Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.331
Peer-review started: November 6, 2019
First decision: December 4, 2019
Revised: December 5, 2019
Accepted: December 22, 2019
Article in press: December 22, 2019
Published online: January 26, 2020
Processing time: 71 Days and 23.7 Hours
Oral-facial-digital syndrome type 1 (OFD1) is a rare ciliopathy mainly with an X-linked dominant pattern of inheritance, which is caused by mutations in the OFD1 gene. The OFD1 protein is located within the centrosomes and basal bodies of the primary cilia. It is reported that approximately 15%–50% cases of OFD1 progress to end-stage renal disease (ESRD) following development of polycystic kidney diseases (PKD). Here we report a pair of childhood male twins who presented only renal failure and PKD caused by an OFD1 mutation in China.
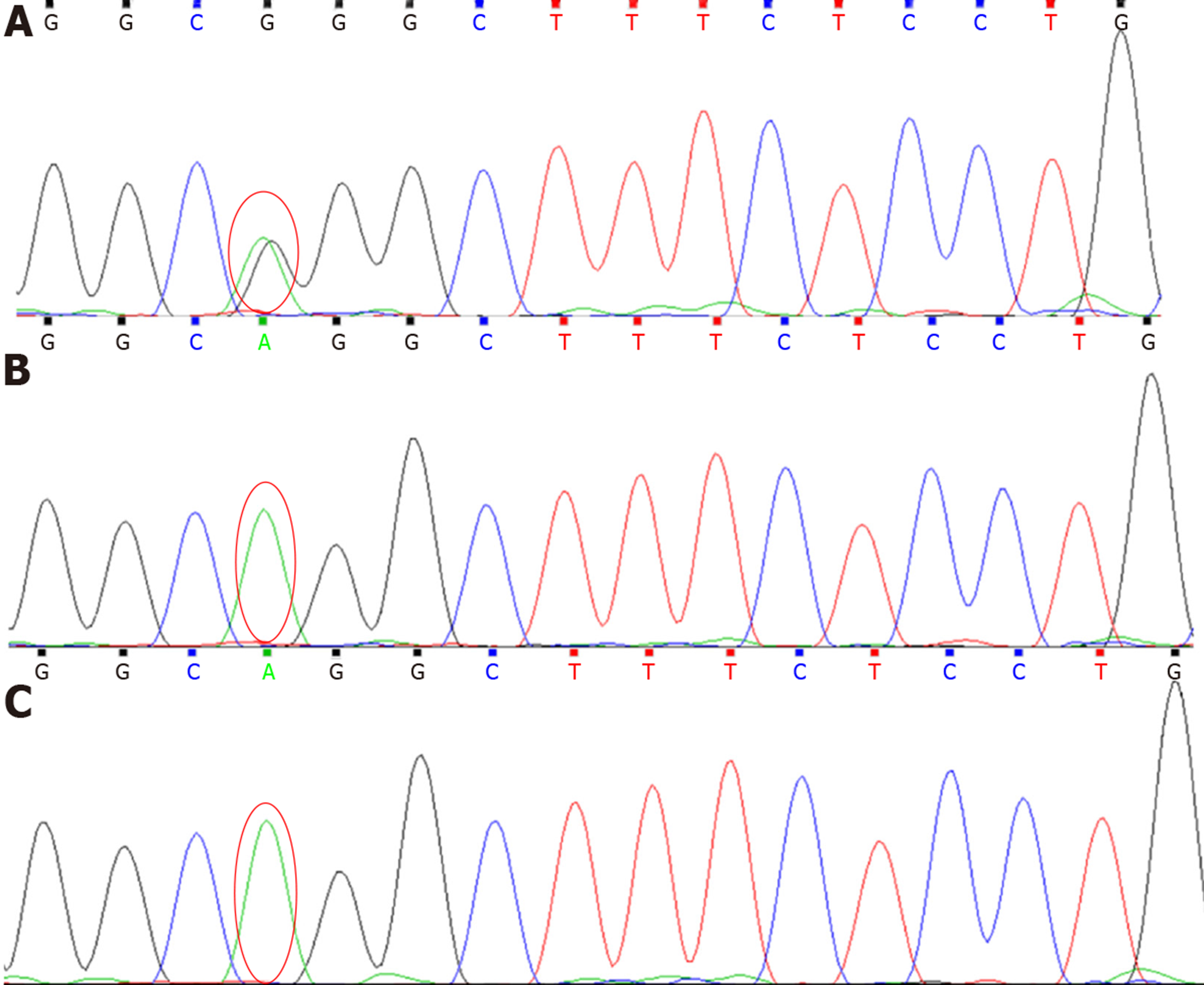
A pair of 14-year male twins were hospitalized with a complaint of abnormal renal function for nine days. They both complained of ankle pain for 3 mo vs 2 wk, respectively. They denied fever, abdominal pain, daytime or nighttime enuresis, urgency, dysuria, or gross hematuria. Laboratory tests at a local hospital showed renal failure (serum creatinine 485 μmol/L vs 442 μmol/L, blood urea nitrogen 14.7 mol/L vs 14.5 mol/L) and anemia (hemoglobin 88 g/L vs 98 g/L). The twins are monozygotic. There was no abnormal birth, past medical, or family history. Clinical data were analyzed and genetic analysis on PKD was carried out in the twins by next-generation sequencing. The results showed that the twins presented low-molecular-weight proteinuria, hyposthenuria, anemia, renal failure, and renal polycystic changes. Genetic tests showed that the twins both carried a hemizygous mutation in exon 19 c.2524G>A (p. G842R) of the OFD1 gene. Their mother heterozygously carried the same mutation as the twins but was without any phenotypes while their father was normal.
We have reported a pair of childhood male twins with an OFD1 mutation who presented ESRD and PKD but without any other phenotypes of OFD1 in China.
Core tip: Oral-facial-digital syndrome type 1 (OFD1) is a rare ciliopathy mainly with an X-linked dominant pattern of inheritance, which is caused by mutations in the OFD1 gene. It is reported that approximately 15%–50% cases of OFD1 progress to end-stage renal disease following development of polycystic kidney diseases. The phenotypic spectrum associated with OFD1 mutations has been recently extended with an X-linked recessive pattern of inheritance. Here we report a pair of childhood male twins who presented only renal failure and polycystic kidney disease caused by an OFD1 mutation with an X-linked recessive fashion of inheritance in China.
- Citation: Zhang HW, Su BG, Yao Y. OFD1 mutation induced renal failure and polycystic kidney disease in a pair of childhood male twins in China. World J Clin Cases 2020; 8(2): 331-336
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/331.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.331
The human OFD1 gene is composed of 23 densely packed exons spanning about 35.59 kb of genomic DNA and maps to chromosome band Xp22.2. The OFD1 protein is located within the centrosomes and basal bodies of the primary cilia. Oral-facial-digital syndrome type 1 (OFD1) is caused by OFD1 mutations with an X-linked dominant pattern of inheritance[1,2]. It is reported that approximately 15%–50% cases of OFD1 progress to end-stage renal disease (ESRD) following the development of polycystic kidney diseases (PKD[3]. The phenotypic spectrum associated with OFD1 mutations has been recently extended with an X-linked recessive pattern of inheritance, such as Joubert syndrome type 10, mental retardation with macrocephaly, Simpson-Golabi-Behmel syndrome type 2, retinitis pigmentosa and so on[4-6].
There has been a report on OFD1 mutation with an atypical presentation with ESRD without evidence of PKD[7]. However, there has been no report on ESRD without any other phenotypes except PKD caused by an OFD1 mutation. Here, we report a pair of childhood male twins with an OFD1 mutation who presented ESRD and PKD but without any other phenotypes.
A pair of 14-year male twins were hospitalized with a complaint of abnormal renal function for nine days.
They both complained of ankle pain for 3 mo vs 2 wk, respectively. They denied fever, abdominal pain, daytime or nighttime enuresis, urgency, dysuria, or hematuria. Laboratory tests at a local hospital showed renal failure (serum creatinine 485 μmol/L vs 442 μmol/L, blood urea nitrogen 14.7 mol/L vs 14.5 mol/L) and anemia (hemoglobin 88 g/L vs 98 g/L).
The twins are monozygotic. There was no abnormal birth, past medical, or family history. There was no family history of renal failure or PKD on their maternal side.
At admission, their blood pressure, weight, and height were all normal. Their physical examination showed no abnormal signs, especially no abnormal dysmorphic features. No neurodevelopmental or ophthalmologic deficits were observed.
Laboratory tests at our hospital revealed Scr 433-486 μmol/L vs 382-425 μmol/L, BUN 20.2-24.4 mmol/L vs 17.1-19.9 mmol/L, serum calcium 1.39-1.80 mmol/L vs 1.62-1.80 mmol/L, serum phosphate 1.42-1.72 mmol/L vs 1.43-1.80 mmol/L, HCO3- 23.6-26.1 mmol/L vs 23.0-26.3 mmol/L, intact parathyroid hormone 248.7-327.7 pg/mL vs 256.2-298.5 pg/mL, hemoglobin 92-101 g/L vs 97-110 g/L, proteinuria 27.4 mg/kg per 24 h vs 25.3 mg/kg per 24 h, urine specific gravity 1.008-1.010 vs 1.007-1.010, urine microalbumin 291 mg/L vs 128 mg/L, urine α1-microglobin 194 mg/L vs 180 mg/L, and low-molecular-weight proteinuria 35.7% vs 42.1% in urine protein electrophoresis. Autoimmune profile was within the normal range, including anti-nuclear antibody, anti-double-stranded DNA antibody, complements C3 and C4, and anti-neutrophil cytoplasmic antibodies (Table 1).
| Feature | Patient 1 | Patient 2 |
| Familial history | - | - |
| Short stature | - | - |
| Frontal bossing | - | - |
| Flat face | - | - |
| Hypertelorism | - | - |
| Epicanthus | - | - |
| Telecanthus | - | - |
| Down-slanting palpebral fissures | - | - |
| Hypoplasia of the nasal alae | - | - |
| Low set ears | - | - |
| Pseudocleft of the upper lip | - | - |
| Cleft lip/tongue/palate | - | - |
| Microretrognathia | - | - |
| Abnormal hairs | - | - |
| Lobulated tongue | - | - |
| Buccal frenulae | - | - |
| Lingual hamartomas | - | - |
| Tooth abnormalities | - | - |
| Cerebellar hypoplasia | - | - |
| Corpus callosum agenesis | - | - |
| Hydrocephalus | - | - |
| Porencephaly | - | - |
| Arachnoid cysts | - | - |
| Hands/Feet | ||
| Brachydactyly | - | - |
| Clinodactyly | - | - |
| Syndactyly | - | - |
| Polydactyly | - | - |
| Polycystic kidney disease | + | + |
| Renal function abnormal | + | + |
| Scr (μmol/L) | 433-589 | 382-564 |
| BUN (mmol/L) | 20.2-28.2 | 17.1-26.6 |
| Proteinuria | + | + |
| Microalbuminuria (mg/L) | 291 | 128 |
| α1-microglobinuria (mg/L) | 194 | 180 |
| Low molecular weight proteinuria (%) | 35.7 | 42.1 |
| Hematuria | - | - |
| Mental retardation | - | - |
Ultrasonography and magnetic resonance imaging (MRI) showed that the renal body was small (7.3-7.5 cm vs 7.2-7.6 cm in length and 3.7-3.8 cm vs 3.5-3.8 cm in width), the border of the cortex and medulla was not clear, polycystic lesions were seen in both kidneys of the twins, and cysts were irregularly distributed and located within the cortex and the medulla. No cysts were found in the liver or pancreas. Brain MRI showed no abnormal findings.
Due to the presence of abnormal renal function, polycystic kidney diseases, and genetic history, an inherited renal cystic disease was suspected.
Genetic analysis was performed using next generation sequencing[8] in the genetics laboratories of MyGenostics biotechnology companies in China, using “the polycystic kidney diseases panel” which covers genes strongly correlated with this disorder[9,10]. The results showed that both the twins carried a hemizygous mutation in exon 19 c.2524G>A (p. G842R) in the OFD1 gene (Figure 1). Their mother heterozygously carried the same mutation as the twins. But the mother showed no anemia, proteinuria, or hematuria, and her renal function was normal. Neither polycystic lesions nor abnormal dysmorphic features were seen in her kidneys. X-inactivation was not analyzed in the mother. The mutation was found in the SNP databases (rs146047094, A = 0.0011/4, 0.007/65, and 0.006/6 in 1000Genomes, ExAC, and GO-ESP, respectively), but only found in females (18 homozygous and 29 heterozygous in ExAC, 2 heterozygous in 1000Genomes). Mutation testing analysis showed that amino acid sequence was changed and protein features (might be) affected. PolyPhen-2 and SIFT analysis showed that the mutation might be healthy or tolerated. However, it was not found in 100 normal Chinese controls. No variations were found in PKD1, PKD2, PKHD1, NPHPn (n = 1-18, 1 L and 2 L), or other related genes.
Within a follow-up period of 12 mo, renal functions of the twins continued to decline rapidly (Scr 589 μmol/L vs 564 μmol/L, BUN 28.2 mol/L vs 26.6 mol/L). Due to symptomatic uremia, they started on peritoneal dialysis and waited for transplant from appropriate living donors.
OFD1 is a rare ciliopathy with an X-linked dominant pattern of inheritance which involves multiple organs including the kidneys, tongue, nasal mucosa, oral mucosa, cranial cartilage, brain, and limbs[11]. The incidence of OFD1 is 1/50000-1/250000 live births[12,13]. PKD is the most common renal involvement[14]. There is also a report of OFD1 mutation in a Chinese boy with Joubert syndrome[15]. However, atypical presentation of OFD1 and ESRD caused by OFD1 mutations without typical polycystic changes were also reported[7].
As for our twins, they both presented with low-molecular-weight proteinuria, hyposthenuria, anemia, renal failure, and renal polycystic changes, suggesting that they had an inherited renal cystic disease. Genetic tests showed that the twins both carried a hemizygous mutation in exon 19 c.2524G>A (p.G842R) of the OFD1 gene. The mutation was found in the SNP databases (ExAC, GO-ESP, and 1000Genomes) but only homozygous or heterozygous in females but not in males. Mutation testing analysis showed changed amino acid sequence and affected protein features (might be), and SIFT and PolyPhen-2 analysis showed that the mutation might be healthy or tolerated. Because no variations were found in PKD1, PKD2, PKHD1, NPHPn, or other related genes in our twins, and the mutation was not found in 100 normal controls in China, we suggested that it might be the genetic cause of our twins with an X-linked recessive pattern of inheritance. The mother heterozygously carried the same mutation as the twins but had no anemia, proteinuria, hematuria, abnormal dysmorphic features and her renal function was normal. Furthermore, our twins presented no malformation of the hands, feet, face, or oral cavity. And no CNS involvement was found. To our knowledge, these are the first two pediatric patients with an OFD1 mutation that presented with renal failure and PKD with an X-linked recessive pattern of inheritance, without classic oral, facial, or digital features. However, it was a pity that we did not analyze fibroblasts from our twins to verify whether the p.G842R mutation affects cilia formation. Xiang et al[16] reported a fetus with Joubert Syndrome terminated at 29+4 wk of gestation, in which genetic analysis showed the c.2524G>A (p.G842R) in the OFD1 gene, and two compound heterozygous variants in the C5orf42 gene (c.3599C>T, p.A1200V in exon 20 and c.3857G>A, p.R1286H in exon 22). They speculated that the later might be responsible for the disorder of the fetus because the 36-year-old normal father also carried the c.2524G>A (p.G842R) in the OFD1 gene. But it is a pity that there was no information on renal function and imaging examination of the father. The pathogenicity of c.2524G>A (p.G842R) in the OFD1 gene needs further studies especially in males in the future.
PKD affects approximately 15%–50% cases of OFD1 who have a higher likelihood of developing renal failure while those without PKD have normal renal function[1,11,14]. Cystic changes can occur at any time point and are distributed and located irregularly within the cortex and the medulla. They are variable in size, multilocular, and thin-walled and contain serous fluid[17,18]. These features are similar to those of our twins. The kidney size is normal or palpably large, but they maintain their reniform shape with minimal changes of renal contour[14]. However, it is interesting that the kidneys of our twins were small in size, which is similar to the report of Sharma et al[7], in which a boy aged 10 years and 6 mo with renal failure but without renal cystic changes had small kidneys (the right kidney 6.6 cm and the left kidney 7.8 cm) detected by renal ultrasound. Furthermore, it seems that the renal size can be small, normal, or large in PKD caused by OFD1 mutations, the same as other renal cystic ciliopathies[19-21].
There were no different features between our monozygotic twins with the same OFD1 mutation. As for renal manifestations, our twins both presented low-molecular-weight proteinuria, besides hyposthenuria, renal failure, and polycystic changes in the kidneys but without microscopic hematuria. This was contrary to other reports that PKD caused by OFD1 mutations mainly presented with microscopic hematuria but no proteinuria[7,11].
We present a pair of twins with an OFD1 mutation inherited in an X-linked recessive fashion. These are the first two childhood patients reported with this condition, who presented only renal failure and cystic kidneys but without classic oral, facial, or digital features of OFD1. Based on this unusual presentation, maybe the phenotypic spectrum of OFD1-associated disorders is broader than previously anticipate. We suggest that OFD1 screening should be considered, especially in males, if the suspicion for ciliopathy remains high in patients with renal failure and polycystic changes.
Manuscript source: Unsolicited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Taheri S, Yorioka N S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Ferrante MI, Giorgio G, Feather SA, Bulfone A, Wright V, Ghiani M, Selicorni A, Gammaro L, Scolari F, Woolf AS, Sylvie O, Bernard L, Malcolm S, Winter R, Ballabio A, Franco B. Identification of the gene for oral-facial-digital type I syndrome. Am J Hum Genet. 2001;68:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Chetty-John S, Piwnica-Worms K, Bryant J, Bernardini I, Fischer RE, Heller T, Gahl WA, Gunay-Aygun M. Fibrocystic disease of liver and pancreas; under-recognized features of the X-linked ciliopathy oral-facial-digital syndrome type 1 (OFD I). Am J Med Genet A. 2010;152A:2640-2645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dollé P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet. 2006;38:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 4. | Thauvin-Robinet C, Thomas S, Sinico M, Aral B, Burglen L, Gigot N, Dollfus H, Rossignol S, Raynaud M, Philippe C, Badens C, Touraine R, Gomes C, Franco B, Lopez E, Elkhartoufi N, Faivre L, Munnich A, Boddaert N, Van Maldergem L, Encha-Razavi F, Lyonnet S, Vekemans M, Escudier E, Attié-Bitach T. OFD1 mutations in males: phenotypic spectrum and ciliary basal body docking impairment. Clin Genet. 2013;84:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Wang J, Chen X, Wang F, Zhang J, Li P, Li Z, Xu J, Gao F, Jin C, Tian H, Zhang J, Li W, Lu L, Xu GT. OFD1, as a Ciliary Protein, Exhibits Neuroprotective Function in Photoreceptor Degeneration Models. PLoS One. 2016;11:e0155860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Franco B, Thauvin-Robinet C. Update on oral-facial-digital syndromes (OFDS). Cilia. 2016;5:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Sharma S, Kalish JM, Goldberg EM, Reynoso FJ, Pradhan M. An Atypical Presentation of a Male with Oral-Facial-Digital Syndrome Type 1 Related Ciliopathy. Case Rep Nephrol. 2016;2016:3181676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Zhang H, Su B, Liu X, Xiao H, Ding J, Yao Y. Mutations in TTC21B cause different phenotypes in two childhood cases in China. Nephrology (Carlton). 2018;23:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, Allen SJ, Soliman NA, Hildebrandt F, Otto EA; GPN Study Group. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet. 2013;132:865-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 10. | Schueler M, Halbritter J, Phelps IG, Braun DA, Otto EA, Porath JD, Gee HY, Shendure J, O'Roak BJ, Lawson JA, Nabhan MM, Soliman NA, Doherty D, Hildebrandt F. Large-scale targeted sequencing comparison highlights extreme genetic heterogeneity in nephronophthisis-related ciliopathies. J Med Genet. 2016;53:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Toprak O, Uzum A, Cirit M, Esi E, Inci A, Ersoy R, Tanrisev M, Ok E, Franco B. Oral-facial-digital syndrome type 1, Caroli's disease and cystic renal disease. Nephrol Dial Transplant. 2006;21:1705-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Feather SA, Woolf AS, Donnai D, Malcolm S, Winter RM. The oral-facial-digital syndrome type 1 (OFD1), a cause of polycystic kidney disease and associated malformations, maps to Xp22.2-Xp22.3. Hum Mol Genet. 1997;6:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Prattichizzo C, Macca M, Novelli V, Giorgio G, Barra A, Franco B; Oral-Facial-Digital Type I (OFDI) Collaborative Group. Mutational spectrum of the oral-facial-digital type I syndrome: a study on a large collection of patients. Hum Mutat. 2008;29:1237-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Saal S, Faivre L, Aral B, Gigot N, Toutain A, Van Maldergem L, Destree A, Maystadt I, Cosyns JP, Jouk PS, Loeys B, Chauveau D, Bieth E, Layet V, Mathieu M, Lespinasse J, Teebi A, Franco B, Gautier E, Binquet C, Masurel-Paulet A, Mousson C, Gouyon JB, Huet F, Thauvin-Robinet C. Renal insufficiency, a frequent complication with age in oral-facial-digital syndrome type I. Clin Genet. 2010;77:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Zhang K, Meng C, Ma J, Gao M, Lv Y, Liu Y, Gai Z. Novel OFD1 frameshift mutation in a Chinese boy with Joubert syndrome: a case report and literature review. Clin Dysmorphol. 2017;26:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Xiang J, Zhang L, Jiang W, Zhang Q, Wang T, Li H, Li H. Prenatal Diagnosis and Genetic Analysis of a Fetus with Joubert Syndrome. Biomed Res Int. 2018;2018:7202168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 17. | Curry NS, Milutinovic J, Grossnickle M, Munden M. Renal cystic disease associated with orofaciodigital syndrome. Urol Radiol. 1992;13:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Feather SA, Winyard PJ, Dodd S, Woolf AS. Oral-facial-digital syndrome type 1 is another dominant polycystic kidney disease: clinical, radiological and histopathological features of a new kindred. Nephrol Dial Transplant. 1997;12:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C:326-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Chung EM, Conran RM, Schroeder JW, Rohena-Quinquilla IR, Rooks VJ. From the radiologic pathology archives: pediatric polycystic kidney disease and other ciliopathies: radiologic-pathologic correlation. Radiographics. 2014;34:155-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Sugimoto K, Miyazawa T, Enya T, Nishi H, Miyazaki K, Okada M, Takemura T. Clinical and genetic characteristics of Japanese nephronophthisis patients. Clin Exp Nephrol. 2016;20:637-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |