Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.377
Peer-review started: November 19, 2019
First decision: November 21, 2019
Revised: November 22, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: January 26, 2020
Processing time: 59 Days and 0.6 Hours
Spinal metastasis of hepatocellular carcinoma (HCC) is rare, with an extremely poor prognosis and results in severe pain. Argon-helium cryotherapy is a local ablation method for HCC.
A 54-year-old man was diagnosed with HCC related to hepatitis B one year ago and underwent surgical tumor resection and tenofovir antiviral treatment. However, a new lesion developed on the right liver after 1 mo. Transarterial chemoembolization was performed four times. One month ago, the patient developed back pain, and metastasis on the 11th thoracic vertebra was detected. Argon-helium cryoablation was performed to treat the right occupancy and metastatic lesion, which immediately alleviated the pain and prolonged survival.
The use of argon-helium cryoablation for thoracic vertebrae with metastasis of HCC achieved favorable results.
Core tip: A 54-year-old man was diagnosed with hepatocellular carcinoma related to hepatitis B one year ago and underwent surgical tumor resection and tenofovir antiviral treatment. However, a new lesion developed on the right liver after 1 mo. Transarterial chemoembolization was performed four times. One month ago, the patient developed back pain, and metastasis on the 11th thoracic vertebra was detected. Argon-helium cryoablation was performed to treat the right occupancy and metastatic lesion, which immediately alleviated the pain and prolonged survival.
- Citation: Tan YW, Ye Y, Sun L. Argon-helium cryoablation for thoracic vertebrae with metastasis of hepatocellular carcinoma-related hepatitis B: A case report. World J Clin Cases 2020; 8(2): 377-381
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/377.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.377
In China, there are still 70 million patients with chronic hepatitis B infection[1], which is the main cause of 80% of hepatocellular carcinoma (HCC) cases; furthermore, > 70% of HCC cases will lose the chance of curative treatment with physicians’ diagnosis[2]. HCC can metastasize to other organs or tissues through blood, lymph, and direct dissemination and local diffusion. Of these cases, 90% metastasized to the lung, and bone metastases of HCC were also common, with an incidence ranging from 3% to 16.2%[3,4]. Common sites of bone metastasis are the vertebrae, ribs, and sternum. Spinal metastasis of HCC is rare, with an incidence of 0.2%–2.2%. HCC with bone metastasis has an extremely poor prognosis with a median survival of 1-2 mo, and results in severe pain[5]. Argon-helium cryotherapy is a local ablation method for HCC[6]. We recently used this method to treat a patient with advanced HCC metastasis to the thoracic spine and achieved good clinical efficacy.
A 54-year-old man was admitted to our hospital complaining of persistent back pain for 1 mo and with a 1-year history of HCC.
One year ago, the patient was found to have a 43 mm × 35 mm occupancy lesion in the left lateral lobe of the liver by enhanced CT. Considering the possibility of HCC which was confirmed by pathology, the patient underwent resection. After 1 mo, the patient was found to have an occupancy lesion in the right lateral lobe of the liver. Transarterial chemoembolization (TACE) was performed four times, and radiotherapy was conducted twice successively. The patient had back pain for 1 mo. CT reexamination showed that the space-occupying lesion in the soft tissue around the left 11th thoracic vertebra was involved in the bone metastases of HCC. Sorafenib was administered for 4 mo.
The patient had a history of chronic hepatitis B for > 10 years, but did not receive antiviral treatment. One year ago, oral tenofovir was administered after the liver occupancy lesion was found. HBV DNA level was lower than the lower limit of monitoring value (< 20 IU/mL).
There was no family aggregation of hepatitis B, no history of blood transfusion and blood product use, no alcohol abuse, and no recent history of hepatotoxic drug use.
Upon admission, temperature was 36.5°C, and blood pressure was 130/80 mmHg. The skin was dark and gloomy, there was no yellow staining of the skin and sclera, and the patient had liver palms. Several spider nevi were noted in the neck and chest, the abdomen was flat, the abdominal wall veins were exposed, and a vertical surgical scar was observed in the middle and right lower abdomen. The abdomen was soft without tenderness and rebound pain. There was no percussion pain in the liver area. Mobility dullness was negative, and bowel sounds were normal. Leg edema was not observed.
Routine blood test results were as follows: Hemoglobin level, 145.00 g/L; red blood cell count, 4.85 × 1012/L; white blood cell count, 2.38 × 109/L; and platelet count, 78.00 × 109/L. Biochemical test results were as follows: Total bilirubin, 12.4 μmol/L; direct bilirubin, 6.1 μmol/L; total protein, 76.8 g/L; prealbumin, 175.6 mg/L; alanine aminotransferase, 29 U/L; aspartate aminotransferase, 31 U/L; cholesterol, 3.10 mmol/L; glomerular filtration rate, 104.30 mL/min/L; glucose, 5.71 mmol/L; urea, 4.47 mmol/L; creatinine, 66.2 μmol/L; uric acid, 377.1 μmol/L; creatine kinase isoenzyme, 31 U/L; C-reactive protein, 3.01 mg/L; and alpha fetoprotein, 32.59 ng/mL.
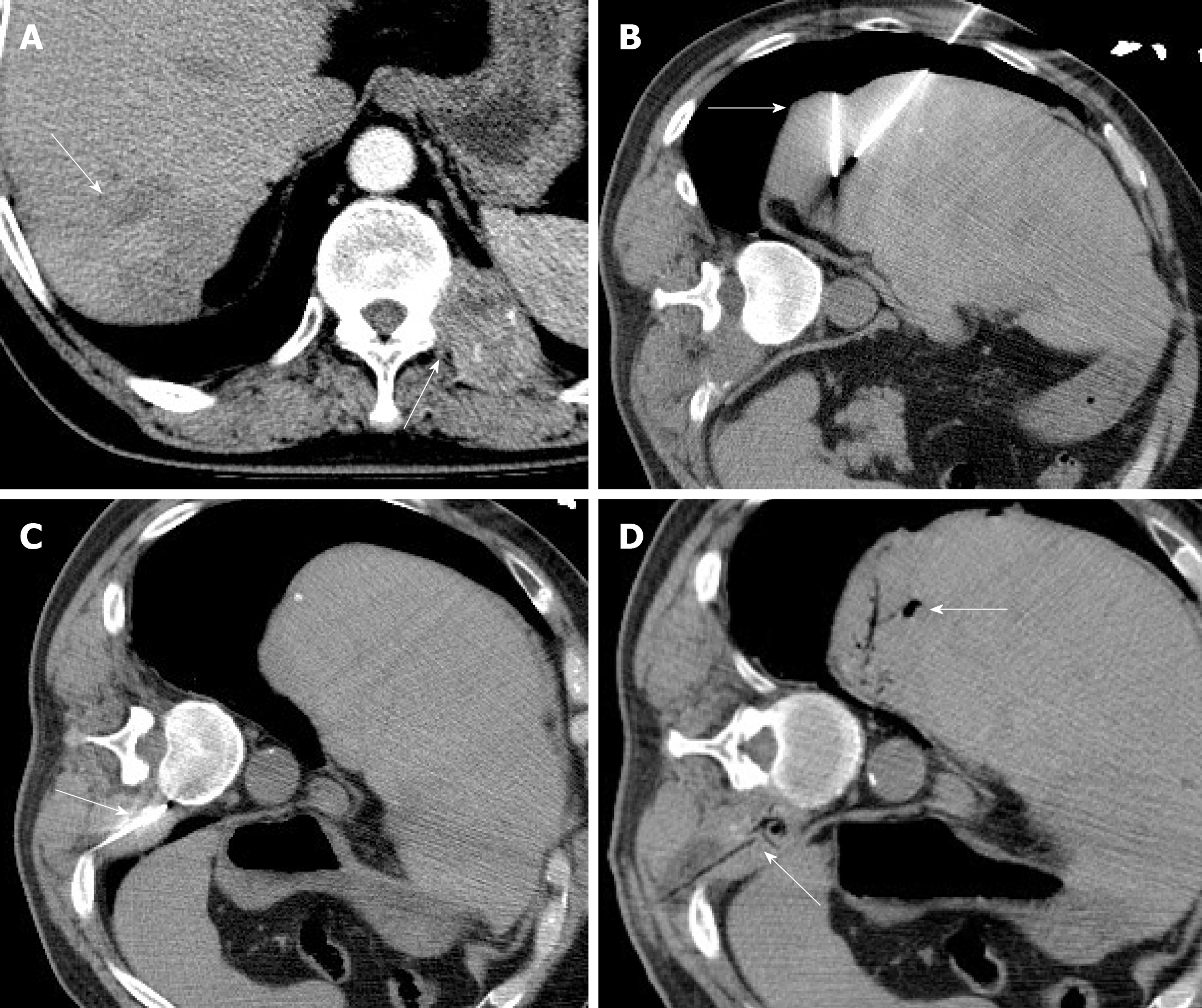
On contrast-enhanced CT scan, the local liver parenchyma in the right posterior lobe was displaced inward. The right posterior lobe of the liver exhibited a low-density patchy shadow with a CT value of approximately 40 HU. The density was not uniform, and the boundary was unclear. On the right posterior lobe of the liver, multiple low-density round shadows of different sizes were noted. A few spots of lipiodol were scattered in the shadow. On the left posterior rib 11, the left transverse process of the 11th thoracic vertebra, vertebral arch plate, and bone structure were damaged, and a soft tissue density shadow was noted (Figure 1A).
The patient was diagnosed with HCC after hepatectomy and TACE with 11th rib and 11th thoracic vertebra metastases on the left side.
Preoperatively, the patient received a full explanation of the disease condition and operative plan, and provided written informed consent. Under the guidance of CT, the right liver cancer with 11 thoracic vertebrae metastasis was treated with targeted cryoablation. Two cryoablations were performed on the right hepatic lobe, and one cryoablation on the left 11th lumbar vertebra (Figures 1B and C). Frozen necrosis gas and low-density hypovascular necrotic tissue were noted in the right posterior lobe and around the 11th lumbar spine (Figure 1D).
Postoperatively, back pain immediately improved and was completely relieved. The patient developed fever on the second postoperative day, the highest temperature being 38.8°C. His high temperature decreased to 38.5°C on the third day, was normal on the fourth day and the patient was then discharged.
Surgical intervention, including tumor resection and liver transplantation, is currently the first-line treatment for HCC[7,8], but the indications for operation and liver donor source limit the curative effect of HCC. Local ablation is an effective method for obtaining a curative effect for HCC[9]. The local ablation methods for HCC mainly include radiofrequency ablation (RFA), microwave ablation, and cryotherapy[10]. RFA is the representative minimally invasive treatment for HCC[11].
The argon-helium cryopreservation system is a minimally invasive treatment method that uses the rapid freezing function of argon to inactivate necrosis of tumor tissue. It originated more than a decade ago[12], but its popularity may be a relative “silent player” due to the limited access to argon and helium. Recently, it has attracted increasing attention as some centers used this technology to treat HCC and obtained favorable results. Wang et al[13] compared this with RFA in a multicenter randomized controlled trial. They found that argon-helium cryoablation had a better effect on local tumor progression than RFA (1-, 2-, and 3-year local tumor progression rates were 3%, 7% and 7%, respectively, for cryoablation and were 9%, 11%, and 11%, respectively, for RFA). The 3- and 5-year survival rates were similar (54% and 35% vs 50% and 34%), and the incidence of major complications was 3.9% and 3.3%, respectively.
Postoperative recurrence of HCC is the main factor in poor prognosis. It is reported that the annual recurrence rate ranges from 51.4% to 61.5% after radical resection of HCC, 43.6% for small HCC (< 5 cm), and higher for local treatment.
In the advanced stage of HCC, multiple organ metastases easily develop. The metastasis pathway can be divided into hematogenous metastasis, lymphatic metastasis, and implant metastasis. Bone metastasis is the most common. Research shows that the incidence of metastasis is approximately 10%. The main sites of bone metastasis are the spine, pelvis, ribs, and long bones, and a few cases showed skull metastasis. The 1-year survival rate of liver cancer with bone metastasis is only approximately 20%.
Patients with HCC and bone metastasis often have severe bone pain and inadequate effective treatment. Our patient underwent surgical treatment, TACE, and radiotherapy, but there was no improvement in pain symptoms. The use of cryoablation achieved favorable results.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Slomiany BL S-Editor: Gong ZM L-Editor: Webster JR E-Editor: Qi LL
| 1. | Chen M, Therneau T, Orsini LS, Qiao YL. Design and rationale of the HCC BRIDGE study in China: a longitudinal, multicenter cohort trial in hepatocellular carcinoma. BMC Gastroenterol. 2011;11:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 3. | Attili VS, Babu KG, Lokanatha D, Bapsy PP, Ramachandra C, Rajshekar H. Bone metastasis in hepatocellular carcinoma: need for reappraisal of treatment. J Cancer Res Ther. 2008;4:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Doval DC, Bhatia K, Vaid AK, Prabhash K, Jena A, Hazarika D. Bone metastases from primary hepatocellular carcinoma simulating multiple myeloma. Hepatobiliary Pancreat Dis Int. 2005;4:308-310. [PubMed] |
| 5. | He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, Wang JH, Sun J, Chen B, Yang P, Pan BS. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115:2710-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Li M, Cui Y, Li X, Guo Y, Wang B, Zhang J, Xu J, Han S, Shi X. Functional Changes of Dendritic Cells in C6 Glioma-Bearing Rats That Underwent Combined Argon-Helium Cryotherapy and IL-12 Treatment. Technol Cancer Res Treat. 2016;15:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6056] [Article Influence: 865.1] [Reference Citation Analysis (3)] |
| 8. | Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, Bagante F, Turcato G, D'Onofrio M, Iacono C. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16:301-11; discussion 311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Mahnken AH, Bruners P, Günther RW. Local ablative therapies in HCC: percutaneous ethanol injection and radiofrequency ablation. Dig Dis. 2009;27:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Jin Q, Chen X, Zheng S. The Security Rating on Local Ablation and Interventional Therapy for Hepatocellular Carcinoma (HCC) and the Comparison among Multiple Anesthesia Methods. Anal Cell Pathol (Amst). 2019;2019:2965173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Curley SA. Radiofrequency ablation leads to excellent local tumor control and durable longterm survival in specific subsets of early stage HCC patients confirming to the Milan criteria. Ann Surg. 2010;252:913-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Lee F, Bahn DK, McHugh TA, Onik GM, Lee FT. US-guided percutaneous cryoablation of prostate cancer. Radiology. 1994;192:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, Bai W, Dong Z, Lu Y, Zeng Z, Lou M, Wang H, Gao X, Chang X, An L, Qu J, Li J, Yang Y. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |