Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.451
Peer-review started: October 30, 2019
First decision: December 4, 2019
Revised: December 21, 2019
Accepted: January 2, 2020
Article in press: January 2, 2020
Published online: January 26, 2020
Processing time: 78 Days and 13.5 Hours
Fungal rhinosinusitis is an infectious and/or allergic disease caused by fungi in the sinus and nasal cavity. Due to the warm and humid climate in Guangxi Zhuang Autonomous Region, the incidence of fungal rhinosinusitis is higher than that in other provinces. However, its physiological mechanism is not yet clear. Not every patient colonized by fungi develops a fungal infection. To a large extent, the immune status of the patient determines the nature of fungal disease in the nasal passages. The pathologic process of progression from harmless fungal colonization to fungal rhinosinusitis is unclear and has not been reported.
We report two patients, one who developed fungal rhinosinusitis 1.5 years after surgery performed to treat an inverted papilloma, and the other with a history of hypertension and cerebral infarction. Both patients recovered from their surgeries. An average time of 2.5 years elapsed from the development of maxillary sinus cysts to the development of fungal rhinosinusitis.
According to these case reports, we speculate that the progression of fungal rhinosinusitis from harmless colonization to disease onset requires approximately one to three years and that the length of the process may be related to underlying diseases, surgical treatment, deficient autoimmune status, and abuse of hormone antibiotics and hormones. Additional data are needed to conduct relevant studies to appropriately prevent and treat fungal rhinosinusitis.
Core tip: Due to the warm and humid climate in Guangxi Zhuang Autonomous Region, the incidence of fungal rhinosinusitis is higher than that in other provinces. But the physiological mechanism is not yet clear. In this paper, we report two such cases in an unprecedented way and describe the complete pathologic progression from harmless fungal colonization to fungal sinusitis. We speculate that the onset process of fungal rhinosinusitis from scratch takes about one to three years, and the length of the process may be related to the basic diseases, surgical treatment, low autoimmune status, and abuse of hormone antibiotics and hormones. These findings may have implications for the treatment and prevention of fungal rhinosinusitis.
- Citation: Wang LL, Chen FJ, Yang LS, Li JE. Analysis of pathogenetic process of fungal rhinosinusitis: Report of two cases. World J Clin Cases 2020; 8(2): 451-463
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/451.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.451
Fungal rhinosinusitis is a comprehensive term that includes primarily inflammatory diseases of the nasal cavity and/or sinus caused by fungal infections. Much controversy exists regarding the classification of fungal rhinosinusitis, but the most widely accepted classification roughly divides fungal rhinosinusitis into two groups according to the histopathological findings as follows: Invasive fungal rhinosinusitis and noninvasive fungal rhinosinusitis. Invasive fungal rhinosinusitis includes three categories: Acute invasive sinusitis, chronic invasive sinusitis, and granulomatous sinusitis. There are three different clinical manifestations of noninvasive fungi: Saprophytic fungal infection, fungal ball, and allergic fungal rhinosinusitis[1,2].
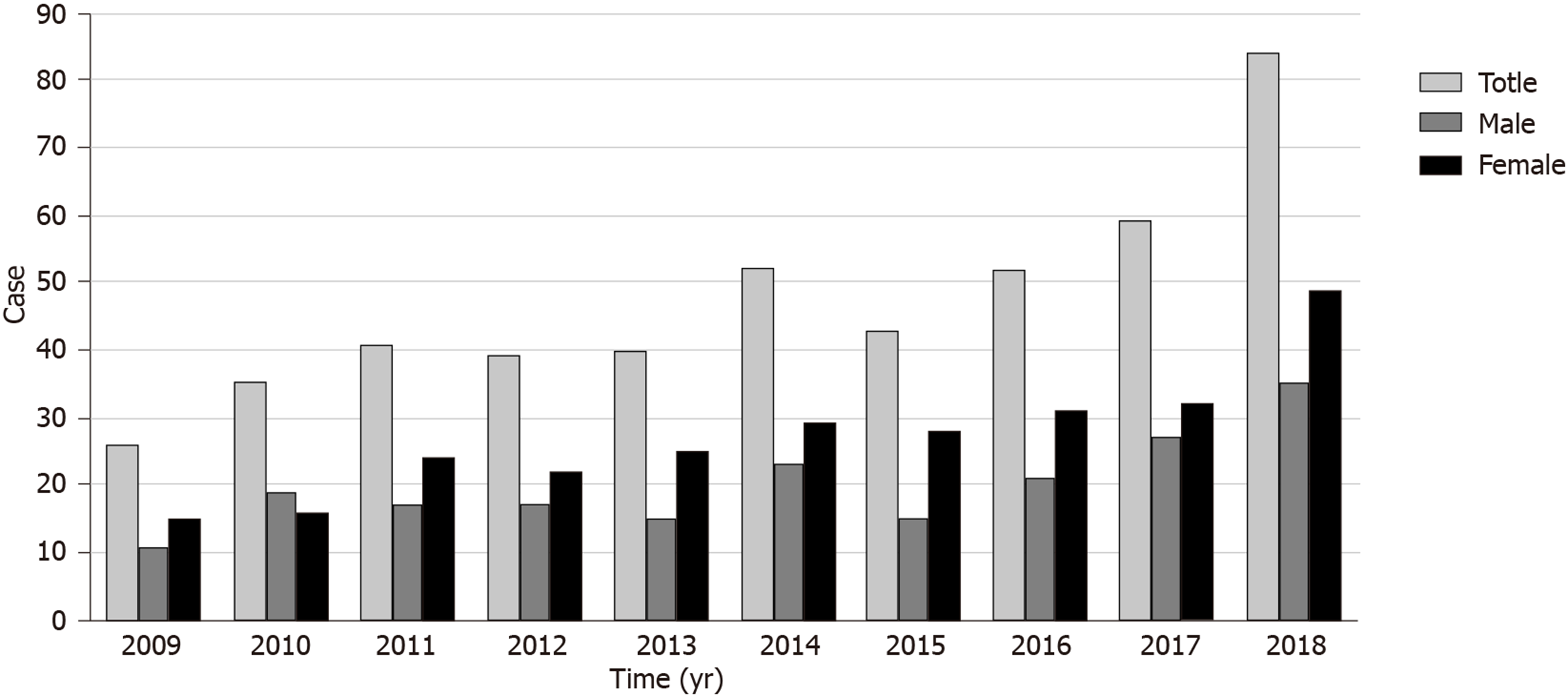
Fungi are ubiquitous, and spores are inhaled with every breath. Guangxi Zhuang Autonomous Region has a subtropical monsoon climate, and the weather is warm and humid, which is more conducive to fungal growth. The incidence of fungal rhinosinusitis in Guangxi is higher than that in other provinces. A statistical analysis of total chronic sinusitis (CRS) and fungal rhinosinusitis patients in our hospital from 2009 to 2018 indicated that 2518 patients with CRS were treated in 10 years, including 471 patients with fungal rhinosinusitis, accounting for 18.71% of CRS patients. In the past 10 years, the overall fungal rhinosinusitis trend in our hospital has increased each year (Figure 1). However, its pathogenesis and causes are still unclear. Anatomical disorders, environmental exposure, microbial toxicity, genetic background, and the immune status of infected hosts are considered to be potential causes[3]. Fungi are found in the nasal mucosa and paranasal sinuses of healthy people and CRS patients. Fungal infections do not occur in every colonized patient, and the immune status of patients largely determines the nature of fungal disease in the nasal cavity[4]. However, the pathophysiological mechanism of the transformation from harmless fungal colonization to fungal rhinosinusitis in patients is still unclear, and there are no relevant literature reports at present.
In our follow-up study of fungal rhinosinusitis patients at the First Affiliated Hospital of Guangxi Medical University, we found two patients with complete progression from harmless fungal colonization to fungal rhinosinusitis. In this paper, by studying the pathogenic progression of these two patients from harmless fungal colonization to fungal rhinosinusitis, we estimated the time required for the pathological transition in these patients and examined the cause of their nasal or sinus infections, which is important for the effective prevention and treatment of fungal rhinosinusitis in the future. Below are the case reports of the two patients.
Case 1: On November 5, 2017, a 52-year-old female patient presented with two months of pain in the right forehead more than one year after the operation for right nasal inverted papilloma.
Case 2: A patient was admitted to the hospital on February 10, 2019 due to repeated aspiration of blood sputum for more than 3 mo.
Case 1: The patient had experienced dull pain in the right forehead since before February, which was intermittent and occasionally accompanied by dizziness, but a diagnosis and treatment had not been performed. The posterior right temporal dull pain was aggravated and paroxysmal, and a small amount of pus was observed; the patient presented to our hospital for treatment. Nevertheless, she was in good spirits, in good health, and sleeping well, and had no substantial alterations in weight or physical manifestations on the upper face.
Case 2: The patient developed aspirational blood stasis after a cold in March, and the blood stasis was intermittent without nasal congestion or purulent sputum. Nevertheless, she was in good spirits, in good health, and sleeping well, and had no substantial alterations in weight or physical manifestations on the upper face.
Case 1: The general health condition was normal. On February 19, 2016, endoscopic resection of the nasal tumors was performed under general anesthesia. The pathological findings indicated an inverted papilloma. The right shoulder fracture caused by the fall 10 years ago had been operated without sequelae. He was diagnosed with tuberculosis as a child and has been cured. The patient had no history of hypertension, diabetes, nerve disease, cerebrovascular disease, neuropsychiatric disease, hepatitis, malaria, mental process, trauma, blood transfusion, or food or drug allergies.
Case 2: The general health status was normal. In 2004, the patient was diagnosed with hypertension, taking amlodipine besylate tablets 1 qd, lisinopril 1 qd, and aspirin 1 qd, and usually had good blood pressure control. A history of cerebral infarction was found on physical examination in 2015. The patient had no history of diabetes, nerve disease, neuropsychiatric disease, hepatitis, tuberculosis, malaria, mental process, trauma, blood transfusion, or food or drug allergies.
Case 1: The patient had no epidemic exposure and no exposure to chemical, radioactive, and toxic substances. The patient had no history of drug abuse, smoking, or drinking. Family history is not special.
Case 2: The patient had no epidemic exposure and no exposure to chemical, radioactive, and toxic substances. The patient had no history of drug abuse, smoking, or drinking. Her family history was not special.
Case 1: The patient’s body temperature was 36.6 °C, pulse was 79/min, respiratory rate was 20/min, and blood pressure was 125/74 mmHg. The patient had a normal conjunctiva, no yellow staining in the sclera, normal pupil, no abnormal secretions in the external auditory canal, and no tenderness in the mastoid handling. At the time of admission, the patient had a normal gingiva and oral mucosa. The tongue color was normal, and the patient did not have enlarged tonsils or thyroid gland. The patient’s external nose was not deformed, and the nasal mucosa was ruddy. The turbinate mucosa was reddish, smooth, and moist, and the probe was soft and elastic. Epithelialization was observed on the right nasal passage, and a little secretion was attached. Among them, a gray-white new creature was seen with a smooth surface. The nasal septum was centered, and there was no congested olfactory sulcus in the Li’s area. There was no empyema in the olfactory sulcus. No abnormality was found in the posterior nostril. The mucosa of the nasopharynx was smooth, the pharyngeal crypts on both sides were symmetrical, without fullness, and no new organism was found in the nasopharynx. The epiglottis had no deformity, lifting well. The ventricular zone was symmetrical, the surface was smooth, and no pre hyperemia union and no abnormality were found. The surface was smooth without hyperemia. There was no hyperemia in bilateral vocal cords, but there was a smooth margin, free movement, and symmetry and good closure of the glottis. No abnormality was found under the glottis. In addition, there were no deformities in the chest, no veins in the chest wall, and no tapping pain in the breastbone. Respiratory movement, intercostal space, and tremor were normal. The patient had normal breathing, obvious respiratory sounds in both lungs, no dry or wet sounds, and no pleural friction sounds.
Case 2: The patient’s body temperature was 36.4 °C, pulse was 92/min, respiratory rate was 20/min, and blood pressure was 143/98 mmHg. The patient had a normal conjunctiva, no yellow staining in the sclera, normal pupil, no abnormal secretions in the external auditory canal, and no tenderness in the mastoid handling. At the time of admission, the patient had a normal gingiva and oral mucosa. The tongue color was normal, and the patient did not have enlarged tonsils or thyroid gland. The patient’s external nose was not deformed, and the nasal mucosa was ruddy. The turbinate mucosa was reddish, smooth, and moist, and the probe was soft and elastic. The nasal passage was unobstructed, and no abnormal secretion was found. The nasal septum was centered, and there was no congested olfactory sulcus in the Li’s area. There was no empyema in the olfactory sulcus. No abnormality was found in the posterior nostril. The mucosa of nasopharynx was smooth, the pharyngeal crypts on both sides were symmetrical, without fullness, and no new organism was found in the nasopharynx. Epiglottis had no deformity, lifting well. The ventricular zone was symmetrical, the surface was smooth, and no pre hyperemia union and no abnormality were found. The surface was smooth without hyperemia. There was no hyperemia in bilateral vocal cords, but there was a smooth margin, free movement, and symmetry and good closure of the glottis. No abnormality was found under the glottis. In addition, there were no deformities in the chest, no veins in the chest wall, and no tapping pain in the breastbone. Respiratory movement, intercostal space, and tremor were normal. The patient had normal breathing, obvious respiratory sounds in both lungs, no dry or wet sounds, and no pleural friction sounds.
Case 1: On October 19, 2017, paranasal sinus computed tomography (CT) showed: (1) Right maxillary sinus inflammation and bleeding; and (2) postoperative changes of the right ethmoid sinus. Nasopharyngoscopy showed that the epithelialization of the right nasal passage was seen, with a little secretion attached. A gray-white new creature was seen, the surface was smooth, and it showed postoperative changes. Routine blood test was abnormal (Tables 1 and 2).
| Project name | Abbreviation | Result | Unit | Abnormal prompt | Reference range |
| White blood cell count | WBC | 9.72 | 10-9/L | H | 3.50-9.50 |
| Red blood cell count | RBC | 4.35 | 10-12/L | 3.80-5.10 | |
| Hemoglobin | HGB | 131.20 | g/L | 115.00-150.00 | |
| Platelet count | PLT | 356.50 | 10-9/L | H | 125.00-350.00 |
| Neutrophil absolute value | NEU | 7.01 | 10-9/L | H | 1.80-6.30 |
| Neutrophil percentage | NEU% | 0.721 | 0.400-0.750 | ||
| Lymphocyte absolute value | LYM | 1.63 | 10-9/L | 1.10-3.20 | |
| Lymphocyte percentage | LYM% | 0.167 | L | 0.200-0.500 | |
| Monocyte absolute value | MONO | 0.63 | 10-9/L | H | 0.10-0.60 |
| Monocyte percentage | MONO% | 0.065 | 0.030-0.100 | ||
| Eosinophil absolute value | EOS | 0.41 | 10-9/L | 0.02-0.52 | |
| Eosinophil percentage | EO% | 0.042 | 0.004-0.080 | ||
| Basophil absolute value | BASO | 0.04 | 10-9/L | 0.00-0.06 | |
| Basophil percentage | BASO% | 0.005 | 0.000-0.010 | ||
| Mean corpuscular volume | MCV | 94.42 | fl | 82.00-100.00 | |
| Mean erythrocyte hemoglobin content | MCH | 30.17 | pg | 27.00-34.00 | |
| Mean erythrocyte hemoglobin concentration | MCHC | 319.50 | g/L | 316.00-354.00 | |
| Red blood cell count volume distribution width CV | RDWCV | 0.13 | 0.11-0.14 | ||
| Mean platelet volume | MPV | 7.56 | fl | L | 9.00-12.00 |
| Platelet specific volume | PCT | 0.27 | 0.11-0.28 | ||
| Platelet volume distribution width | PDW | 0.16 | 0.15-0.18 | ||
| Hematocrit | HCT | 0.411 | 0.350-0.450 |
| Project name | Abbreviation | Result | Unit | Abnormal prompt | Reference range |
| White blood cell count | WBC | 5.58 | 10-9/L | 3.50-9.50 | |
| Red blood cell count | RBC | 3.93 | 10-12/L | 3.80-5.10 | |
| Hemoglobin | HGB | 122.20 | g/L | 115.00-150.00 | |
| Platelet count | PLT | 296.20 | 10-9/L | 125.00-350.00 | |
| Neutrophil absolute value | NEU | 2.44 | 10-9/L | 1.80-6.30 | |
| Neutrophil percentage | NEU% | 0.438 | 0.400-0.750 | ||
| Lymphocyte absolute value | LYM | 2.00 | 10-9/L | 1.10-3.20 | |
| Lymphocyte percentage | LYM% | 0.359 | 0.200-0.500 | ||
| Monocyte absolute value | MONO | 0.34 | 10-9/L | 0.10-0.60 | |
| Monocyte percentage | MONO% | 0.062 | 0.030-0.100 | ||
| Eosinophil absolute value | EOS | 0.75 | 10-9/L | H | 0.02-0.52 |
| Eosinophil percentage | EO% | 0.134 | H | 0.004-0.080 | |
| Basophil absolute value | BASO | 0.04 | 10-9/L | 0.00-0.06 | |
| Basophil percentage | BASO% | 0.008 | 0.000-0.010 | ||
| Mean corpuscular volume | MCV | 92.90 | fl | 82.00-100.00 | |
| Mean erythrocyte hemoglobin content | MCH | 31.12 | pg | 27.00-34.00 | |
| Mean erythrocyte hemoglobin concentration | MCHC | 335.00 | g/L | 316.00-354.00 | |
| RBC volume distribution width CV | RDWCV | 0.13 | 0.11-0.14 | ||
| Mean platelet volume | MPV | 7.31 | fl | L | 9.00-12.00 |
| Platelet specific volume | PCT | 0.22 | 0.11-0.28 | ||
| Platelet volume distribution width | PDW | 0.16 | 0.15-0.18 | ||
| Hematocrit | HCT | 0.365 | 0.350-0.450 |
Case 2: Nasal endoscopy performed at Binjiang Hospital (November 21, 2018) suggested bleeding from the right nose. Nasopharynx CT (November 21, 2018) performed at 303 Hospital indicated inflammation of the right maxillary sinus, hypertrophy of bilateral lower turbinate mucosa, and slightly deviated nasal septum. The patient was negative for EB virus antibodies. Routine blood test was normal (Table 3).
| Project name | Abbreviation | Result | Unit | Abnormal prompt | Reference range |
| White blood cell count | WBC | 6.21 | 10-9/L | 3.50-9.50 | |
| Red blood cell count | RBC | 4.91 | 10-12/L | 3.80-5.10 | |
| Hemoglobin | HGB | 143.20 | g/L | 115.00-150.00 | |
| Mean corpuscular volume | MCV | 87.42 | fl | 82.00-100.00 | |
| Mean erythrocyte hemoglobin content | MCH | 29.13 | pg | 27.00-34.00 | |
| Mean erythrocyte hemoglobin concentration | MCHC | 333.30 | g/L | 316.00-354.00 | |
| RBC volume distribution width CV | RDWCV | 0.13 | 0.11-0.14 | ||
| Platelet count | PLT | 218.70 | 10-9/L | 125.00-350.00 | |
| Neutrophil absolute value | NEU | 3.47 | 10-9/L | 1.80-6.30 | |
| Neutrophil percentage | NEU% | 0.559 | 0.400-0.750 | ||
| Lymphocyte absolute value | LYM | 1.91 | 10-9/L | 1.10-3.20 | |
| Lymphocyte percentage | LYM% | 0.308 | 0.200-0.500 | ||
| Monocyte absolute value | MONO | 0.55 | 10-9/L | 0.10-0.60 | |
| Monocyte percentage | MONO% | 0.089 | 0.030-0.100 | ||
| Eosinophil absolute value | EOS | 0.23 | 10-9/L | 0.02-0.52 | |
| Eosinophil percentage | EO% | 0.037 | 0.004-0.080 | ||
| Basophil absolute value | BASO | 0.04 | 10-9/L | 0.00-0.06 | |
| Basophil percentage | BASO% | 0.007 | 0.000-0.010 | ||
| Hematocrit | HCT | 0.430 | 0.350-0.450 | ||
| Platelet specific volume | PCT | 0.174 | 0.11-0.28 | ||
| Mean platelet volume | MPV | 7.98 | fl | L | 9.00-12.00 |
| Platelet volume distribution width | PDW | 0.17 | 0.15-0.18 |
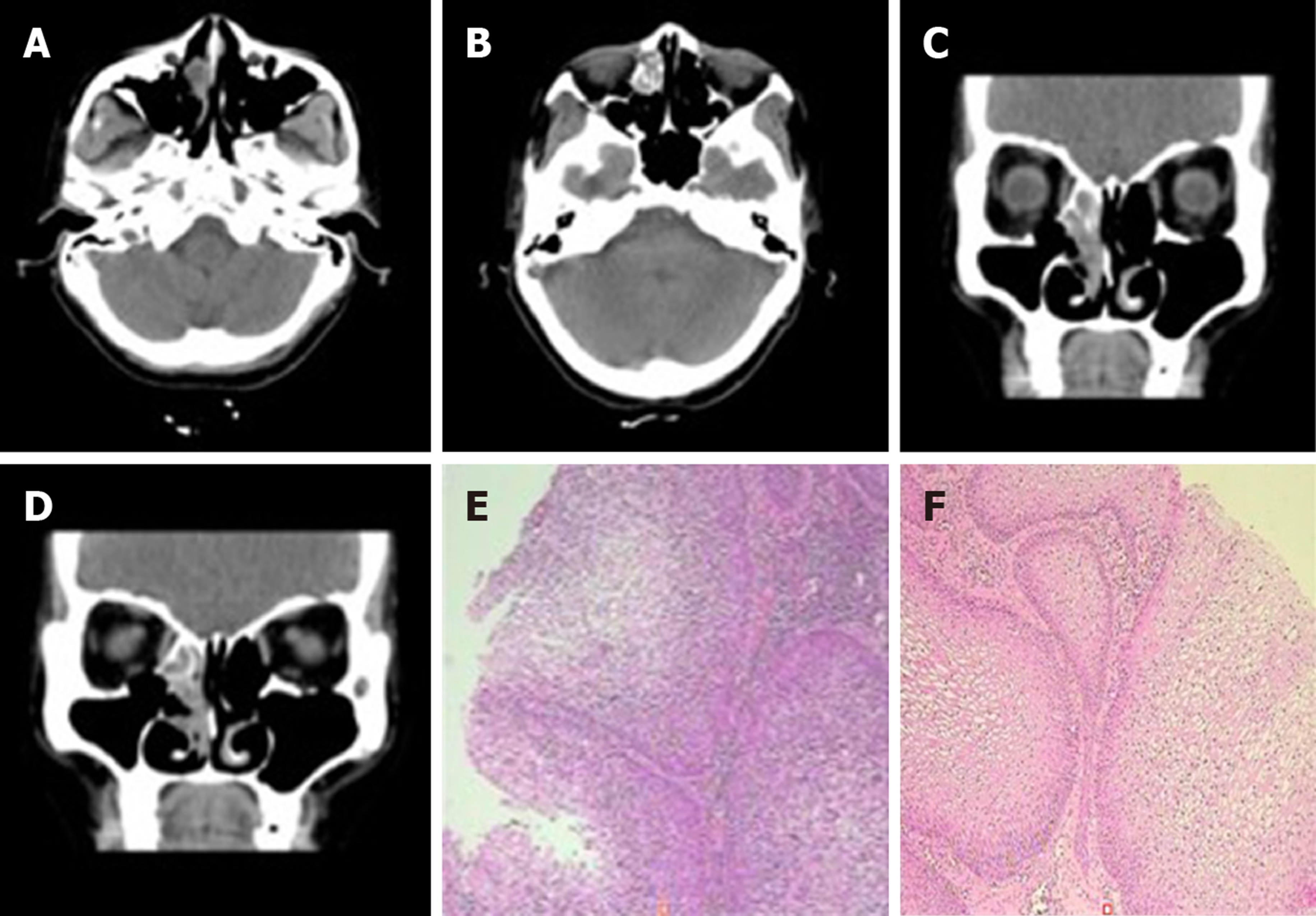
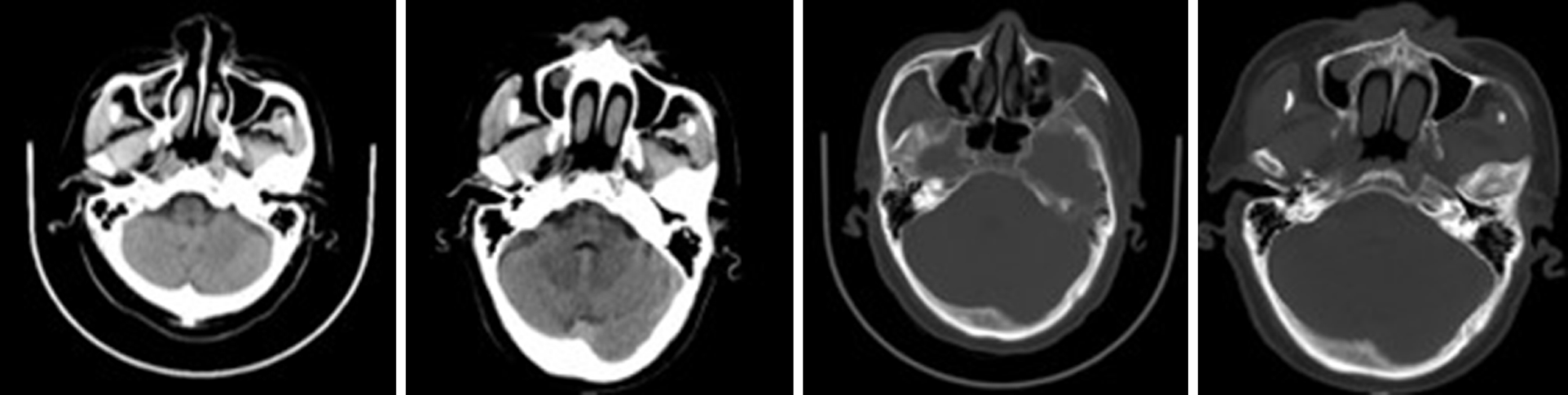
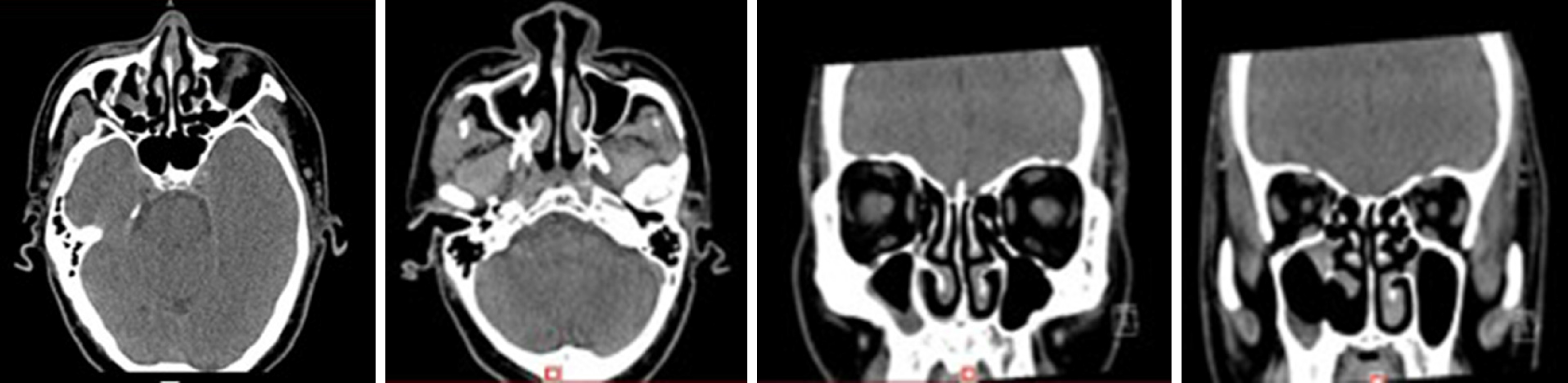
Case 1: There was a preoperative examination of the first operation on February 4, 2016. Axial and sagittal paranasal sinus CT scans showed dense shadows in the soft tissue of the right inferior nasal tract with small, low-density shadows, which were indistinct from the inferior turbinate and ethmoid sinus, and bone destruction in the ethmoid sinus (Figure 2A-D). Histopathologic findings showed that the nasal mass was an inverted papilloma and that the maxillary sinus exhibited chronic inflammation of the mucous membrane (Figure 2E-F).
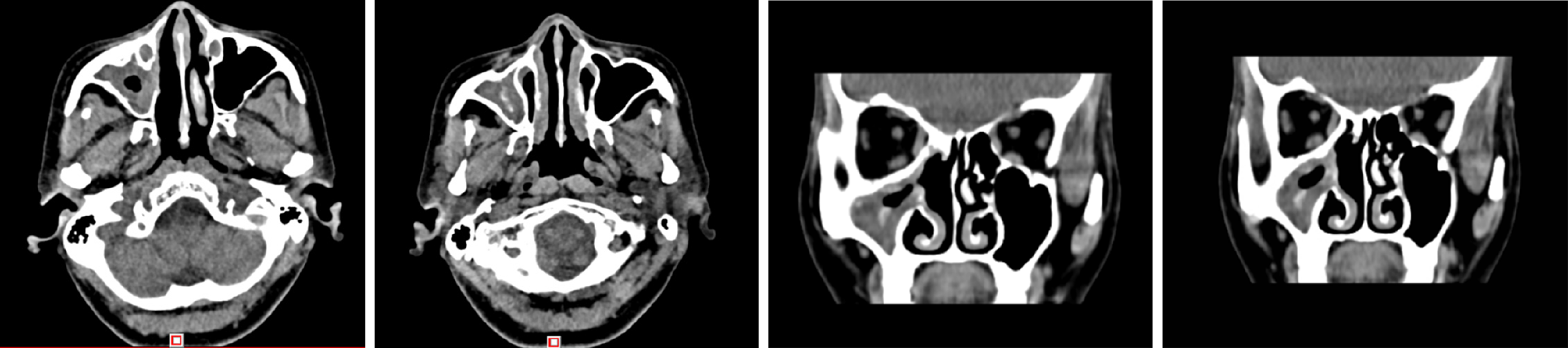
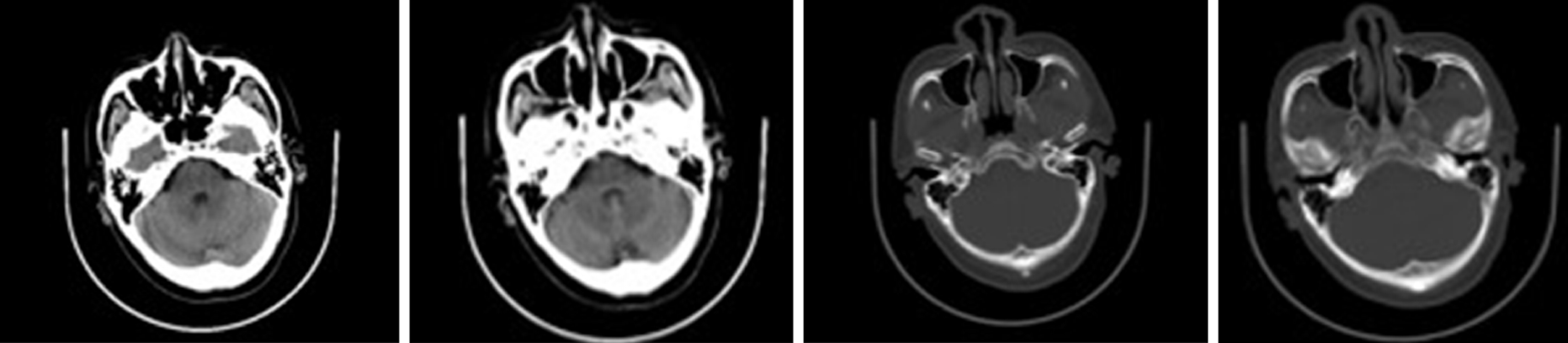
There was a preoperative examination of the second operation on October 19, 2017. Axial and sagittal paranasal sinus CT scans showed that most of the ethmoid plates in the right ethmoid sinus were missing. Patchy, dense shadows were visible in the right maxillary sinus, and strips of dense shadows were visible in the right maxillary sinus. No abnormal bone tissue was observed in the sinus wall (Figure 3).
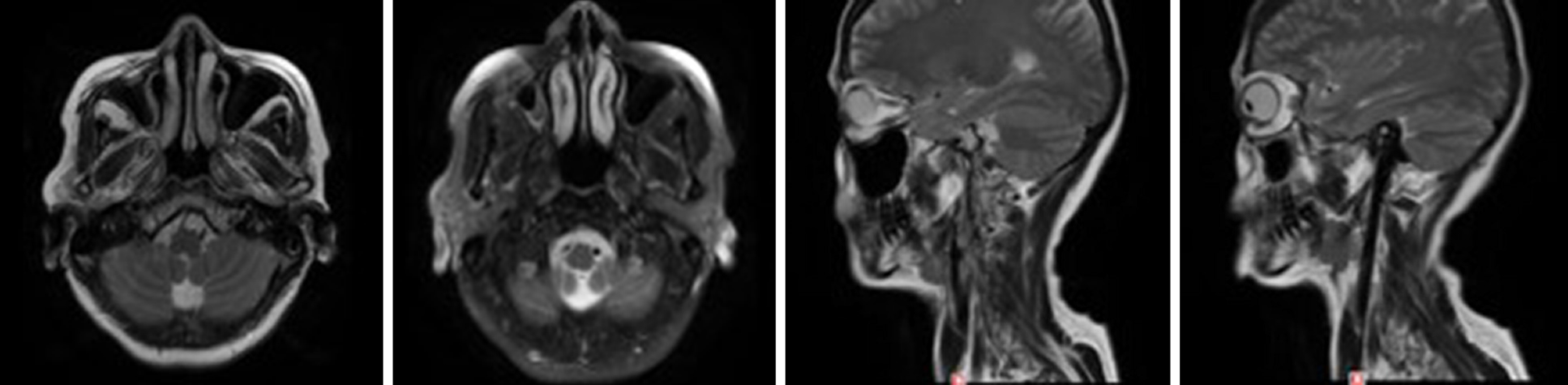
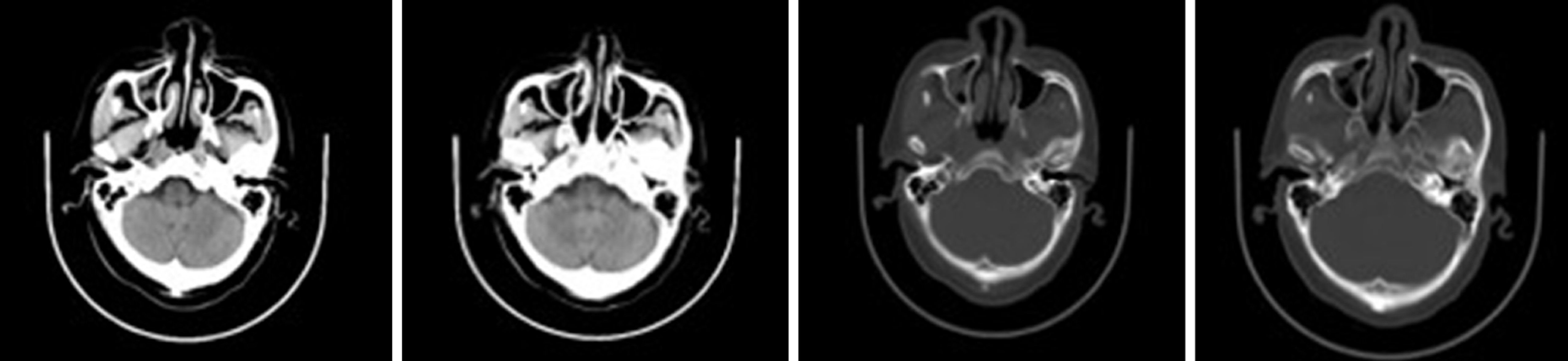
There was a postoperative re-examination on December 28, 2018. Axial and sagittal magnetic resonance imaging of the right maxillofacial region showed that most of the ethmoid plates, and the middle turbinate and part of the upper turbinate of the right ethmoid sinus were absent, indicating postoperative changes. The opening of the right maxillary sinus was satisfactory, the mucosa was slightly thickened on the medial wall, and no abnormal signal or shadow was observed in the nasal cavity (Figure 4).
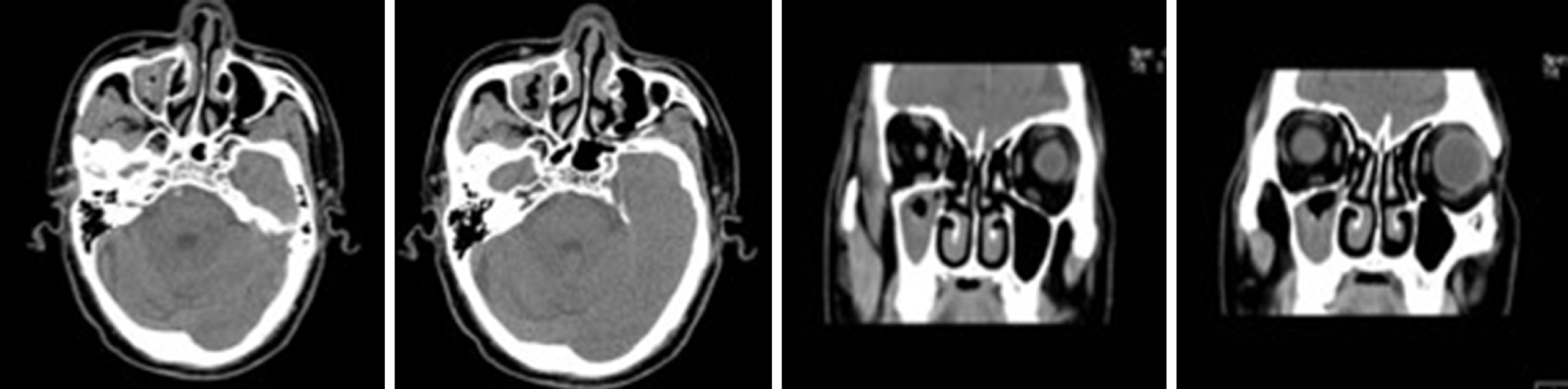
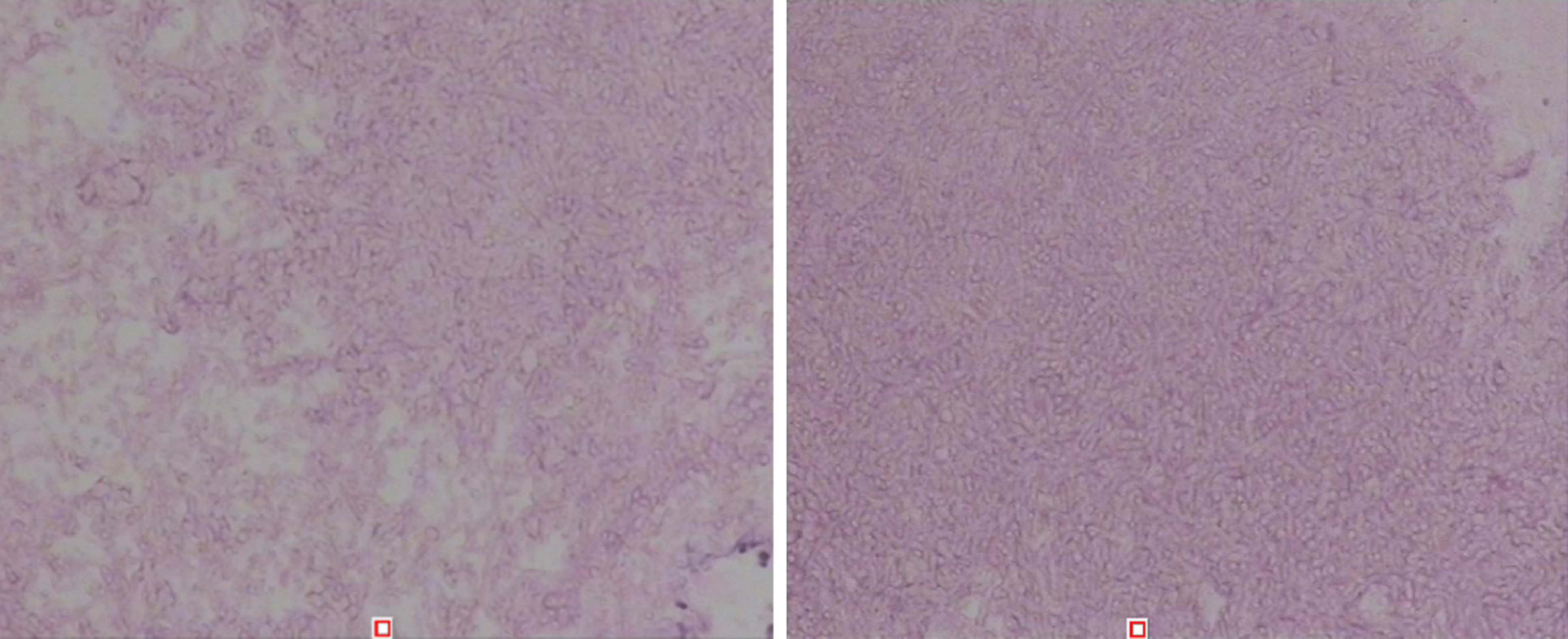
Case 2: The patient exhibited a right maxillary sinus cyst on a head CT scan performed during a physical examination in 2016 (Figure 5). In 2017, another head CT scan (Figure 6) revealed that the maxillary sinus cyst had disappeared, and no abnormalities were found in the remaining sinuses. In 2018, during a physical examination, a head CT scan (Figure 7) showed new maxillary sinus inflammation. The patient was admitted to the hospital on February 10, 2019. Nasal endoscopy showed right epistaxis, and nasal CT showed a rounded, dense shadow in the right maxillary sinus (Figure 8). A diagnosis of right maxillary sinusitis was considered. Endoscopic sinus surgery was performed on the right upper maxillary sinus, and postoperative pathology showed (Figure 9) a large amount of fungal flora in the right maxillary sinus. Three months after the operation, a repeat sinus CT scan was performed (Figure 10).
The two cases were pathologically diagnosed as maxillary sinus aspergillosis.
Transnasal endoscopic maxillary sinus surgery was performed.
After the operation, hemostasis, fluid replacement, and anti-inflammatory treatment were routinely administered.
A fungal ball is the most common noninvasive fungal sinusitis finding; it is usually located on one side of the maxillary sinus and is more common in middle-aged and elderly women[5,6]. These patients are mainly characterized by runny and stuffy nose, dried blood in the nose, headaches, and facial pain. However, as host immune function deteriorates, the fungal ball may develop into an invasive form that erodes the sinus wall, causing facial pain or obstructing the sinus opening and causing secondary bacterial infection. Currently, the clinical diagnosis of fungal rhinosinusitis relies mainly on imaging examination. On CT, fungal rhinosinusitis is characterized by microcalcification in the diseased sinus. Surgery is the first choice for treatment of symptomatic fungal spherical sinusitis, which involves minimally invasive procedures and clearing of the field. Surgery can clear the lesions, remove the obstructive factors, reconstruct the normal anatomy, protect the sinus mucosa, and return the patient to normal physiological function[7,8]. Antifungal therapy is usually not required after complete excision of the fungal ball, but removal of the fungal ball for pathological diagnosis is a common procedure[9,10].
The first patient was found to have a mass during a physical examination in January 2016, and the tumor was surgically removed in February. In September 2017, the patient began to have headaches in the right frontal part of the head. In November, the patient visited again. A paranasal sinus CT scan showed a high-density shadow in the maxillary sinus (Figure 3), raising the possibility of maxillary sinus mycosis. The postoperative pathological diagnosis was right maxillary sinus aspergillosis. Approximately one and a half years were required for the patient to develop fungal rhinosinusitis, which may be related to the deficient immune status caused by the recurrence of the patient's inverted papilloma or to the bacterial changes caused by the invasiveness of the first surgical procedure.
The second patient underwent a physical examination in May 2016. A head CT scan showed a submucosal cyst in the right maxillary sinus. In May 2017, a CT scan of the maxillary sinus cavity showed that the cyst had disappeared. In May 2018, a head CT scan obtained during a physical examination showed new maxillary sinus inflammation. No treatment was administered for two years. In November 2018, the patient had repeated aspiration of blood sputum and was admitted to the hospital in February 2019. The postoperative pathology showed a large number of fungal colonies in the right maxillary sinus, which were PAS (+) and PASD (+) upon staining, and the diagnosis of aspergillosis was clear. The time required for progression of the maxillary sinus cyst to fungal rhinosinusitis was 2.5 years. The patient had been diagnosed with hypertension in 2004 and usually had good blood pressure control. A physical examination revealed a history of cerebral infarction in 2015. We conclude that the occurrence of fungal rhinosinusitis was associated with decreased immunity due to cardiovascular disease.
Based on the analysis of the above two cases, we speculate that the pathological transition from fungal colonization to fungal rhinosinusitis requires approximately one to three years. The duration may be related to underlying disease, surgical treatment, decreased immunity, and use of hormone antibiotics and hormones. Not every colonized patient develops fungal infection. The interaction of fungi with the host immune system determines the occurrence or disappearance of infection. Studies have shown that many factors may cause chronic fungal rhinosinusitis; 10% of patients are exposed to a harmful work environment (mining), 10% of patients smoke, and 20% have had surgery for CRS. Leszczyńska et al[11] suggested that hypertension and confirmed type 2 diabetes are predominant in patients with fungal rhinosinusitis. Successful antifungal immunity depends on the innate and adaptive immune systems. Innate immune responses to fungi are mainly orchestrated by phagocytes and the epithelium. Toxins secreted by fungi, such as candidalysin, can directly damage epithelial membranes and trigger a danger-response signaling pathway that activates epithelial immunity[12]. C-type lectin receptors (CLRs), which include Dectin-1 and Dectin-2, are essential pattern recognition receptors involved in fungal recognition and initiation of protective antifungal immunity[13]. Upon binding of its ligands, Dectin-1 activates a number of cellular responses, including phagocytosis, reactive oxygen species production, and the production of numerous cytokines via multiple signaling pathways[14]. The best characterized Dectin-1 signaling pathway is the Syk-CARD9 pathway, which leads to activation of the canonical NF-κB subunits, c-Rel, and p65 and, subsequently, production of pro-IL-1β, IL-6, IL-10, IL-23, and TNF-α[15]. Dectin-1-mediated cytokine production has been shown to influence T-cell polarization. Dectin-1-/- mice infected intratracheally with Aspergillus fumigatus showed reduced levels of key Th17 cytokines (IL-17 and IL-23) and a dominant IL-12p40/IFN-γ-producing Th1 response, with increased lung fungal burdens relative to wild-type infected animals[16]. The importance of Th1 and Th17 immunity in antifungal defense mechanisms has been described in both mice and humans. Th1 cells secrete IFN-γ, GM-CSF, and TNF, which affect the maturation and killing ability of phagocytes as well as antigen-presenting cell function. The synergistic action of TNF with IFN-γ induces macrophage reactive oxygen species production in vitro, which is thought to contribute to in vivo growth arrest of intracellular fungal pathogens including Histoplasma capsulatum and Coccidioides immitis[17,18]. In humans, deficiencies in receptors for either IL-12 or IFN-γ have been associated with increased susceptibility to coccidioidomycosis and histoplasmosis[19,20]. Notably, IFN-γ immunotherapy has been shown to improve the outcome of patients with aspergillosis, cryptococcosis, or coccidioidomycosis[21]. Th17 cells produce cytokines, including IL-17A, IL-17F, and IL-22, that promote neutrophil trafficking and fungicidal activity and are involved in the induction of antimicrobial peptides such as S100A7, S100A8, S100A9, and β-defensins from epithelial cells and keratinocytes that defend against fungal overgrowth[22]. Th17 cells are essential for preventing mucosal fungal infections, such as chronic mucocutaneous candidiasis, and Malassezia skin infections[23]. In humans, a deficiency in the IL-17/IL-17R axis and in signaling components, including genetic defects in STAT1 and STAT3 (hyper-IgE syndrome), results in increased susceptibility to chronic mucocutaneous candidiasis[24]. CLRs have been implicated in Th2-mediated fungal allergic responses. For instance, in mice, following repeated lung exposure to Aspergillus fumigatus, CD4+ T cells produce IL-4, IL-5, and IL-13 in a Dectin-1-dependent manner (Clec7a-/- mice)[24]. Moreover, intratracheal administration of A. versicolor spores in mice exacerbated house dust mite-induced allergic asthma through the production of IL-4 and IL-13[25].
From the blood test results of the patients, we found that the number of eosinophils before surgery was increased in the first patient and that the number of eosinophils before surgery was normal in the second patient. To our knowledge, the presence of prevalent eosinophilic infiltration of the inflamed mucosa is the most constant pathological finding in CRS. This feature is equally observed both in allergic and nonallergic patients. Such a pathological finding might be due to a prevalent Th2 immune response and a similar pattern of cytokines. The eosinophils in the sinus mucosa have been proposed to be mainly responsible for the initial tissue damage and can alter the epithelial innate immunity. In turn, tissue damage may promote bacterial aggression in the sinus mucosa; in particular, Staphylococcus aureus can cause superantigenic stimulation of the adaptive immune system through the production of exotoxins, and its biofilms have been associated with eosinophilic inflammation and elevated eosinophilic cationic protein and IL-5 levels[26]. CRS affects 1% to 9% of the general population and exhibits two traditional clinical phenotypes, CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP). CRSwNP is characterized by T-helper cell type 2-skewed inflammation, whereas CRSsNP exhibits a T-helper cell type 1-mediated inflammatory response[27]. Iinuma et al[28] classified patients with CRSwNP as having non-eosinophilic chronic rhinosinusitis (NECRS) or eosinophilic CRS (ECRS). Some findings have suggested major differences in the pathophysiology of the two forms of CRSwNP. CD4+ T cells, basophils, and IgE+ mast cells could contribute to the eosinophilic infiltration and overall pathogenesis of ECRS, but this was not found for non-eosinophilic chronic rhinosinusitis[29]. Several pathological findings and clinical observations have suggested that CRS and asthma may be part of the same disease process, characterized by diffuse eosinophilic inflammation of the respiratory airways, from the nostrils to the bronchi, which constitutes the pathological basis of the so-called “United Airways Disease” theory[30]. The histological features of CRS and comorbid asthma largely overlap; both the sinuses and bronchial mucosa show eosinophilic inflammation and signs of airway remodeling. CRS and asthma may also interact via the systemic route through the release of pro-inflammatory cytokines in the blood, including IL-5 from peripheral sites; then, those mediators act on the bone marrow, leading to the maturation and mobilization of inflammatory cells, including eosinophils and basophils[26]. Some studies have shown that, following initial fungal allergen exposure of naïve mice, basophils are rapidly recruited to the airways, produce IL-4, and influence the development of the adaptive immune response[31]. The mechanism underlying eosinophil infiltration in fungal diseases has not been determined, but if a common mechanism could be identified, it would lead to a breakthrough in the treatment of many diseases.
In the above two cases, we have described in detail the pathogenic process from fungal colonization to fungal sinusitis. The pathological change in these two cases required approximately one to three years. At present, few related reports have been published on these subjects domestically or abroad. We have highlighted the central role of CLRs, such as Dectin-1, in inducing and modulating cytokine production and directing antifungal immune responses. However, many of the important mechanisms shaping these responses are yet to be fully understood. The formation of fungal rhinosinusitis may be related to the reduced immune function caused by endoscopic sinus surgery and hypertension in the above cases, which requires further discussion. Further understanding of other mechanisms that shape T-cell immunity during fungal infections is of great significance for the development of emerging therapies.
We would like to thank the two patients for allowing us to use their personal information.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poddighe D S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Chakrabarti A, Das A, Panda NK. Controversies surrounding the categorization of fungal sinusitis. Med Mycol. 2009;47 Suppl 1:S299-S308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W, Kita H, Marple B, Panda N, Vlaminck S, Kauffmann-Lacroix C, Das A, Singh P, Taj-Aldeen SJ, Kantarcioglu AS, Handa KK, Gupta A, Thungabathra M, Shivaprakash MR, Bal A, Fothergill A, Radotra BD. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2009;119:1809-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 308] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Montone KT. Pathology of Fungal Rhinosinusitis: A Review. Head Neck Pathol. 2016;10:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Callejas CA, Douglas RG. Fungal rhinosinusitis: what every allergist should know. Clin Exp Allergy. 2013;43:835-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Gorovoy IR, Kazanjian M, Kersten RC, Kim HJ, Vagefi MR. Fungal rhinosinusitis and imaging modalities. Saudi J Ophthalmol. 2012;26:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Seo YJ, Kim J, Kim K, Lee JG, Kim CH, Yoon JH. Radiologic characteristics of sinonasal fungus ball: an analysis of 119 cases. Acta Radiol. 2011;52:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Lop-Gros J, Gras-Cabrerizo JR, Bothe-González C, Montserrat-Gili JR, Sumarroca-Trouboul A, Massegur-Solench H. Fungus ball of the paranasal sinuses: Analysis of our serie of patients. Acta Otorrinolaringol Esp. 2016;67:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Nomura K, Asaka D, Nakayama T, Okushi T, Matsuwaki Y, Yoshimura T, Yoshikawa M, Otori N, Kobayashi T, Moriyama H. Sinus fungus ball in the Japanese population: clinical and imaging characteristics of 104 cases. Int J Otolaryngol. 2013;2013:731640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Nicolai P, Lombardi D, Tomenzoli D, Villaret AB, Piccioni M, Mensi M, Maroldi R. Fungus ball of the paranasal sinuses: experience in 160 patients treated with endoscopic surgery. Laryngoscope. 2009;119:2275-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Ledderose GJ, Braun T, Betz CS, Stelter K, Leunig A. Functional endoscopic surgery of paranasal fungus ball: clinical outcome, patient benefit and health-related quality of life. Eur Arch Otorhinolaryngol. 2012;269:2203-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Leszczyńska J, Stryjewska-Makuch G, Lisowska G, Kolebacz B, Michalak-Kolarz M. Fungal sinusitis among patients with chronic rhinosinusitis who underwent endoscopic sinus surgery. Otolaryngol Pol. 2018;72:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 13. | Speakman EA, Dambuza IM, Salazar F, Brown GD. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends Immunol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | Shiokawa M, Yamasaki S, Saijo S. C-type lectin receptors in anti-fungal immunity. Curr Opin Microbiol. 2017;40:123-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Gringhuis SI, Wevers BA, Kaptein TM, van Capel TM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TB. Selective C-Rel activation via Malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathog. 2011;7:e1001259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med. 2011;208:369-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Kroetz DN, Deepe GS. The role of cytokines and chemokines in Histoplasma capsulatum infection. Cytokine. 2012;58:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Castro-Lopez N, Hung CY. Immune Response to Coccidioidomycosis and the Development of a Vaccine. Microorganisms. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Jannière L, Rose Y, de Suremain M, Kong XF, Filipe-Santos O, Chapgier A, Picard C, Fischer A, Dogu F, Ikinciogullari A, Tanir G, Al-Hajjar S, Al-Jumaah S, Frayha HH, AlSum Z, Al-Ajaji S, Alangari A, Al-Ghonaium A, Adimi P, Mansouri D, Ben-Mustapha I, Yancoski J, Garty BZ, Rodriguez-Gallego C, Caragol I, Kutukculer N, Kumararatne DS, Patel S, Doffinger R, Exley A, Jeppsson O, Reichenbach J, Nadal D, Boyko Y, Pietrucha B, Anderson S, Levin M, Schandené L, Schepers K, Efira A, Mascart F, Matsuoka M, Sakai T, Siegrist CA, Frecerova K, Blüetters-Sawatzki R, Bernhöft J. Freihorst J, Baumann U, Richter D, Haerynck F, De Baets F, Novelli V, Lammas D, Vermylen C, Tuerlinckx D, Nieuwhof C, Pac M, Haas WH, Müller-Fleckenstein I, Fleckenstein B, Levy J, Raj R, Cohen AC, Lewis DB, Holland SM, Yang KD, Wang X, Wang X, Jiang L, Yang X, Zhu C, Xie Y, Lee PP, Chan KW, Chen TX, Castro G, Natera I, Codoceo A, King A, Bezrodnik L, Di Giovani D, Gaillard MI, de Moraes-Vasconcelos D, Grumach AS, da Silva Duarte AJ, Aldana R, Espinosa-Rosales FJ, Bejaoui M, Bousfiha AA, Baghdadi JE, Ozbek N, Aksu G, Keser M, Somer A, Hatipoglu N, Aydogmus C, Asilsoy S, Camcioglu Y, Gülle S, Ozgur TT, Ozen M, Oleastro M, Bernasconi A, Mamishi S, Parvaneh N, Rosenzweig S, Barbouche R, Pedraza S, Lau YL, Ehlayel MS, Fieschi C, Abel L, Sanal O, Casanova JL. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore). 2010;89:381-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis. 2009;49:e62-e65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Verma A, Wüthrich M, Deepe G, Klein B. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2014;5:a019612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | De Luca A, Zelante T, D'Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, Puccetti P, Romani L. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Kolls JK, Khader SA. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 2010;21:443-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125:1389-1390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Zhang Z, Biagini Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA, Reponen T, Vesper SJ, Forde F, Ruff B, Bass SA, LeMasters GK, Bernstein DI, Lockey J, Budelsky AL, Khurana Hershey GK. β-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. J Allergy Clin Immunol. 2017;139:54-65.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Poddighe D, Brambilla I, Licari A, Marseglia GL. Pediatric rhinosinusitis and asthma. Respir Med. 2018;141:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, Schleimer RP, Ledford D. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 28. | Iinuma T, Okamoto Y, Yamamoto H, Inamine-Sasaki A, Ohki Y, Sakurai T, Funakoshi U, Yonekura S, Sakurai D, Hirahara K, Nakayama T. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2015;114:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Baba S, Kondo K, Suzukawa M, Ohta K, Yamasoba T. Distribution, subtype population, and IgE positivity of mast cells in chronic rhinosinusitis with nasal polyps. Ann Allergy Asthma Immunol. 2017;119:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Hellings PW, Hens G. Rhinosinusitis and the lower airways. Immunol Allergy Clin North Am. 2009;29:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Poddighe D, Mathias CB, Freyschmidt EJ, Kombe D, Caplan B, Marseglia GL, Oettgen HC. Basophils are rapidly mobilized following initial aeroallergen encounter in naïve mice and provide a priming source of IL-4 in adaptive immune responses. J Biol Regul Homeost Agents. 2014;28:91-103. [PubMed] |