Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.436
Peer-review started: November 4, 2019
First decision: December 4, 2019
Revised: December 5, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 26, 2020
Processing time: 74 Days and 1.6 Hours
Neuroblastoma (NB) is the most common type of extracranial solid tumour in children. The overall prognosis of NB is poor, but at the same time, NB shows significant clinical diversity. NB can demonstrate spontaneous regression or can differentiate into benign ganglioneuroma.
This study retrospectively analyzed the clinical data of a patient with spontaneous regression of stage III NB who was admitted in May 2015. Studies of the spontaneous regression of NB published from October 1946 to September 2019 were retrieved through PubMed. The clinical manifestations, diagnosis, treatment, and follow-up results were analysed.
Spontaneous regression of stage III NB is rare in the clinic. The report of this case is an important supplement to the study of the spontaneous regression of NB.
Core tip: Neuroblastoma (NB) is the most common type of extracranial solid tumour in children. The overall prognosis of NB is poor, but at the same time, NB shows significant clinical diversity. NB can demonstrate spontaneous regression or can differentiate into benign ganglioneuroma. This study retrospectively analyzed the clinical data of a patient with spontaneous regression of stage III NB who was admitted in May 2015. Studies of the spontaneous regression of NB published from October 1946 to September 2019 were retrieved through PubMed. The clinical manifestations, diagnosis, treatment, and follow-up results were analysed. Spontaneous regression of stage III NB is rare in the clinic. The report of this case is an important supplement to the study of the spontaneous regression of NB.
- Citation: Liu J, Wu XW, Hao XW, Duan YH, Wu LL, Zhao J, Zhou XJ, Zhu CZ, Wei B, Dong Q. Spontaneous regression of stage III neuroblastoma: A case report. World J Clin Cases 2020; 8(2): 436-443
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/436.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.436
Neuroblastoma (NB) is the most common type of extracranial solid tumour in children. It originates from primitive neural crest cells[1]. NB occurs mainly in the retroperitoneum (mainly, the adrenal gland), mediastinum, pelvic cavity and neck as well as in the central nervous system[2]. The overall prognosis of NB is poor, but at the same time, NB shows significant clinical diversity. NB can demonstrate spontaneous regression or can differentiate into benign ganglioneuroma[3]. The spontaneous regression of stage IV NB has been reported in the literature[4], but the occurrence of stage III NB is extremely rare, with only a few reports (Table 1)[5-7]. This paper reports the case of a patient with spontaneous regression of stage III NB admitted in May 2015. A literature review was conducted to explore the regularity and mechanism of regression and to summarize the diagnosis and treatment. This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. Medical records and pictures were obtained via informed consent from the patient’s parents or legal guardians, and written informed consent was obtained from the parents.
An 11-mo-old girl had an abdominal mass during a physical examination two days before her hospitalization.
Two days ago, the patient had abdominal ultrasound examination which revealed an abdominal mass.
The patient had a history of dilated cardiomyopathy.
The patient had no family history.
The abdomen was flat and soft, and a hard mass was palpated in the right abdomen. The boundary was not clear, and the activity was poor. There was no tenderness or rebound pain in the abdomen, and the mass could not be palpated under the ribs near the liver and spleen.
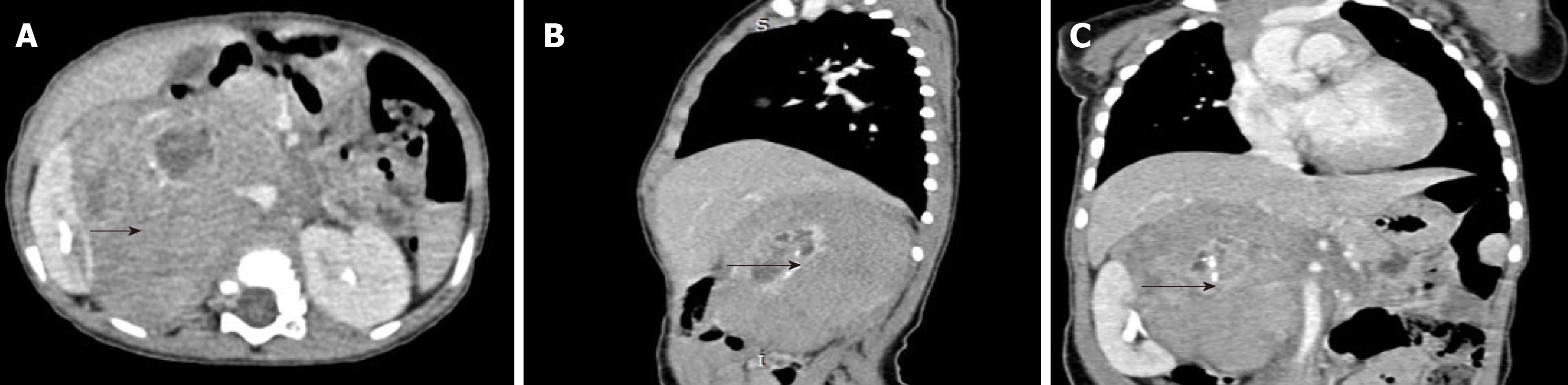
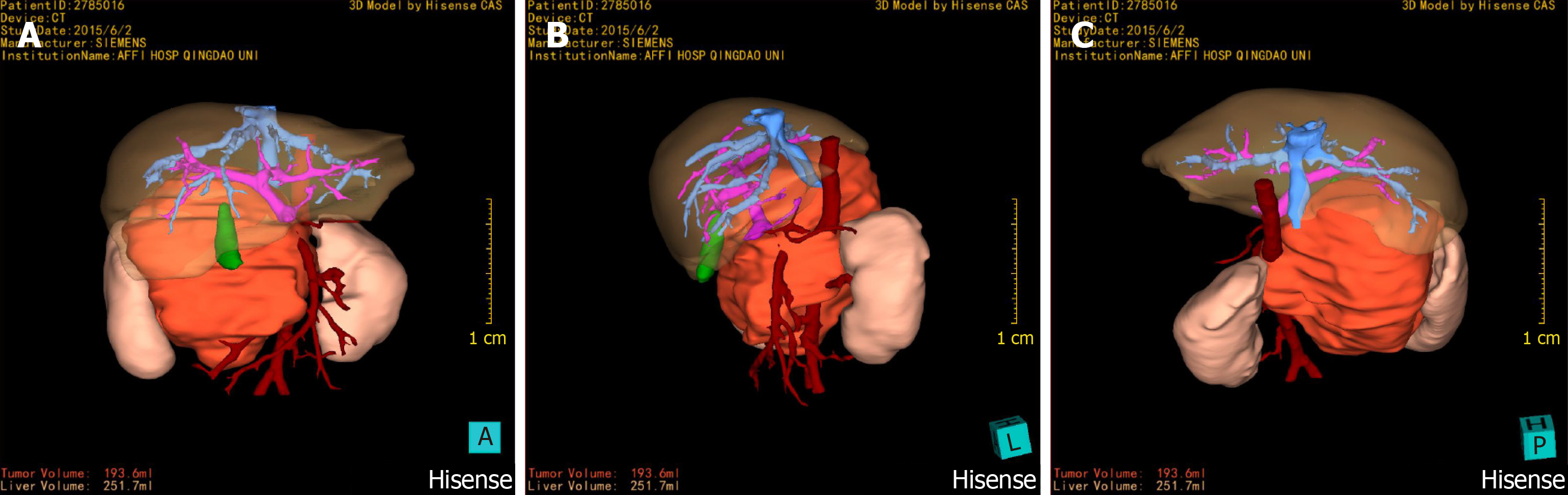
Abdominal enhanced computed tomography (CT) examination was performed on June 2, 2015, and three-dimensional (3D) reconstruction was performed by using a computer-assisted surgical system (Hisense CAS)[8]. The CT results showed mass shadows of retroperitoneal lobulated soft tissue with a maximum cross section of 93 mm × 82 mm, and these shadows were considered to be possible NB. Hisense CAS 3D imaging showed that the tumour volume was 193.6 mL, and part of the boundary was infiltrating, with lesions surrounding the major retroperitoneal vessels such as the inferior vena cava, renal artery, renal vein, and abdominal aorta (Figures 1 and 2).
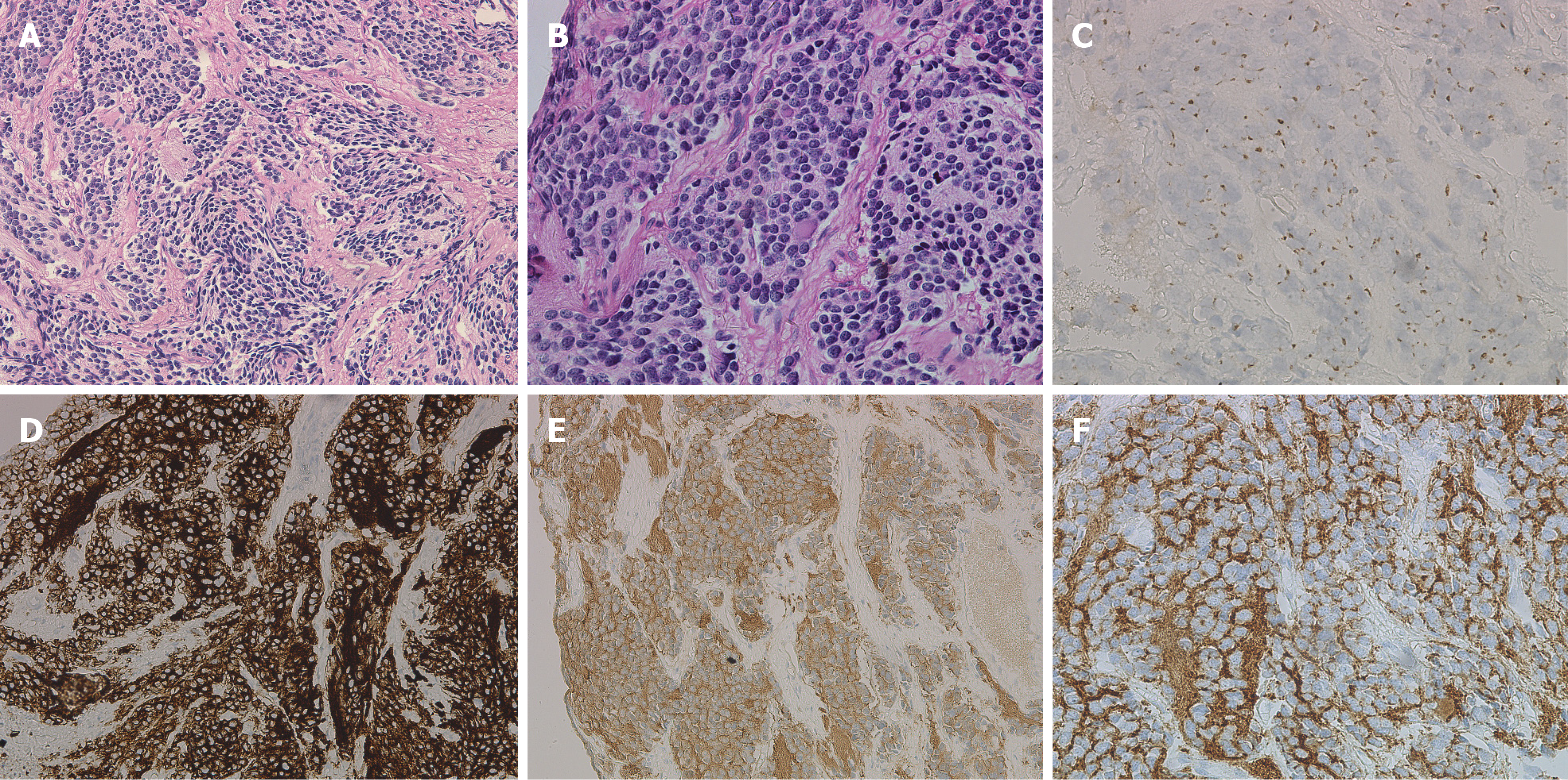
Tumour biopsy specimens were diagnosed as NB. Immunohistochemical staining showed that Syn, NSE, and CD56 were positive, CgA was suspiciously positive, and CD99 was negative (Figure 3).
The normal bone marrow biopsy results suggested that the patient should be diagnosed with stage III NB.
After discussion with the treatment group, it was suggested that the tumour could not be completely removed and that chemotherapy should be administered first. However, the parents of the children refused preoperative chemotherapy due to concerns about the side effects of chemotherapy drugs and left the hospital by themselves.
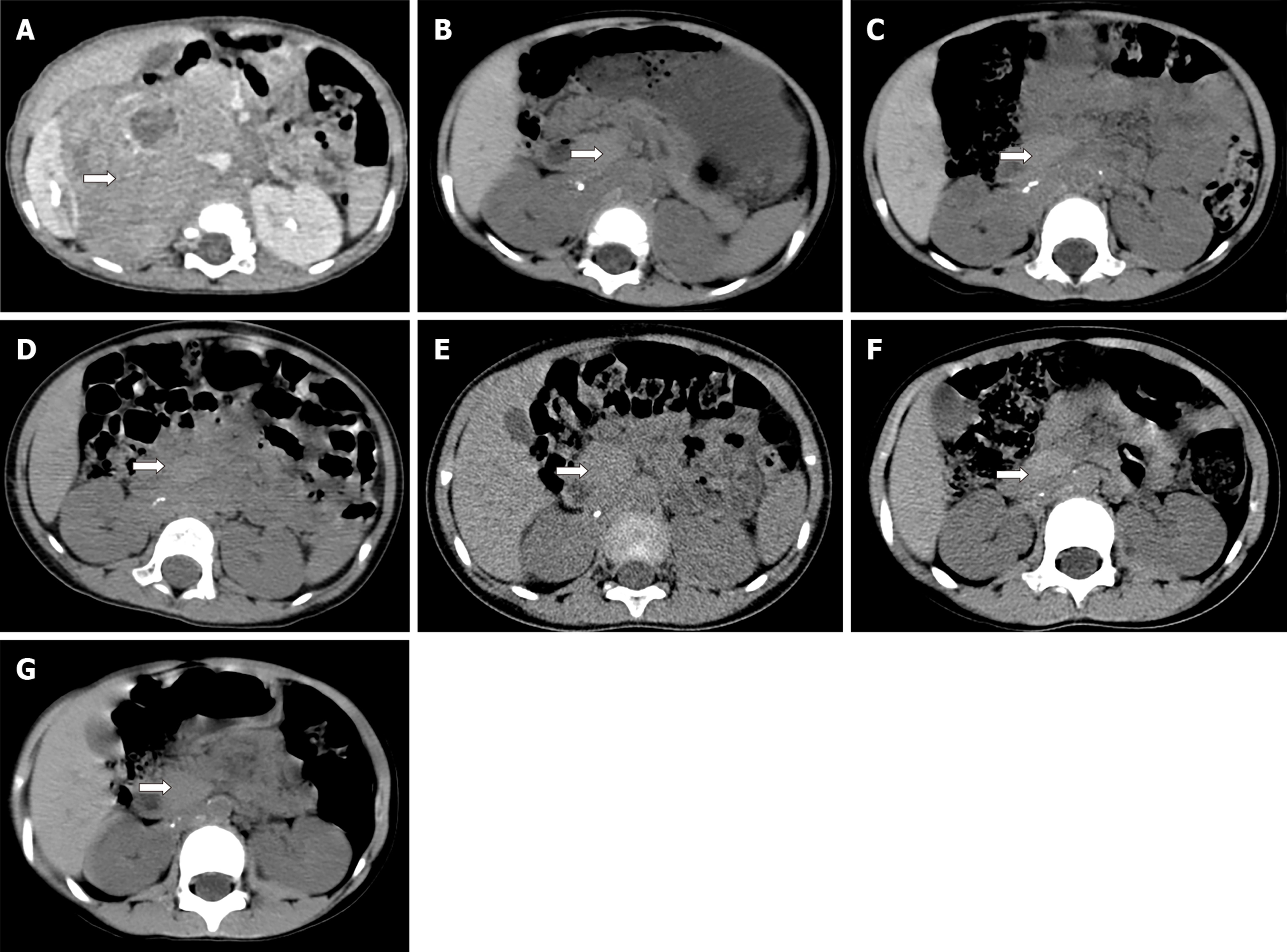
Abdominal B-ultrasound re-examination after 2 mo showed that the retroperitoneal tumour volume was approximately 46 mm × 52 mm × 46 mm, which was significantly smaller than before. Considering the possibility of spontaneous regression of the tumour, it was recommended that observation and treatment be continued. During the follow-up period from 2015 to 2019, the child was re-examined via abdominal CT six times (Figure 4 and Table 2). The patient was followed for 4 years without recurrence or distant metastasis. The child generally has a good condition, with growth and development similar to those of children of the same age.
| Age (mo) | Abdominal CTexamination time | Maximum transverse value of abdominal CT (mm) | Image number1 |
| 11 | June, 2015 | 93 × 82 | 4A |
| 16 | October, 2015 | 35 × 21 | 4B |
| 18 | December, 2015 | 31 × 19 | 4C |
| 26 | August, 2016 | 28 × 17 | 4D |
| 38 | August, 2017 | 26 × 15 | 4E |
| 50 | October, 2018 | 23 × 14 | 4F |
| 61 | July, 2019 | 11 × 8 | 4G |
NB is one of the most common types of malignant solid tumours in infants. It is not only insidious in onset but also rapidly develops. Despite some progress in treatment, the 5-year overall survival rate of the disease in high-risk groups is still very low, which has become a difficult problem in medical research[9]. Cole et al[10] first reported a special biological behaviour of spontaneous regression of tumours in 1956 and found that the incidence of spontaneous regression of malignant tumours was approximately 1/60000-100000, with the highest NB regression rate. At present, the mechanism of NB spontaneous regression is not very clear, and it is still a hot research topic. Nakagawara et al[11] found that nerve growth factor could promote the growth and differentiation of NB tumour cells, and apoptosis occurred within 1 wk after removing the factor. Zhu et al[12] found that UNC5D could indirectly regulate the effect of nerve growth factor on NB. Some scholars believe that the host immune response may be one of the reasons for the natural regression of NB. Inflammation and the tumour microenvironment may have an important impact on the prognosis of NB[13]. Decock et al[14] described the characteristics of the DNA methylation in patients with stage IVS NB for the first time, showing that such patients have unique DNA methylation patterns. This finding may be helpful for the study of new mechanisms of DNA-specific tumour biology and spontaneous regression in patients with stage IVS NB. Brodeur[15] reported that the expression of TrkA in NB is related to good clinical and biological characteristics. The prognosis of these patients is good, but the case did not undergo TrkA test due to the small amount of tumor fine needle aspiration samples.
Some scholars have also studied the law of spontaneous regression of NB. Evans et al[16] reported in 1971 that there was a stage with the highest degradation rate in NB, namely, the IVS stage. The age of onset of patients with stage IVS NB is less than 1 years old. The stage of primary tumours is usually stage I, IIA, or IIB. The distant metastasis of tumours is limited to the skin, liver, and/or bone marrow. In addition, bone marrow biopsy results require that the proportion of neuroblasts in all nucleated cells must be less than 10%. The results of bone marrow scanning by metaiodoben-zylguanidine imaging should be negative[17]. Some scholars believe that spontaneous regression usually occurs within 6 mo after birth. For this age group or for the limited types of NB with diameters less than 5 cm, we can use the method of “Wait and See” without implementing any treatment measures[18]. Yoneda et al[19] studied the abovementioned observations and treatment of stages I and II NB with diameters less than 5 cm. A total of eight children were enrolled in the group. The study found that five children had spontaneous regression. Fritsch et al[20] used the same treatment, and three of five patients had spontaneous regression. These studies regularly detected tumour markers via magnetic resonance imaging, abdominal B-mode ultrasound, or chest X-ray during follow-up. If there were signs of tumour enlargement, tumour marker elevation, or metastasis, and if parents have the intention of surgery, the researchers immediately stopped observations and provided surgical treatment[19]. It was found that spontaneous regression also exists in children aged over 6 months and with tumours larger than 5 cm in diameter, but the researchers also pointed out that the “Wait and See” strategy for stage III NB and large tumours should be carried out cautiously[7].
Some scholars believe that spontaneous regression of NB is not necessarily confined to stage IVS and may occur at any stage of the disease[15]. However, the literature published from October 1946 to September 2019 was searched through PubMed for studies on stage III NB spontaneous regression, and only three studies were found to have reported cases of stage III NB spontaneous regression (Table 1). In 1995, Iwata et al[5] reported a tumour size of approximately 55 mm × 38 mm × 36 mm in the case of a 6-mo-old male child with NB. In a patient with upper respiratory tract infections awaiting surgical treatment, re-examination showed that the tumour shrank by approximately 50% when the patient was re-hospitalized for surgery, but the patient finally underwent surgical treatment and complete resection. NB was diagnosed postoperatively, and no further treatment was given. A 3-year follow-up showed that the patient survived well without recurrence or metastasis. Hero et al[6] studied a total of 340 NB patients from 1995 to 2004, including 70 patients with stage III NB. The researchers completed follow-up for up to 10 years and found that 44 of all patients (including those with stages I, II, III, IV, and IVS) had spontaneous regression, including 11 cases of stage III NB. Fawzy et al[7] reported a total of 32 NB children aged less than 1.5 years from 2007 to 2016, among whom four were stage III patients, three were treated with chemotherapy intervention, and only one had spontaneous regression, with good follow-up survival. However, the authors did not specify the specific follow-up time of this patient.
Generally, spontaneous regression of stage III NB is extremely rare. At present, the mainstream view of treatment is still based on the combination of chemotherapy and surgery. Treatment requires that primary tumours be removed and metastatic lymph nodes be cleared on the premise of patient safety. If the tumours cannot be completely removed because they surround important vessels or organs, partial excision of the tumours is feasible during the operation. Postoperative radiotherapy or chemotherapy can be used to further treat residual tumours[21].
This paper reports that the tumour gradually shrank during the follow-up period. As of the date of publication, the patient has been followed for 4 years. No tumour recurrence or metastasis has been found, and the child has good survival. We realize that although NB spontaneous regression is more common in stage IVS, it may still occur in other stages of the disease. If there are obvious signs of regression in the course of diagnosis and treatment, the tumour can also be temporarily observed and treated, but regular follow-up is required. If there are signs of tumour enlargement or other metastasis, observation and waiting for treatment should be stopped in time. In conclusion, stage III spontaneous regression of NB is rare in the clinic. The report of this case is an important supplement to the study of the spontaneous regression of NB.
We are grateful to our radiology department for their help with the radiological parameter’s measurement.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Munoz M, Kupeli S S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Zhi Y, Lu H, Duan Y, Sun W, Guan G, Dong Q, Yang C. Involvement of the nuclear factor-κB signaling pathway in the regulation of CXC chemokine receptor-4 expression in neuroblastoma cells induced by tumor necrosis factor-α. Int J Mol Med. 2015;35:349-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Louis CU, Shohet JM. Neuroblastoma: molecular pathogenesis and therapy. Annu Rev Med. 2015;66:49-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 3. | Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1600] [Cited by in RCA: 1621] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 4. | Nakagawara A, Li Y, Izumi H, Muramori K, Inada H, Nishi M. Neuroblastoma. Jpn J Clin Oncol. 2018;48:214-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 5. | Iwata M, Koshinaga T, Okabe I, Kurosu Y, Esumi M. Biological characteristics of neuroblastoma with spontaneous tumor reduction: a case report. J Pediatr Surg. 1995;30:722-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Hero B, Simon T, Spitz R, Ernestus K, Gnekow AK, Scheel-Walter HG, Schwabe D, Schilling FH, Benz-Bohm G, Berthold F. Localized infant neuroblastomas often show spontaneous regression: results of the prospective trials NB95-S and NB97. J Clin Oncol. 2008;26:1504-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 7. | Fawzy M, El Zomor H, El Menawi S, Elkinaai N, Ahmed G, Refaat A, Elahmadawy M, Zaghloul M. Watch and See Strategy in Selected Neuroblastoma Case Scenarios: Success and Limitations. J Pediatr Hematol Oncol. 2019;41:e384-e387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Zhang G, Zhou XJ, Zhu CZ, Dong Q, Su L. Usefulness of three-dimensional(3D) simulation software in hepatectomy for pediatric hepatoblastoma. Surg Oncol. 2016;25:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1595] [Cited by in RCA: 1706] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 10. | Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Nakagawara A, Brodeur GM. Role of neurotrophins and their receptors in human neuroblastomas: a primary culture study. Eur J Cancer. 1997;33:2050-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Zhu Y, Li Y, Haraguchi S, Yu M, Ohira M, Ozaki T, Nakagawa A, Ushijima T, Isogai E, Koseki H, Nakamura Y, Kong C, Mehlen P, Arakawa H, Nakagawara A. Dependence receptor UNC5D mediates nerve growth factor depletion-induced neuroblastoma regression. J Clin Invest. 2013;123:2935-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Vanichapol T, Chutipongtanate S, Anurathapan U, Hongeng S. Immune Escape Mechanisms and Future Prospects for Immunotherapy in Neuroblastoma. Biomed Res Int. 2018;2018:1812535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Decock A, Ongenaert M, De Wilde B, Brichard B, Noguera R, Speleman F, Vandesompele J. Stage 4S neuroblastoma tumors show a characteristic DNA methylation portrait. Epigenetics. 2016;11:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Brodeur GM. Spontaneous regression of neuroblastoma. Cell Tissue Res. 2018;372:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 16. | Evans AE, D'Angio GJ, Randolph J. A proposed staging for children with neuroblastoma. Children's cancer study group A. Cancer. 1971;27:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Twist CJ, Naranjo A, Schmidt ML, Tenney SC, Cohn SL, Meany HJ, Mattei P, Adkins ES, Shimada H, London WB, Park JR, Matthay KK, Maris JM. Defining Risk Factors for Chemotherapeutic Intervention in Infants With Stage 4S Neuroblastoma: A Report From Children's Oncology Group Study ANBL0531. J Clin Oncol. 2019;37:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Cozzi DA, Mele E, Ceccanti S, Natale F, Clerico A, Schiavetti A, Dominici C. Long-term follow-up of the "wait and see" approach to localized perinatal adrenal neuroblastoma. World J Surg. 2013;37:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Yoneda A, Oue T, Imura K, Inoue M, Yagi K, Kawa K, Nishikawa M, Morimoto S, Nakayama M. Observation of untreated patients with neuroblastoma detected by mass screening: a "wait and see" pilot study. Med Pediatr Oncol. 2001;36:160-162. [PubMed] [DOI] [Full Text] |
| 20. | Fritsch P, Kerbl R, Lackner H, Urban C. "Wait and see" strategy in localized neuroblastoma in infants: an option not only for cases detected by mass screening. Pediatr Blood Cancer. 2004;43:679-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Adkins ES, Sawin R, Gerbing RB, London WB, Matthay KK, Haase GM. Efficacy of complete resection for high-risk neuroblastoma: a Children's Cancer Group study. J Pediatr Surg. 2004;39:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |