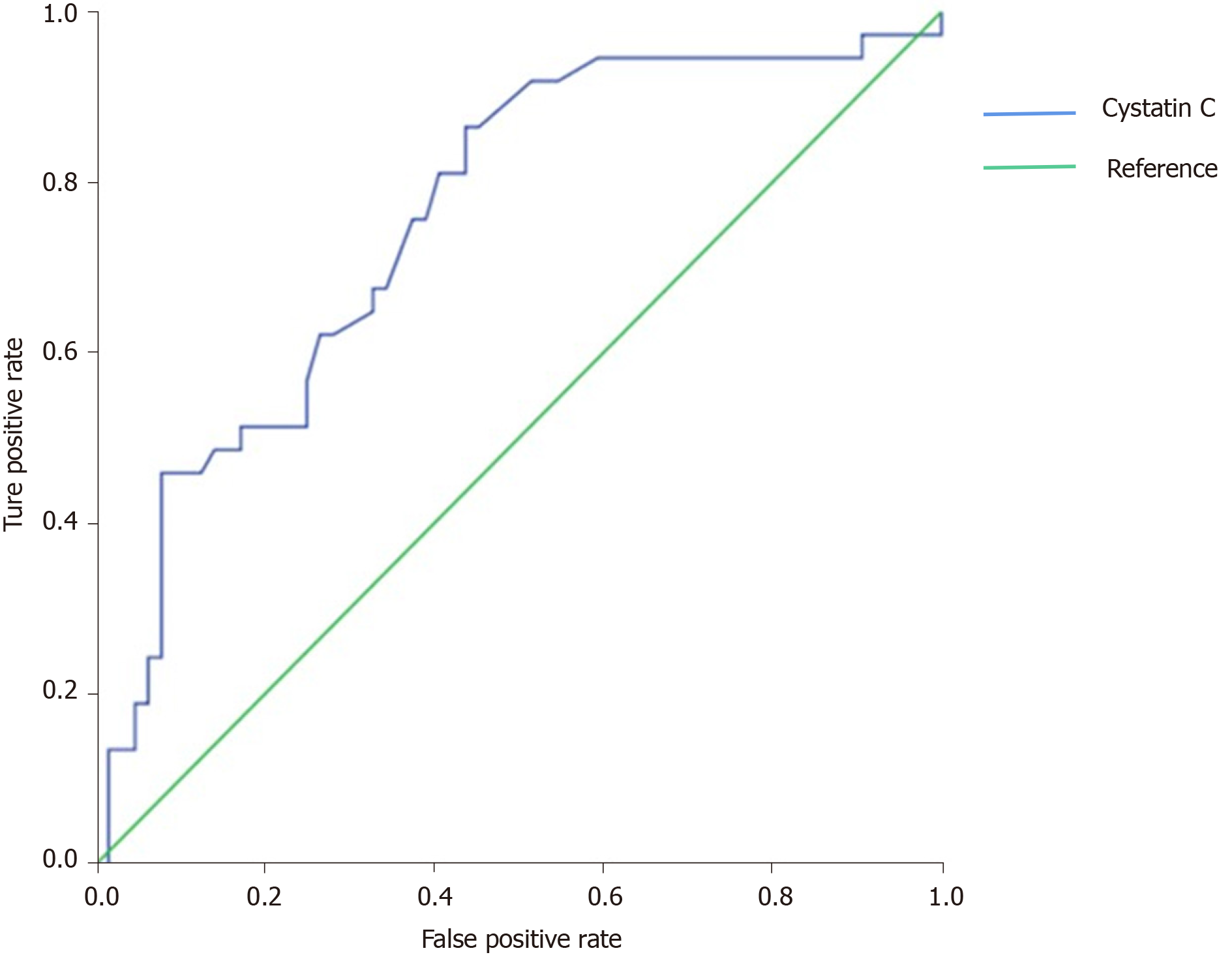
There were significant differences in age, sCys C, creatinine, C-reactive protein, serum albumin, creatine kinase-MB, alkaline phosphatase, lactate dehydrogenase, neutrophil count, and lymphocyte count between the two groups (P < 0.001). Multivariate logistic regression analysis showed that sCys C was an independent risk factor for death in patients with COVID-19 (Odds ratio = 1.812, 95% confidence interval [CI]: 1.300-2.527, P < 0.001). The area under the ROC curve was 0.755 (95%CI: 1.300-2.527), the cut-off value was 0.80, the specificity was 0.562, and the sensitivity was 0.865.
sCys C is an independent risk factor for death in patients with COVID-19. Patients with a sCys C level of 0.80 mg/L or greater are at a high risk of death.
Core Tip: The novel coronavirus epidemic has become a major public health event in the world. The mortality rate of severe and critical patients is high. It is very important to find an available, cheap, and sensitive biomarker to predict mortality. Cystatin C is a predictor of renal injury. We compared 101 severe and critical patients with coronavirus disease 2019 (COVID-19) in Wuhan, China, and found that patients with a cystatin C level of 0.80 mg/L or greater were at a high risk of death. Therefore, cystatin C may have an important role in the prognosis of severe and critical patients with COVID-19.
- Citation: Li Y, Yang S, Peng D, Zhu HM, Li BY, Yang X, Sun XL, Zhang M. Predictive value of serum cystatin C for risk of mortality in severe and critically ill patients with COVID-19. World J Clin Cases 2020; 8(20): 4726-4734
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4726.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4726
The outbreak of coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. As of June 17, 2020, more than 8 million people have been infected worldwide. The World Health Organization has reported more than 440000 deaths and 130000 new confirmed cases daily. Most patients with COVID-19 have mild symptoms, but approximately 5%-20%[1] have severe symptoms, including acute respiratory distress syndrome, septic shock, and multiple organ failure. The mortality rate of patients with severe COVID-19 was higher than that of patients with mild COVID-19. The later the diagnosis is made, the higher the risk of death.
In addition to the impacts of COVID-19 on the respiratory system and immune system, the kidney is also one of the main organs being affected. Whether COVID-19 patients will develop acute kidney injury (AKI), which may affect their prognosis, is an important issue and worthy of clinical attention. Some studies have shown that AKI is an independent risk factor for poor prognosis in patients with COVID-19[2]. In addition to the direct toxicity of coronaviruses, factors attributed to AKI include systemic hypoxia, coagulation abnormalities, and rhabdomyolysis which may be related to drugs or hyperventilation[3]. Early detection of AKI in patients with COVID-19 is conducive to establishing standardized AKI cluster prevention and treatment (5R principle): Risk screening, early recognition, timely response, renal replacement therapy, and renal recovery, which are key to improve the cure rate and reduce the mortality rate of patients with COVID-19.
Serum cystatin C (sCys C) is a newly developed index with a high sensitivity and specificity for evaluating renal function, which is not affected by age, sex, weight, inflammation, and other factors. It is considered an endogenous marker superior to serum creatinine (sCr)[4]. sCys C is a cysteine protease inhibitor, also known as γ-trace basic protein or post-gamma globulin, which is encoded by the Cys C gene that can be continuously transcribed and expressed in all nucleated cells at a constant speed. It has no tissue specificity with a small molecular weight (13 kDa), being positively charged under physiological conditions. It is freely filtered in the glomeruli, completely reabsorbed by the renal tubular epithelial cells, and degraded within cells without returning to the blood. Renal tubular epithelial cells do not secrete sCys C into the lumen, therefore its serum concentration is mainly determined by glomerular filtration rate which is an important indicator of glomerular filtration[5]. In addition, it has been reported that sCys C can be used to evaluate the survival rate of patients with sepsis[6].
The purpose of this study was to investigate the effect of COVID-19 on renal function and assess the predictive value of sCys C for risk of mortality in critically ill patients with COVID-19.
This single-center retrospective study included adults diagnosed with COVID-19 admitted to the ward of a designated hospital in Wuhan, Hubei Province, from January to March 2020 without any history of chronic kidney disease. The diagnostic criteria were based on the COVID-19 Diagnosis and Treatment Protocol (trial version 7) formulated by the National Health Commission of China, with the epidemiological history and clinical expression of COVID-19, and the etiological or serological evidence (positive novel coronavirus nucleic acid test or serum specific antibody test) considered. Patients recruited in this study were all severe or critically ill patients. Severe condition was determined when subjects had any of the following conditions: (1) Shortness of breath with a respiration rate ≥ 30 times/min; (2) Oxygen saturation ≤ 93% in the resting state; and (3) PaO2/FiO2 ≤ 300 mmHg. Subjects were considered critical if any of the following conditions arose: (1) Respiratory failure and need for mechanical ventilation; (2) Shock; and (3) Patients with other organ failure that should be monitored in the intensive care unit (ICU).
This survey was a retrospective study collecting only the clinical data of patients. Since it did not bring risks to patients’ physiology and did not interfere with patients’ treatment plan, and researchers protected patients’ information from disclosure, Xuanwu Hospital of Capital Medical University agreed to exempt this study from ethical review.
All subjects enrolled in our study had a case report form, and data were collected within 12 h after hospital admission, including sex, age, medical history, complications, sCys C, creatine kinase-MB (CK-MB), serum creatinine (sCr), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGTP), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total bilirubin, C-reactive protein (CRP), neutrophil count, albumin, uric acid, lymphocyte count, hemoglobin, platelet count, and blood calcium. All data were obtained from the first laboratory examination after admission. Discharge or death was considered the study outcome.
All data were analyzed using SPSS version 22.0. Continuous data (sCr, AST, ALT, etc.) with a normal distribution are presented as the mean ± SD. Non-normally distributed variables are presented as medians with interquartile ranges and analyzed using a non-parametric test. Categorical data (sex, medical history, etc.) were analyzed by the
There were a total of 101 cases including 18 critically ill patients and 83 severe patients at baseline, and they were divided into two groups: Discharge group (64 cases) and death group (37 cases). All data were obtained from the first laboratory examination after admission within 12 h after the diagnosis of severe and critical COVID-19. The median length of hospital stay was 15 d. Comparison of the baseline data between the two groups showed that the group of patients who died had the following characteristics: Advanced age (P < 0.001), higher levels of sCys C (P < 0.001), CK-MB (P < 0.001), sCr (P = 0.001), AST (P < 0.001), ALT (P < 0.05), GGTP(P < 0.05), ALP (P < 0.05), LDH (P < 0.001), total bilirubin (P < 0.05), CRP (P < 0.001), and neutrophil count (P < 0.05), and lower levels of albumin (P < 0.001), calcium ion concentration (P < 0.001), lymphocyte count (P < 0.001), and platelet count (P < 0.05) (Table 1).
| Characteristic | Discharged | Dead | P value |
| Age, yr | 54.09 ± 14.95 | 71.76 ± 10.012 | 0.000 |
| Male sex, n (%) | 30 (46.9) | 23 (62.2) | 0.138 |
| Critically ill patients, n (%) | 6 (9.4) | 12 (32.4) | 0.004 |
| Hypertension, n (%) | 26 (40.6) | 20 (54.1) | 0.192 |
| Diabetes, n (%) | 20 (31.3) | 7 (18.9) | 0.177 |
| Combined with renal insufficiency, n (%) | 9 (14.1) | 3 (8.1) | 0.373 |
| Coronary heart disease, n (%) | 5 (7.8) | 7 (18.9) | 0.097 |
| sCys C, mg/L | 0.8 (0.7, 0.9) | 1 (0.9, 1.3) | 0.000 |
| CK-MB activity, U/L | 12 (9, 14) | 19(13, 35) | 0.000 |
| Creatinine, µmol/L | 64.5 (57.5, 79.1) | 78.2 (70, 94.2) | 0.001 |
| Aspartate aminotransferase, U/L | 29 (19, 40) | 44 (31, 73) | 0.000 |
| Alanine aminotransferase, U/L | 29 (17, 52) | 44 (23, 58) | 0.028 |
| Glutamyl transpeptidase, U/L | 25 (17, 49) | 37 (26, 86) | 0.005 |
| Alkaline phosphatase, U/L | 47 (41, 58) | 68 (52, 95) | 0.000 |
| Lactic acid dehydrogenase, U/L | 228 (188, 283) | 548 (399, 623) | 0.000 |
| Total bilirubin, µmol/L | 9.6 (7.4, 13.5) | 13.4 (8.5, 18.6) | 0.024 |
| Albumin, g/L | 32.7 (30.4, 35.9) | 28 (24.8, 29.4) | 0.000 |
| Uric acid, µmol/L | 229.2 (176, 290.8) | 233.8 (177.2, 366.7) | 0.521 |
| Calcium, mmol/L | 1.97 (1.91, 2.04) | 1.73 (1.03, 1.88) | 0.000 |
| C reactive protein, mg/L | 11.1 (3.55,29.13) | 92.29 (42.57, 116.45) | 0.000 |
| Neutrophils, G/L | 3 (2.21, 4.27) | 7.28 (5.63, 9.35) | 0.000 |
| Lymphocytes, G/L | 1.02 (0.79, 1.39) | 0.58 (0.42, 0.88) | 0.000 |
| Hemoglobin, g/L | 128 (119, 139) | 127 (105, 138) | 0.540 |
| Platelets, G/L | 211 (159, 277) | 147(109, 229) | 0.011 |
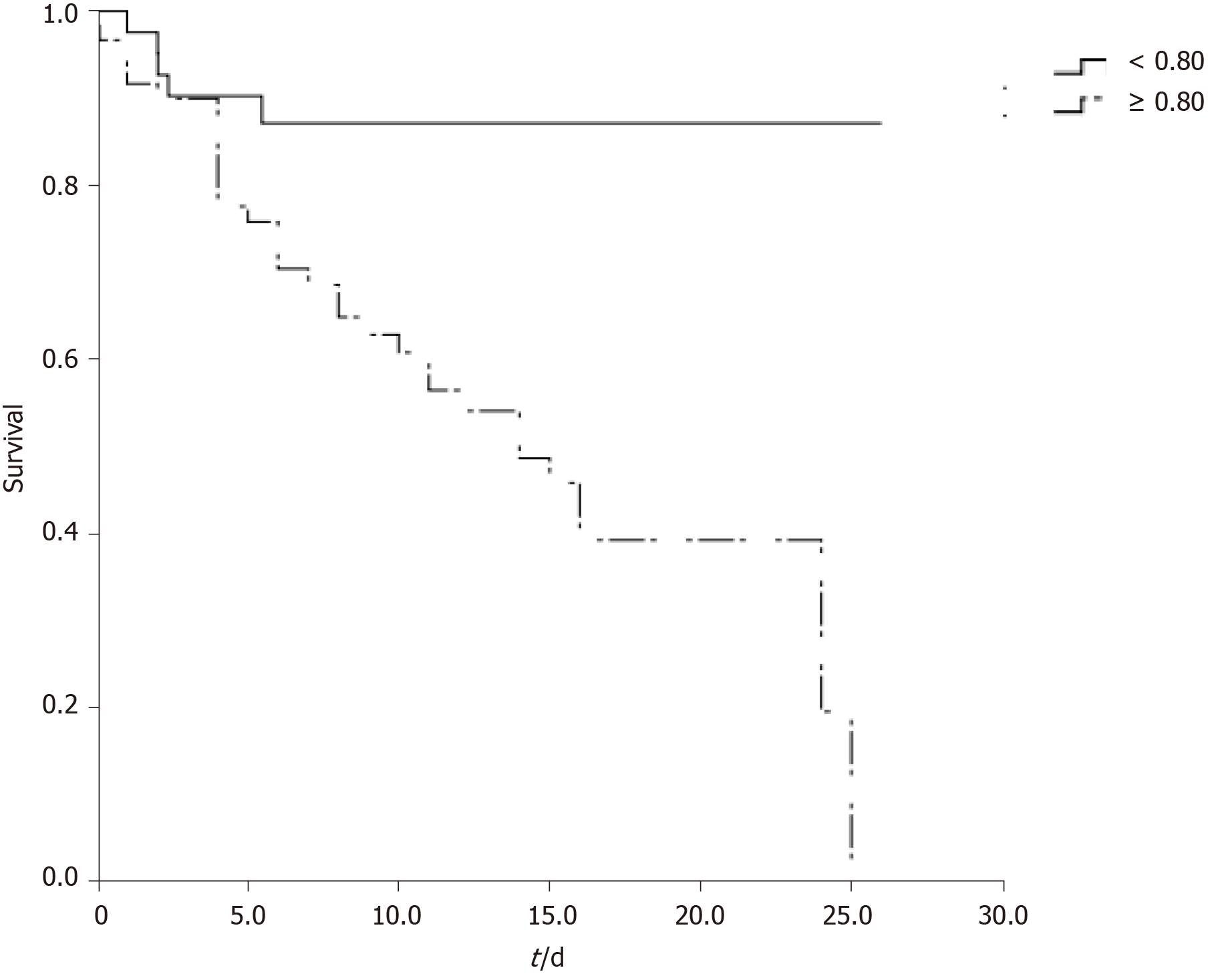
By using sCys C, sCr, and the differences in factors as explanatory variables in forward Cox regression analysis to explore the independent factors (Table 2), sCys C was found to be an independent risk factor for death, but the sCr level was not. The ROC curve was used to determine the optimal threshold for predicting the death of severe and critical COVID-19 patients. The results are shown in Figure 1. The area under the curve, optimal threshold, specificity, and sensitivity were 0.755, 0.80, 0.562, and 0.865, respectively. Patients with a sCys C level of 0.80 mg/L or greater were at a high risk of death. The patients were divided into two groups based on a cut-off of 0.80 mg/L, and the survival curve was obtained, as shown in Figure 2. The results demonstrated that this threshold was effective in differentiating high-risk groups (log-rank = 13.375, P < 0.001).
| B | SE | Wald | P value | Exp(B) | 95%CI | |
| sCys C | 0.595 | 0.17 | 12.279 | 0.000 | 1.812 | 1.300, 2.527 |
| CK-MB | 0.037 | 0.014 | 7.149 | 0.008 | 1.037 | 1.010, 1.066 |
| AST | 0.026 | 0.008 | 10.875 | 0.001 | 1.027 | 1.011, 1.043 |
| Albumin | -0.221 | 0.041 | 28.531 | 0.000 | 0.802 | 0.739, 0.869 |
| Calcium | -3.157 | 0.555 | 32.347 | 0.000 | 0.043 | 0.014, 0.126 |
From January to March 2020, the clinical data of 101 severe and critically ill patients in a designated hospital in Wuhan, Hubei Province, China were analyzed retrospectively, including 37 dead patients (death group) and 64 surviving patients (discharge group). The risk factors for prognosis were assessed by comparing the laboratory examination results of the discharge and death groups. Multivariate analysis showed that sCys C, CK-MB, AST, albumin, and serum calcium were independent risk factors for death in severe and critically ill patients. The predictive value of CK-MB in patients with COVID-19 has been confirmed by many studies. Wang et al[7] showed that serum cardiac troponin (cTnI) and CK-MB levels in ICU patients were significantly higher than those in non-ICU patients (P = 0.004), suggesting that increased CK-MB and cTnI levels represent a poor prognosis in patients with COVID-19. Zhou et al[1] also illustrated that an increase in CK-MB and cTnI levels in COVID-19 patients was significantly correlated with hospital death (P = 0.043). AST and albumin are indicators for liver function, and previous literature supported the predictive value of abnormal liver function in the prognosis of COVID-19 patients. A meta-analysis showed that abnormal liver function indicators such as AST, ALT, bilirubin, and albumin can predict the severity and prognosis of COVID-19 patients[8]. Lei et al[9] included 5771 adult COVID-19 patients from Hubei Province, and retrospectively analyzed the time distribution of liver function indicators in patients and correlated them with death. The results indicated that abnormal liver function, especially elevated AST, was closely related to the risk of death, which was consistent with the results of this study[9]. In addition, many studies have shown that a decrease in serum calcium is a risk factor for disease severity and death in patients with COVID-19[10,11].
Presently, the predictive value of sCys C in the prognosis of patients with COVID-19 is rarely reported, but studies have confirmed that sCys C can predict the risk of AKI and death in critically ill patients such as those with acute cerebral infarction, acute myocardial infarction, heart failure, and sepsis[12,13]. This indicates that sCys C has a predictive value in the prognosis of critically ill patients. However, the predictive value of sCys C for severe and critically ill COVID-19 patients has not been confirmed. In this study, the sCys C level in the death group was significantly higher than that in the discharge group, suggesting that the sCys C level was related to the risk of death in COVID-19. Moreover, multivariate regression analysis showed that the sCys C level was an independent risk factor for the death of patients with COVID-19. To further clarify the correlation between sCys C value and prognosis of COVID-19, we used the ROC curve to assess the cut-off value of sCys C in predicting the death of patients. The results showed that sCys C had an AUC of 0.755, sensitivity of 0.865, and specificity of 0.562, which indicated that sCys C had appreciated value in predicting the death of patients with severe and critical COVID-19. The cut-off value was 0.80 mg/L, that is, patients with sCys C ≥ 0.80 mg/L are at a high risk of death. The low specificity may be related to the low sample size and retrospective study. Clinicians should be vigilant when patients have sCys C ≥ 0.80 mg/L so that early intervention could be given to improve the prognosis.
In this study, the sCr level of the dead patients was significantly higher than that of the survivors (P < 0.01). However, sCr was not an independent risk factor for death when other different factors were included in the Cox regression equation. Accordingly, sCys C was deemed superior to sCr in predicting the risk of death in severe and critically ill patients with COVID-19. sCys C is a member of the cystatin family. It is freely filtered by the glomerulus, reabsorbed by the proximal tubules, and completely catabolized without returning to the blood stream. Therefore, its concentration in the blood is not affected by age, sex, or liver function[14,15]. sCys C is generally considered an earlier and more sensitive marker of renal insufficiency than the conventional indicators of renal function such as sCr and blood urea nitrogen (BUN). Numerous studies have shown that there is no significant increase in sCr or BUN levels in the early stage of renal injury, and their elevations can only be observed in the middle and late stages of renal injury. Abnormal levels of indicators usually indicate that renal damage is serious and irreversible[16]. Xiang et al[17] showed that BUN, sCr, and sCys C (biochemical indicators of renal function) were significantly increased in patients with severe COVID-19, indicating that severe acute respiratory syndrome coronavirus 2 infection may damage the kidney. To date, a number of studies have revealed that patients with COVID-19 may have renal function damage, and the clinical manifestations include rise in sCr, hematuria, albuminuria, and AKI[18,19]. It has been reported that the incidence of AKI in patients with COVID-19 ranged from 0.9% to 29%[20-22]. For patients who required intensive care, the incidence of AKI increased significantly to 50%[4]. Autopsy results indicated that patients with COVID-19 had acute proximal renal tubular injury that was characterized by loss of brush margin, vacuolar degeneration, dilation of the tubular lumen with cell fragments, necrosis, and epithelial exfoliation[19]. Pei et al[23] showed that the total mortality rate of patients with renal involvement was 11.2%, while the mortality rate of patients without renal involvement was 1.2%, suggesting that renal complications were related to the mortality rate of COVID-19. In addition, another study[24] found that the levels of sCys C were positively correlated with inflammatory reaction indexes such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α, and hsCRP, and promoted the occurrence and development of inflammatory reaction. Therefore, in the pathological state of COVID-19 invading lung tissue, sCys C is synthesized and released in large quantities, and the level of sCys C is increased, which regulates the cathepsin activity released from necrotic or inflammatory cells. It is suggested that clinicians should pay close attention to the sCys C level and its changes.
Since the specificity of the prediction was relatively low, interpretation with caution in clinical practice is suggested. This was a single-center retrospective study and the sample size was relatively small and we lacked clinical data of surviving patients after discharge, thus we were unable to evaluate the effect of sCys C on the long-term prognosis of patients with COVID-19. Our research population was Chinese, further research based on other populations is needed before we can infer the results and draw general conclusions.
In conclusion, sCys C can be used as a means for early diagnosis of severe and critical COVID-19 patients with acute renal function damage, providing predictive information for clinical prognosis. Clinicians should improve their understanding pertaining to kidney injury in severe and critically ill patients with COVID-19. Early detection and effective intervention of renal involvement may help reduce potential death in patients with COVID-19.
Coronavirus disease 2019 (COVID-19) is an acute infectious disease caused by a new coronavirus, which is clinically categorized into mild, moderate, severe, and critical illness. Severe and critically ill patients progress rapidly with dyspnea, hypoxemia, and even life-threatening complications such as multiple organ dysfunction syndrome, sepsis, and shock. Early detection of risk factors that affect the prognosis of severe and critically ill patients while using active and effective treatment measures is very important and can effectively reduce the potential mortality rate.
A rapid, effective, and accurate biomarker is urgently needed to predict the prognosis of patients with COVID-19. Serum cystatin C (sCys C) has a predictive value in the prognosis of critically ill patients such as those with acute cerebral infarction, acute myocardial infarction, heart failure, and sepsis. However, the predictive value of sCys C for severe and critically ill COVID-19 patients has not been confirmed. We designed this study to confirm whether sCys C can be used as a prognostic indicator for COVID-19 patients.
The main objective of this study was to determine the predictive value of sCys C for the prognosis of severe and critically ill COVID-19 patients.
A total of 101 severe or critically ill patients with COVID-19 were divided into a discharge group (64 cases) and a death group (37 cases). We compared the general information, underlying diseases, and laboratory examination indexes of the two groups. Multivariate Cox regression was used to explore the relationship between sCys C and prognosis. In addition, we used the receiver operating characteristic curve to assess the cut-off value of sCys C in predicting the death of patients.
sCys C, creatine kinase-MB, aspartate aminotransferase, albumin, and serum calcium were independent risk factors for death in severe and critically ill patients. sCys C had an AUC of 0.755, sensitivity of 0.865, and specificity of 0.562. Patients with a sCys C level of 0.80 or greater were at a high risk of death.
sCys C is superior to sCr in predicting the risk of death in severe and critically ill patients with COVID-19. Patients with sCys C ≥ 0.80 mg/L are at a high risk of death.
sCys C could be a marker of mortality in severe and critically ill COVID-19 patients.
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Yeh YC S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18195] [Article Influence: 3639.0] [Reference Citation Analysis (0)] |
| 2. | Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98:209-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 939] [Cited by in RCA: 1019] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 3. | Adapa S, Aeddula NR, Konala VM, Chenna A, Naramala S, Madhira BR, Gayam V, Balla M, Muppidi V, Bose S. COVID-19 and Renal Failure: Challenges in the Delivery of Renal Replacement Therapy. J Clin Med Res. 2020;12:276-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Delanaye P, Cavalier E, Morel J, Mehdi M, Maillard N, Claisse G, Lambermont B, Dubois BE, Damas P, Krzesinski JM, Lautrette A, Mariat C. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol. 2014;15:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Liu J. Evaluation of serum cystatin C for diagnosis of acute rejection after renal transplantation. Transplant Proc. 2012;44:1250-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Al-Beladi FI. Cystatin C is an early marker of contrast-induced nephropathy in patients with sepsis in the intensive care unit. Saudi J Kidney Dis Transpl. 2015;26:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14766] [Article Influence: 2953.2] [Reference Citation Analysis (0)] |
| 8. | Parohan M, Yaghoubi S, Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: A systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50:924-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 9. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 10. | Cappellini F, Brivio R, Casati M, Cavallero A, Contro E, Brambilla P. Low levels of total and ionized calcium in blood of COVID-19 patients. Clin Chem Lab Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem. 2020;57:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 12. | Zhi H, Zhang M, Cui X, Li Y. [Renal echography and cystatin C for prediction of acute kidney injury: very different in patients with cardiac failure or sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:1258-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Fu Z, Yang X, Shen M, Xue H, Qian G, Cao F, Guo J, Dong W, Chen Y. Prognostic ability of cystatin C and homocysteine plasma levels for long-term outcomes in very old acute myocardial infarction patients. Clin Interv Aging. 2018;13:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Jung YJ, Lee HR, Kwon OJ. Comparison of serum cystatin C and creatinine as a marker for early detection of decreasing glomerular filtration rate in renal transplants. J Korean Surg Soc. 2012;83:69-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Simonsen O, Grubb A, Thysell H. The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest. 1985;45:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 224] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Jickling GC, Sharp FR. Biomarker panels in ischemic stroke. Stroke. 2015;46:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Xiang J, Wen J, Yuan X, Xiong S, Zhou X, Liu C, Min X. Potential biochemical markers to identify severe cases among COVID-19 patients. 2020 Preprint. Available from: medRxiv:2020.03.19.20034447. [DOI] [Full Text] |
| 18. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1165] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 19. | Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang HC, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1054] [Cited by in RCA: 1269] [Article Influence: 253.8] [Reference Citation Analysis (0)] |
| 20. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30112] [Article Influence: 6022.4] [Reference Citation Analysis (3)] |
| 21. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12973] [Article Influence: 2594.6] [Reference Citation Analysis (1)] |
| 22. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18875] [Article Influence: 3775.0] [Reference Citation Analysis (7)] |
| 23. | Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J Am Soc Nephrol. 2020;31:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 24. | Akerfeldt T, Helmersson J, Larsson A. Postsurgical inflammatory response is not associated with increased serum cystatin C values. Clin Biochem. 2010;43:1138-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |