Published online Oct 26, 2020. doi: 10.12998/wjcc.v8.i20.4700
Peer-review started: May 17, 2020
First decision: July 25, 2020
Revised: July 29, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: October 26, 2020
Processing time: 161 Days and 21.3 Hours
Chronic pulmonary aspergillosis (CPA) is a rare syndrome that is often accompanied by gradual lung tissue destruction. Voriconazole is usually employed as the first-line agent for CPA treatment. However, some patients can develop hepatotoxicity and often were forced to stop voriconazole treatment.
To record the improving trend of liver function and the therapeutic effects in patients after lowering the trough concentration of voriconazole.
This study retrospectively analyzed 12 adult CPA patients who developed hepatotoxicity during the voriconazole treatment. In these patients, the oral dose was reduced to 3/4 or 1/2 of the standard dose (4 mg/kg, twice daily), and the lower limit of voriconazole trough concentration was maintained more than 0.5 µg/mL. The trend of remission of liver toxicity after drug reduction in 12 patients was recorded. During the same period, 25 patients who received standard doses served as the control group. Data from the two groups were collected and analyzed for different parameters such as demographic characteristics, underlying pulmonary disorders, laboratory tests, and therapeutic effect. The differences between the two groups were statistically compared.
Hepatotoxicity occurred in 12 patients within 28-65 d after oral voriconazole treatment. Hepatotoxicity was mainly manifested by the significantly increased level of gamma-glutamyltransferase and a slight increase of alanine aminotransferase and aspartate aminotransferase. The oral dose of voriconazole was reduced to approximately 3 mg/kg in seven patients and approximately 2 mg/kg in five patients. The average trough concentrations for the 12 patients before and after voriconazole oral dose reduction were 3.17 ± 1.47 µg/mL (1.5-6.0 µg/mL) and 1.70 ± 0.78 µg/mL (0.6-3.3 µg/mL), respectively (P = 0.02). After lowering the trough concentrations, the hepatotoxicity was alleviated in all the patients. However, gamma-glutamyltransferase levels declined slowly. After 4 mo of treatment, 7 of the 12 patients were successfully treated in the low trough concentrations group (41.7%). Similarly, 8 of the 25 patients in the standard treatment dose group (32.0%) were effectively treated. There was no statistical difference between the groups (P = 0.72).
Reducing the lower limit of the voriconazole trough concentration to 0.5 µg/mL can alleviate the hepatotoxicity and maintained certain clinical efficacy in CPA patients; however, patients should be closely monitored.
Core Tip: In this retrospective study, we evaluated 12 adult chronic pulmonary aspergillosis patients who developed hepatotoxicity during voriconazole treatment. The hepatotoxicity mainly manifested as a significant increase in the level of gamma-glutamyltransferase. In some patients, gamma-glutamyltransferase can reach up to 6-20 times the upper limit. Overall findings recommend that reducing the lower limit of the voriconazole trough concentration to 0.5 µg/mL can alleviate the hepatotoxicity of chronic pulmonary aspergillosis patients and maintain the clinical efficacy.
- Citation: Teng GJ, Bai XR, Zhang L, Liu HJ, Nie XH. Remission of hepatotoxicity in chronic pulmonary aspergillosis patients after lowering trough concentration of voriconazole. World J Clin Cases 2020; 8(20): 4700-4707
- URL: https://www.wjgnet.com/2307-8960/full/v8/i20/4700.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i20.4700
Chronic pulmonary aspergillosis (CPA) is a rare syndrome that is usually accompanied by mild dysfunction of the immune system followed by gradual lung tissue destruction[1]. Patients with CPA often present with pulmonary symptoms such as a persistent and/or productive cough, breathlessness, hemoptysis, weight loss, and fatigue[2]. These patients require long-term antifungal therapy to improve the symptoms and prevent the development of pulmonary fibrosis. Due to the chronicity of the disease, oral antifungal therapy is often preferred.
Voriconazole is a broad-spectrum triazole antifungal agent, which can be quickly absorbed after oral administration. The oral bioavailability of the drug can reach up to 90%-96% along with good permeability to the tissues and body fluids. In our hospital, voriconazole is often used as the first-line agent for CPA treatment. However, some patients have complained of adverse drug reactions during the treatment process, which eventually leads to discontinuation of the therapy. Common adverse reactions include hepatotoxicity, neurotoxicity, skin rash, and visual disturbances. Among these, hepatotoxicity is the most common adverse reaction. The adverse reactions of voriconazole are largely influenced by its concentration in the blood, especially the trough concentration. Reducing the trough concentration of voriconazole can reduce adverse drug reactions so that patients can eventually tolerate long-term treatment.
The cytochrome P450 2C19 (CYP2C19) polymorphism also influences the trough concentration of voriconazole and is a significant factor contributing to the highly variable pharmacokinetics of voriconazole[3-6]. The values for plasma concentration of the CYP2C19 poor metabolizer (PM) are reportedly approximately 3 times higher than the extensive metabolizer[7]. However, there is a lack of clinically relevant studies on the relationship between CYP2C19 gene status and administration regimen.
Considering the effect of the trough concentration and CYP2C19 genetic status on the adverse drug reactions, in this study, we retrospectively analyzed 12 patients who developed hepatotoxicity during voriconazole treatment. The trough concentration was reduced by minimizing the oral voriconazole dosage. Changes in the liver function and overall treatment efficacy were recorded.
This retrospective study included adult CPA patients admitted to Xuanwu Hospital Capital Medical University between January 2013 and January 2019. The patients’ selection criteria were: (1) Positive diagnosis with CPA as per the definitions of the European society for clinical microbiology and infectious diseases and the European respiratory society[8]; (2) Patients who experienced hepatotoxicity during oral voriconazole treatment, leading to a reduced oral dose of voriconazole; and (3) Patients who continued treatment for more than 4 mo after the drug dose was reduced.
The trough concentration of voriconazole was monitored. In accordance with the requirements of the Chinese Pharmacological Society's voriconazole medication guidelines[9], the patient maintained a plasma trough concentration greater than 0.5 µg/mL after reducing the dose. During this period, a total of 21 CAP patients received voriconazole reduction therapy in our hospital. The adverse reactions mainly included hepatotoxicity in 16 patients, visual impairment in 2 patients, hallucination in 2 patients, and erythematous photosensitive rash in 1 patient. Of the 16 patients with hepatotoxicity, 3 patients with CYP2C19*1/*1 genotype showed a significant decrease in trough concentrations after the dose reduction, ranging from 0.23 to 0.48 µg/mL. All three patients were treated with other antifungal drugs instead of voriconazole. One patient with CYP2C19*1/*3 genotype could not be followed up after 1 mo of treatment. As mentioned, these patients were not included in this study. Finally, 12 patients with hepatotoxicity were included in this retrospective study.
During the same period, 25 patients with standard oral voriconazole dose (about 4 mg/kg, twice daily) and no adverse reactions served as the control group.
Demographic characteristics, underlying lung disease, CYP2C19 genotype, cereal concentration, and chest CT changes were collected on these 37 patients. The difference in treatment effect between the two groups was compared.
Treatment response was categorized as effective, stable, or failure. The response was considered effective if there was an improvement of CPA-related symptoms, laboratory tests, and pulmonary computed tomography (CT). Patients with improved CPA-related symptoms or laboratory tests, but without any significant changes in the CT scans constituted the stable group. Patients with aggravating CT scans were part of the treatment failure group.
SPSS 19.0 statistical software was used for all statistical analyses. Categorical data were presented as frequencies and percentages. The continuous data are presented as mean and standard deviation or as median and ranges. Chi-square or Fisher’s exact tests were used to compare the categorical variables. For normally distributed continuous variables, the independent samples t-test was used. For non-normally distributed variables the Mann-Whitney U test was applied. Statistical significance was set at 5% for all the analyses.
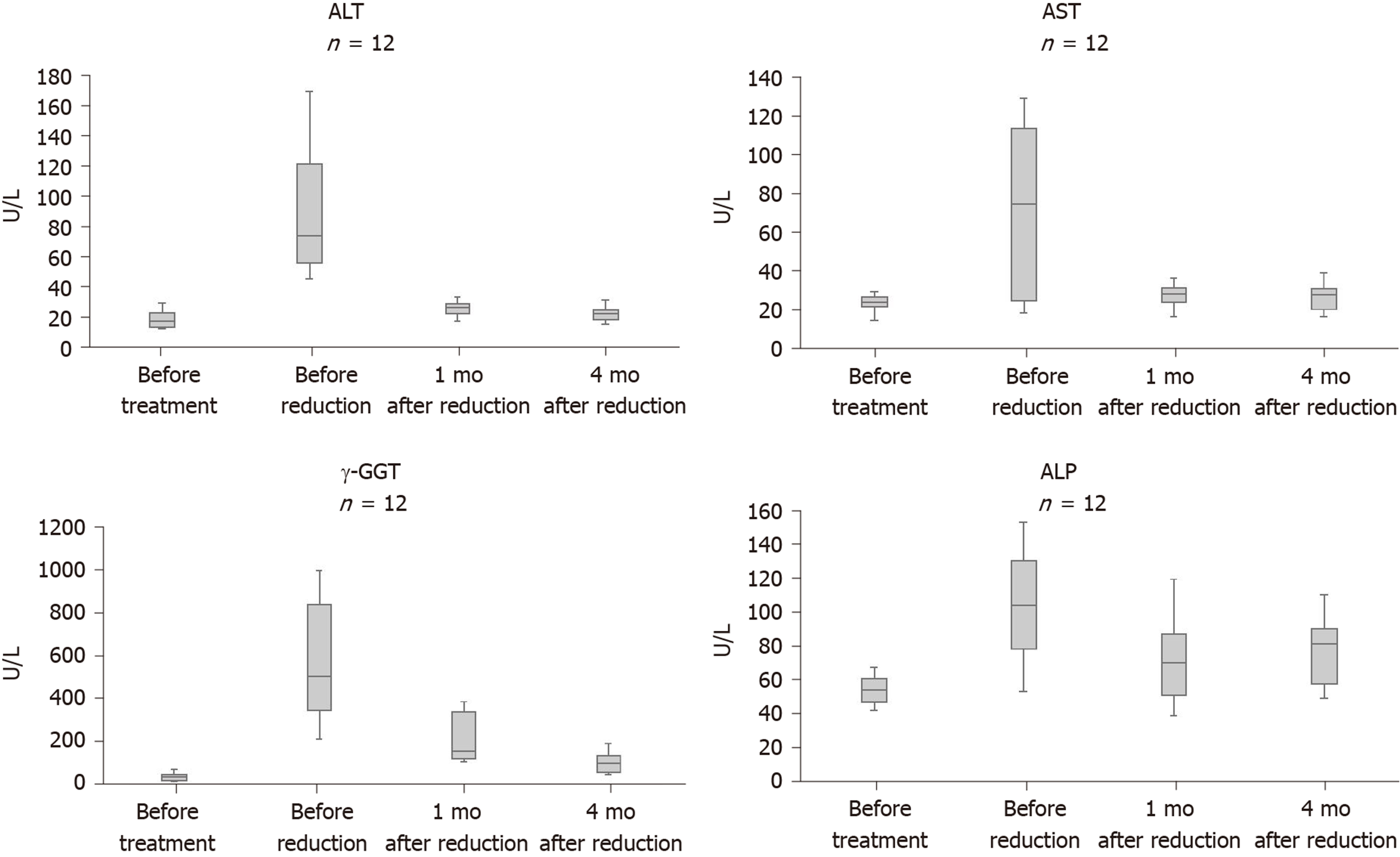
Before oral voriconazole treatment of CPA, 12 patients had normal liver function and no history of hepatitis. The patients were treated with a reduced dose due to hepatotoxicity within 28 to 65 d after oral administration of voriconazole (4 mg/kg). Gamma-glutamyltransferase (γ-GGT) levels were significantly elevated in all 12 patients, ranging from 213 to 996 U/L (normal range: 7-50 U/L). Four patients had alanine aminotransferase (normal range: 5-40 U/L) or aspartate aminotransferase (normal range: 8-40 U/L) more than 3 times the upper limit. The increase in alkaline phosphatase was not significant in 12 patients, with a maximum of only 153 U/L (normal range: 40-150 U/L). No patient had elevated bilirubin.
The oral dose of voriconazole was reduced to approximately 3 mg/kg in seven patients and approximately 2 mg/kg in five patients. The average trough concentrations for the 12 patients before and after voriconazole oral dose reduction were 3.17 ± 1.47 µg/mL (1.5-6.0 µg/mL) and 1.70 ± 0.78 µg/mL (0.6-3.3 µg/mL), respectively (P = 0.02). After lowering the trough concentrations, the abnormal liver function of all 12 patients was improved. Alanine aminotransferase and aspartate aminotransferase significantly improved and returned to normal levels within 1 mo. However, γ-GGT levels declined slowly. After 1 mo of drug reduction, γ-GGT levels dropped by 42.4%-75.4%, ranging from 106 to 384 U/L. After 4 mo, only three patients had γ-GGT levels returned to normal levels, and there were still five patients whose GGT level was 2 times higher than the upper limit (Figure 1).
The CYP2C19 genotypes in 12 patients were CYP2C19*1/*2 (n = 4), CYP2C19*1/*3 (n = 5), and CYP2C19*2/*2 (n = 3).
Among the total 37 patients, voriconazole was indicated as the first-line treatment for CPA in 30 patients (81.1%) and second-line treatment in 7 patients (18.9%). These second-line treatment patients had previously received itraconazole therapy with a mean treatment time of 42.5 d. The mean voriconazole trough concentration of 12 patients in the reduced dose group and 25 patients in the standard dose group were 1.70 ± 0.78 µg/mL (0.6-3.3 µg/mL) and 2.22 ± 0.94 µg/mL (0.8-4.1 µg/mL), respectively. There was no significant difference in blood drug concentration between the two groups (P = 0.10). A comparison of different parameters between the two groups is shown in Table 1.
| Characteristics | Reduced-dose group, n = 12 | Standard-doses group, n = 25 | P value |
| Demographics | |||
| Age, median range | 70.0 ± 15.8 | 62.8 ± 9.6 | 0.17 |
| Male/female | 8/4 | 17/8 | 1.00 |
| Underlying diseases leading to CPA | |||
| Chronic obstructive pulmonary diseases | 3 | 9 | 0.71 |
| Tuberculosis sequelae | 4 | 1 | 0.03 |
| Bronchiectasis | 4 | 8 | 1.00 |
| Lung cancer survivor | 0 | 2 | 1.00 |
| Interstitial lung disease | 5 | 3 | 0.83 |
| Steroid therapy | |||
| Inhaled glucocorticoid | 6 | 13 | 0.91 |
| Oral glucocorticoid | 7 | 16 | 1.00 |
| Additional predisposing factors | |||
| Diabetes | 2 | 3 | 1.00 |
| Smoking | 7 | 15 | 1.00 |
| Laboratory test after treatment | |||
| CRP | 5.1 ± 3.7 | 6.6 ± 5.1 | 0.38 |
| ESR | 24.3 ± 25.6 | 15.9 ± 15.0 | 0.31 |
| Total immunoglobulin E | 206.8 ± 269.9 | 279.7 ± 191.7 | 0.35 |
| Trough plasma concentration | 1.70 ± 0.78 µg/mL (0.6-3.3 µg/mL) | 2.22 ± 0.94 µg/mL (0.8-4.1 µg/mL) | 0.10 |
| Therapeutic effect after 4 mo | |||
| Effective | 5 | 8 | 0.72 |
| Stable + failure | 5 + 2 | 14 + 3 |
After the 4 mo treatment, in the reduced dose group, 5 of the 12 patients (41.7%) were clinically effective. In the standard dose group, 8 of the 25 patients (32%) were clinically effective after 4 mo. There was no statistically significant difference between the two groups (P = 0.72).
The treatment guidelines for CPA recommends switching to other antifungal agents such as posaconazole or itraconazole in the event of adverse drug reactions due to oral voriconazole[10]. The adverse reaction rate of itraconazole is higher (40%-50% of patients) and the symptoms include gastrointestinal upset, hair loss, peripheral neuropathy, hypertension, and ankle edema[11]. Also, due to the high price of posaconazole, it is difficult for many patients to afford it for a long time. Before giving up voriconazole treatment completely, we tried to adjust the trough concentrations of voriconazole to reduce the adverse drug reactions, so that patients can tolerate long-term drug treatment.
Recently, several independent studies have found that trough concentration is closely related to the adverse events such as hepatotoxicity, visual disturbances, and hallucinations[6,12-15]. A reduction in the voriconazole dose can lower its trough concentration and reduce or eliminate the adverse drug reactions[16]. Japanese scholars have reported that patients with hepatotoxicity have improved liver function after reducing the trough concentrations of voriconazole[17]. Our findings further corroborate these observations. All 12 patients had different levels of improvement in liver function after reducing the concentration of voriconazole.
In 12 patients, the hepatotoxicity of voriconazole mainly manifested as a significant increase in γ-GGT, higher than 200 U/L in all patients. γ-GGT is an enzyme that is present in hepatocytes and biliary epithelial cells[18]. In certain diseases, γ-GGT serum levels can be > 10 times the reference value. The guidelines of the Chinese pharmacology society recommend that voriconazole be withdrawn when the patient γ-GGT level is 5 times above the upper limit[9]. However, in this study, γ-GGT levels were 6-20 times higher than the upper limit in eight patients. After reducing the lower limit of trough concentration to 0.5 µg/mL, all patients could tolerate long-term treatment, and no further deterioration of liver function was observed. This result suggests that patients without underlying liver disease can tolerate the significant increase in γ-GGT. Voriconazole reduction treatment could be tried instead of withdrawal.
The optimal trough concentration of voriconazole in patients with CPA is currently uncertain. Many studies have used a voriconazole trough plasma concentration > 1 µg/mL as the lower cut-off in patients with invasive pulmonary aspergillosis[10,19]. However, it may not be the best choice for CPA patients. Based on various in vitro studies, voriconazole minimum inhibitory concentrations are reportedly between 0.5 and 1 µg/mL for most of the Aspergillus species[20]. A meta-analysis involving 21 studies (including 1158 patients) demonstrated that the trough concentration of voriconazole should be maintained above 0.5 µg/mL[21]. In 2018, a practice guideline of the Chinese Pharmacological Society recommend 0.5 µg/mL as the lower limit of the voriconazole trough concentration[9]. Our retrospective study confirms that the trough concentration of voriconazole above 0.5 µg/mL could maintain good therapeutic effects. For patients with CPA who require long-term oral voriconazole therapy, maintaining a relatively safe lower trough concentration of 0.5 μg/mL may be a better option.
The CYP2C19 polymorphism influences the concentration of voriconazole. With respect to CYP2C19 extensive metabolizer, PM has a higher trough concentration of voriconazole. It is proposed in the Clinical Pharmacogenetics Implementation Consortium guidelines that patients with CYP2C19 PM, voriconazole should be administered at a preferably lower than standard dosage with careful therapeutic drug monitoring[22]. CYP2C19 PM prevalence is 14.7% in China, which is much higher than that in European Caucasians and Africa (2.1% and 3.7%, respectively)[9]. For Chinese patients, adjusting the dose of voriconazole according to the CYP2C19 genotype may be more necessary. In our study, three patients with CYP2C19 PM (CYP2C19*2/*2) had the voriconazole dose reduced to 2 mg/kg twice daily, but the trough concentration remained 1.4-3.9 µg/mL. The results suggest that patients with CYP2C19 genotype PM could try 1/2 of the standard dose of voriconazole for treatment. However, the clinical status of all patients treated with a low trough concentration of voriconazole should be closely monitored, if the disease worsens or resistance is suspected, other antifungal agents should be considered in time.
In conclusion, reducing the lower limit of the voriconazole trough concentration to 0.5 µg/mL can alleviate the hepatotoxicity and maintained certain clinical efficacy in CPA patients. The CYP2C19 polymorphism influences the trough concentration of voriconazole, patients with CYP2C19 genotype PM could try 1/2 of the standard dose of voriconazole for treatment. However, patients should be closely monitored.
Voriconazole is often used as the first-line agent for chronic pulmonary aspergillosis (CPA) treatment. However, some patients develop hepatotoxicity that eventually leads to discontinuation of treatment.
The optimal trough concentration of voriconazole in patients with CPA is currently uncertain.
After reducing the lower limit of trough concentration to 0.5 µg/mL, the improvement trend of liver function and therapeutic effect on the patients were recorded.
This study retrospectively analyzed 12 adult CPA patients who developed hepatotoxicity during voriconazole treatment between January 2013 to January 2019. In these patients, the oral dose was reduced and the lower limit of voriconazole trough concentration was maintained at more than 0.5 µg/mL. Data were collected and analyzed for parameters.
Hepatotoxicity was mainly manifested as significantly increased level of gamma-glutamyltransferase in 12 patients. After lowering the trough concentrations, the hepatotoxicity was alleviated in all patients. However, gamma-glutamyltransferase levels declined slowly. After reducing the lower limit of trough concentration to 0.5 µg/mL, a certain clinical effects was still maintained.
Reducing the lower limit of the voriconazole trough concentration to 0.5 µg/mL can help alleviate hepatotoxicity and maintain a certain clinical efficacy in CPA patients; however, patients should be closely monitored.
This single-center retrospective study had a small sample size. Hence, additional multi-center prospective randomized study studies should be conducted to reveal the optimal trough concentration of voriconazole in CPA patients.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anand A S-Editor: Yan JP L-Editor: Filipodia P-Editor: Liu JH
| 1. | Segal BH. Aspergillosis. N Engl J Med. 2009;360:1870-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 518] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 2. | Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003;37 Suppl 3:S265-S280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 346] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45:649-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 371] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 4. | Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol. 2009;49:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Berge M, Guillemain R, Trégouet DA, Amrein C, Boussaud V, Chevalier P, Lillo-Lelouet A, Le Beller C, Laurent-Puig P, Beaune PH, Billaud EM, Loriot MA. Effect of cytochrome P450 2C19 genotype on voriconazole exposure in cystic fibrosis lung transplant patients. Eur J Clin Pharmacol. 2011;67:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Kim SH, Yim DS, Choi SM, Kwon JC, Han S, Lee DG, Park C, Kwon EY, Park SH, Choi JH, Yoo JH. Voriconazole-related severe adverse events: clinical application of therapeutic drug monitoring in Korean patients. Int J Infect Dis. 2011;15:e753-e758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Ikeda Y, Umemura K, Kondo K, Sekiguchi K, Miyoshi S, Nakashima M. Pharmacokinetics of voriconazole and cytochrome P450 2C19 genetic status. Clin Pharmacol Ther. 2004;75:587-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47:45-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 593] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 9. | Chen K, Zhang X, Ke X, Du G, Yang K, Zhai S. Individualized Medication of Voriconazole: A Practice Guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Ther Drug Monit. 2018;40:663-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Alastruey-Izquierdo A, Cadranel J, Flick H, Godet C, Hennequin C, Hoenigl M, Kosmidis C, Lange C, Munteanu O, Page I, Salzer HJF; on behalf of CPAnet. Treatment of Chronic Pulmonary Aspergillosis: Current Standards and Future Perspectives. Respiration. 2018;96:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Maghrabi F, Denning DW. The Management of Chronic Pulmonary Aspergillosis: The UK National Aspergillosis Centre Approach. Curr Fungal Infect Rep. 2017;11:242-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 667] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 13. | Matsumoto K, Ikawa K, Abematsu K, Fukunaga N, Nishida K, Fukamizu T, Shimodozono Y, Morikawa N, Takeda Y, Yamada K. Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int J Antimicrob Agents. 2009;34:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Hamada Y, Seto Y, Yago K, Kuroyama M. Investigation and threshold of optimum blood concentration of voriconazole: a descriptive statistical meta-analysis. J Infect Chemother. 2012;18:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Mihăilă RG. Voriconazole and the liver. World J Hepatol. 2015;7:1828-1833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Park WB, Kim NH, Kim KH, Lee SH, Nam WS, Yoon SH, Song KH, Choe PG, Kim NJ, Jang IJ, Oh MD, Yu KS. The effect of therapeutic drug monitoring on safety and efficacy of voriconazole in invasive fungal infections: a randomized controlled trial. Clin Infect Dis. 2012;55:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 17. | Matsumoto K, Abematsu K, Shigemi A, Kanazawa N, Watanabe E, Yokoyama Y, Ikawa K, Morikawa N, Takeda Y. Therapeutic drug monitoring of voriconazole in Japanese patients: analysis based on clinical practice data. J Chemother. 2016;28:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 18. | Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1175] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 19. | Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69:1162-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 522] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 20. | Moriyama B, Kadri S, Henning SA, Danner RL, Walsh TJ, Penzak SR. Therapeutic Drug Monitoring and Genotypic Screening in the Clinical Use of Voriconazole. Curr Fungal Infect Rep. 2015;9:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Jin H, Wang T, Falcione BA, Olsen KM, Chen K, Tang H, Hui J, Zhai S. Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J Antimicrob Chemother. 2016;71:1772-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Moriyama B, Obeng AO, Barbarino J, Penzak SR, Henning SA, Scott SA, Agúndez J, Wingard JR, McLeod HL, Klein TE, Cross SJ, Caudle KE, Walsh TJ. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin Pharmacol Ther. 2017;102:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |