Published online Jan 26, 2020. doi: 10.12998/wjcc.v8.i2.264
Peer-review started: November 11, 2019
First decision: November 22, 2019
Revised: December 15, 2019
Accepted: January 1, 2020
Article in press: January 1, 2020
Published online: January 26, 2020
Processing time: 67 Days and 0.4 Hours
Peutz-Jeghers syndrome (PJS) is an autosomal dominant inherited disease easily causing secondary malignant changes without effective treatments.
To assess the clinical characteristics, diagnosis, and treatment of malignant changes secondary to PJS.
The clinical data of five patients with malignant changes secondary to PJS diagnosed and treated at Beijing Friendship Hospital from June 2014 to January 2017 were retrospectively analyzed; the follow-up ended in May 2018.
There were three male and two female patients with an average age of 43.6 years. Intestinal obstruction, intussusception, and abdominal pain were the first symptoms. Computed tomography and gastrointestinal imaging combined with endoscopy helped evaluate the depth of tumor infiltration and determine the need for radical resection. Three patients underwent surgery. Postoperative pathology confirmed adenocarcinoma, genetic test indicated STK11 mutation, and the patients received chemotherapy, including one who succumbed to tumor progression 6 months post-surgery. Other two patients underwent endoscopic resection, and postoperative pathology confirmed high grade intraepithelial neoplasia. The surviving patients had no recurrence by May 2018.
Endoscopy combined with computed tomography and gastrointestinal imaging is of great significance in the diagnosis and treatment of PJS, and pathological examination and gene detection are the gold standards for detecting malignant changes secondary to PJS. Some malignant polyps can be removed under endoscopy, and surgery is feasible when malignant polyps cannot be removed under an endoscope. For patients unable to achieve R0 resection, clinical symptoms should be relieved, and postoperative adjuvant chemotherapy could improve long-term prognosis. Meanwhile, close and regular surveillance should be conducted to prevent severe complications.
Core tip: Peutz-Jeghers syndrome (PJS) is an autosomal dominant inherited disease easily causing secondary malignant changes without effective treatments. Pathological examination and gene detection are the gold standards for detecting malignant PJS polyps. Patients with PJS have higher malignant tumor susceptibility. Hence, we need to surveil regularly and take positive measures for removing Peutz-Jeghers polyps.
- Citation: Zheng Z, Xu R, Yin J, Cai J, Chen GY, Zhang J, Zhang ZT. Malignant tumors associated with Peutz-Jeghers syndrome: Five cases from a single surgical unit. World J Clin Cases 2020; 8(2): 264-275
- URL: https://www.wjgnet.com/2307-8960/full/v8/i2/264.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i2.264
Peutz-Jeghers syndrome (PJS) is an autosomal dominant inherited disorder characterized by multiple hamartoma polyps in the gastrointestinal tract and mucocutaneous pigmentation. PJS is caused by mutation of the serine/threonine kinase 11 (STK11) gene located on chromosome 19p13.3, with an incidence of about 1/29000-1/120000. Therefore, patients with PJS are at a high risk for malignant tumors[1]. Studies have reported[1,2] that PJS patients have a higher risk of malignant changes of digestive tract polyps and of malignant tumors outside the digestive tract compared with the normal population. The pathogenic location is more common in the colorectum, stomach, and small intestine, followed by the breast, reproductive system, pancreas, and lung[2]. In recent years, with the increasing disease awareness and the popularity of endoscopic screening, the detection and malignancy rates of PJS have considerably risen. Some scholars proposed that asymptomatic PJS patients could be observed dynamically without special treatment. However, others thought that even asymptomatic patients need active treatment to prevent malignancy and gastrointestinal emergency when gastrointestinal polyps are found. Therefore, there are still controversies regarding the diagnosis and treatment of PJS[3]. Even if patients with malignant PJS were actively treated, the prognosis would still be poor. Therefore, the present study aimed to analyze cases of malignant changes secondary to PJS treated at our hospital, summarizing the clinical characteristics of PJS and combined carcinogenesis, in order to guide clinical treatment and improve patient prognosis.
The present study retrospectively analyzed 46 cases of PJS patients, between June 2014 and January 2017, diagnosed by biopsy, endoscopic resection, and postoperative pathology. Among these patients, five cases from different pedigrees were diagnosed with malignant changes secondary to PJS. The inclusion criteria were: (1) Gastrointestinal polyps, with histology consistent with the characteristics of PJS, including smooth muscles from the muscularis mucosa extending like branches to the submucosal layer of polyps and becoming PJS polyps; (2) A family history of PJS in combination with PJS polyps; (3) A family history of PJS in combination with typical mucocutaneous pigmentation; and (4) Typical mucocutaneous pigmentation combined with PJS polyps. The subjects included three males and two females, with an average age of 43.6 years (range, 29-67 years). All patients were treated at the General Surgery Department of Beijing Friendship Hospital, Capital Medical University. Three of them received surgical treatment and two underwent endoscopic submucosal dissection (ESD) therapy. The patients were followed through outpatient service and telephone or letter after discharge, until May 2018 (Table 1). This study was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University.
| Number | Age | Diagnostic criteria | Diagnostic method1 | Clinical manifestation | Operation method | Postoperative pathology2 | Follow-up time |
| 1 | 42 | Pigmentation | Gastroscopy(+)4 gene detection(+)4 | Intussusception | Total gastrectomy | Stomach: Multiple PJS polyps3 and highly differentiated adenocarcinoma in gastric fundus and body | 25 mo |
| Gastrointestinal polyps | Colonoscopy(+)4 | Incomplete intestinal obstruction | Hepatic metastasis resection | Intestine: Multiple PJS polyps3 | |||
| Family history of PJS3 | Upper digestive tract radiography(+)4 | Abdominal pain | Small intestinal polyp resection | Liver: Infiltrating adenocarcinoma | |||
| CT(+)4 | Anti-acid, heartburn | Intussusception reduction | Gene detection: STK11 mutation | ||||
| T4bN3M1 | |||||||
| 2 | 29 | Gastrointestinal polyps | Gastroscopy(+)4 | Abdominal pain | Subtotal gastrectomy | Stomach: Multiple PJS polyps3, with most tumors developing into highly differentiated adenocarcinoma | 20 mo |
| Family history of PJS3 | Colonoscopy(+)4 | Abdominal distension | Resection of small intestinal polyps | Intestine: Multiple PJS polypsc | |||
| CT(+)4 | T2N0M0 | ||||||
| Upper digestive tract radiography(+)4 | |||||||
| 3 | 67 | Gastrointestinal polyps | Gastroscopy(+)4 | Intussusception | Gastrojejunostomy | Greater omentum: Infiltrating adenocarcinoma | The patient died of liver metastasis, abdominal lymph node metastasis, and cachexia 6 mo after surgery |
| Pigmentation | Colonoscopy(+)4 | Incomplete intestinal obstruction | Intussusception reduction | Meso-ileum: Infiltrating adenocarcinoma | |||
| Upper digestive tract radiography(+)d | Abdominal distension | Resection of small intestinal polyps | Abdominal wall nodule: Infiltrating adenocarcinoma | ||||
| CT(+)d | Nausea and vomit | Intestine: Adenocarcinoma infiltrating intestinal wall; PJSc polyps of intestine | |||||
| T4bNxM1 | |||||||
| 4 | 29 | Gastrointestinal polyps | Gastroscopy(+)4 | Abdominal pain | Colonic polyp ESD | PJS3 polyps, with some high-grade intraepithelial neoplasia and clean margins | 42 mo |
| Family history of PJS3 | Abdominal distension | T1aN0M0 | |||||
| 5 | 51 | Gastrointestinal polyps | Gastroscopy(+)4 | Incomplete intestinal obstruction | Colonic polyp ESD | Stomach: Fundic gland polyp | 47 mo |
| Family history of PJS3 | Colonoscopy(+)4 | Abdominal distension | Gastric polyp ESD | Intestine: PJS3 polyps, with some high-grade intraepithelial neoplasia and clean margins | |||
| MRI(-)5 | T1aN0M0 |
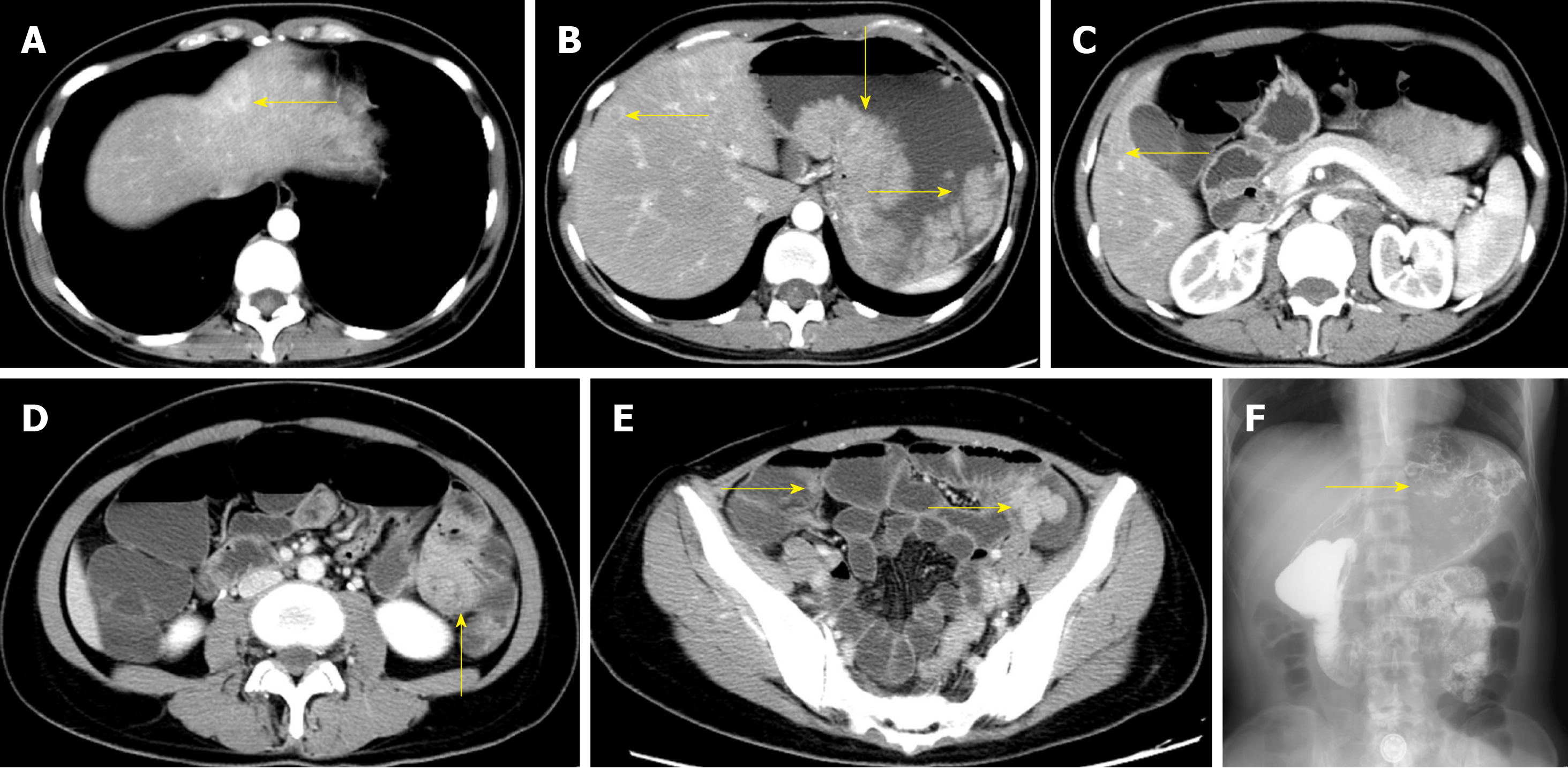
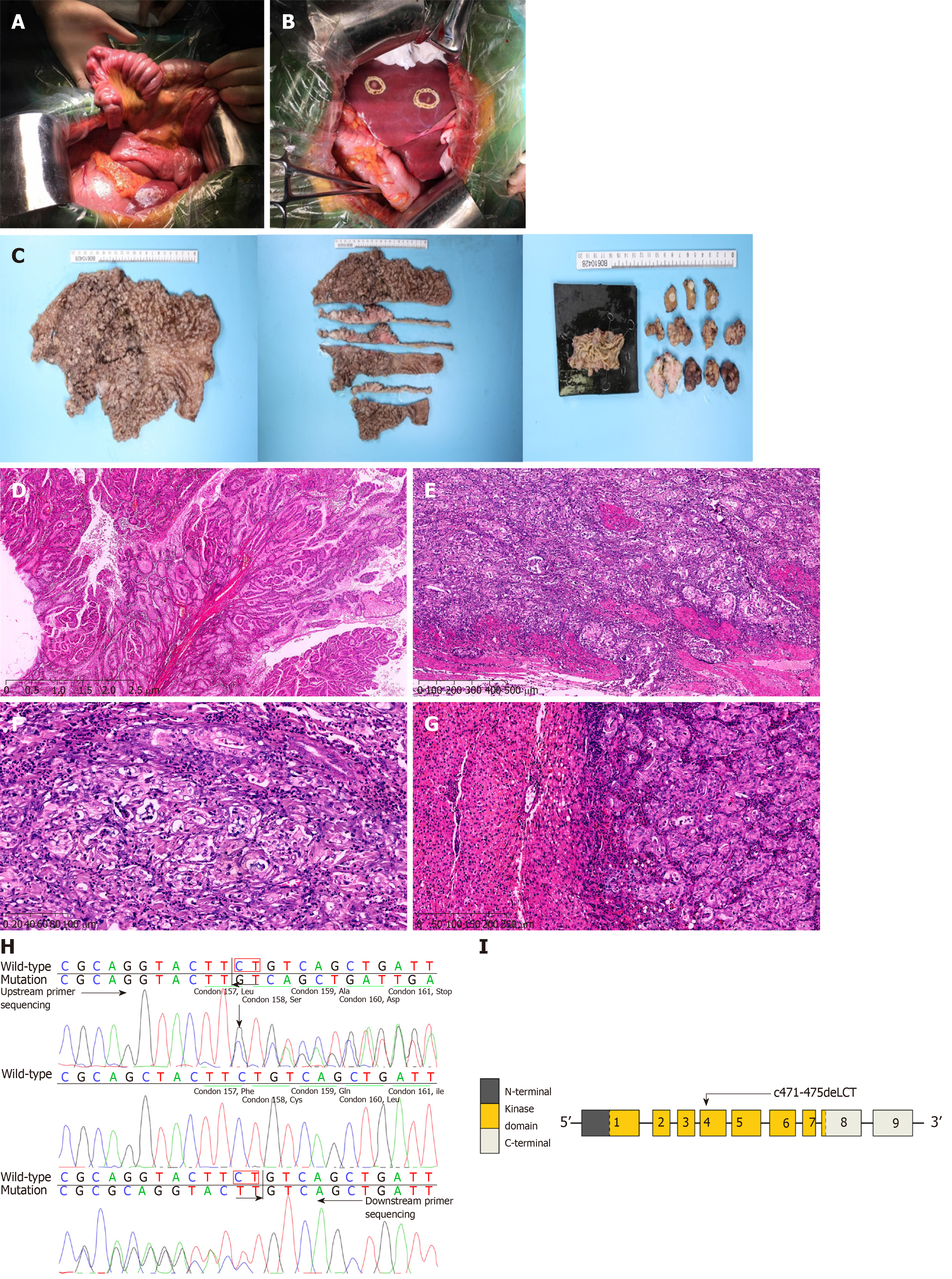
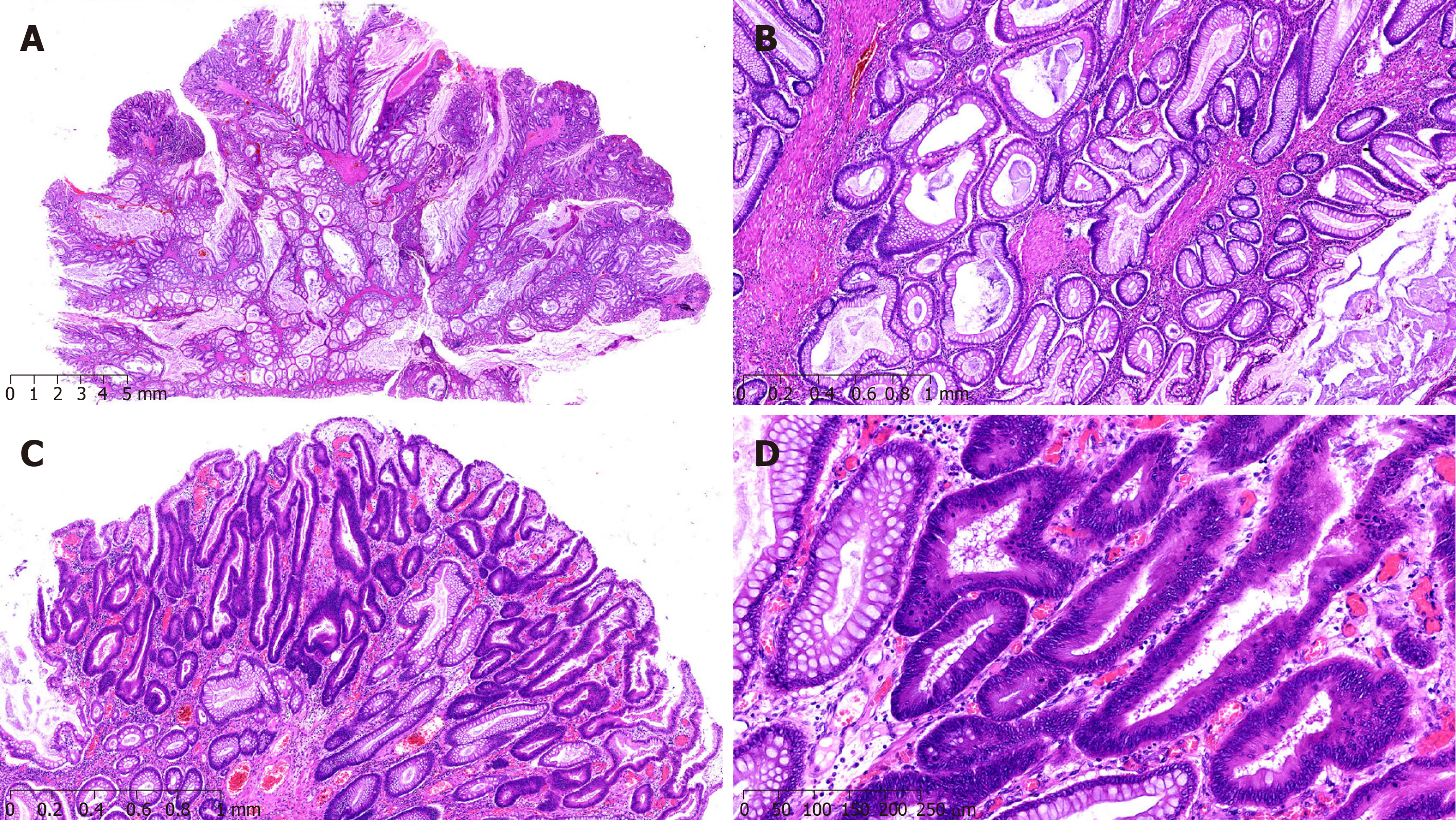
A 42-year-old woman was admitted to the hospital due to repeated upper abdominal pain for 14 years and aggravated with low back pain for 2 mo. Four years ago, gastrointestinal endoscopy indicated PJS, so endoscopic polypectomy and colonoscopic polypectomy were performed successively for three times. Postoperative pathology findings were consistent with P-J polyps, and abdominal pain was relieved later. Two months ago, upper abdominal distention and pain appeared again, with gradual aggravation, accompanied by pain in the left low back, especially at night, with acid reflux, heartburn, 1/3 less food intake than before, and melena. The lowest hemoglobin level was 62 g/L, and the body weight decreased by about 10 kg. The body mass index (BMI) was 16.7 kg/m2, and there was melanin pigmentation on the lips, eyelids, fingertips, and palms. Fathers and sons in her family suffered from PJS. Gastroscopy showed multiple polyps in the stomach, and those around the cardia were uplifted. The stomach was partly villous, and the erosion extended to the bottom of the stomach. Abdominal enhanced computed tomography (CT) showed polyps mostly in the gastric cavity and small intestine, with lesions in soft tissue masses of the stomach; malignant changes could not be ruled out, and multiple metastatic tumors may occur in the liver. Full gastrointestinal contrast CT showed many filling defect areas in the gastric cavity, and the largest was in the gastric fundus area of the cardia, with a size of approximately 7.6 cm × 4.2 cm. The level of CA199 was > 1200 U/mL, and that of CEA was 6.94 ng/mL (Figure 1). Exploratory laparotomy was performed after admission. There were no malignant tumor cells in the peritoneal lavage, and a total of three 1-2 cm nodules were examined in the II, IV, and V segments of the liver. They were considered liver metastases. The small intestine was overlapped at 100 cm of the flexor ligament, with an overlapping length of about 50 cm. After that, multiple polyps could be found, with the lengths of 2-4 cm. The tumor near the cardia at the bottom of the stomach was found to be a polypoid change, with a size of 15 cm × 10 cm and complete serosa, so palliative total gastrectomy (Roux-en-Y anastomosis), resection of liver metastases, intussusception reduction, and intestinal polyp resection were performed. Postoperative pathology showed multiple PJ polyps in the stomach, and some gastric fundus polyps turned to highly differentiated adenocarcinomas. The cancer infiltrated the muscle layer to reach the serosa, and metastasis was observed in 16/22 lymph nodes. There were multiple PJ polyps in the small intestine, and some had focal dysplasia. All three liver nodules were infiltrated by adenocarcinomas (Figure 2). Genetic test indicated STK11 mutation. The patient recovered well after surgery and received eight cycles of XELOX chemotherapy. The follow-up time was 25 months, with no clear signs of tumor recurrence.
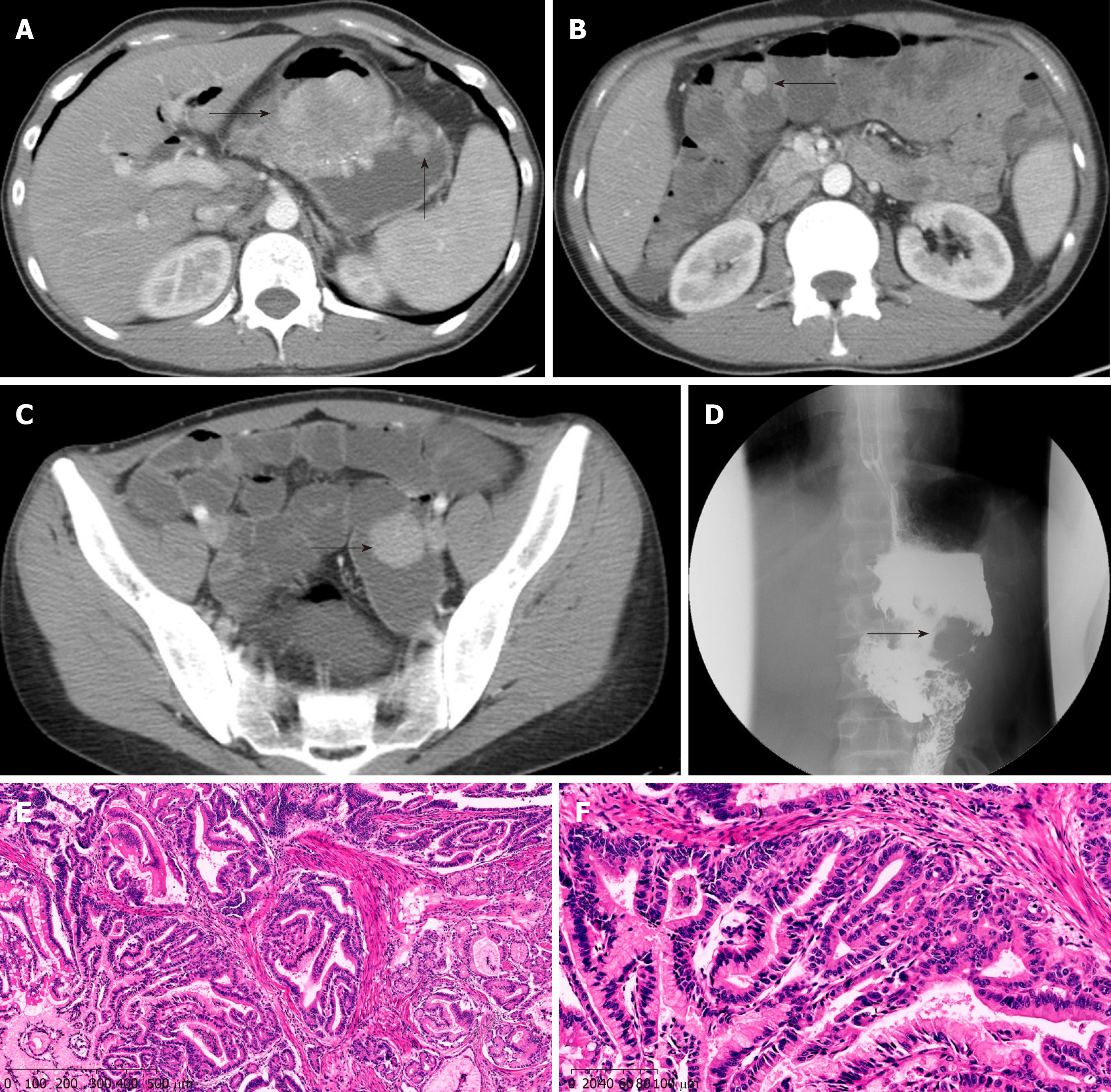
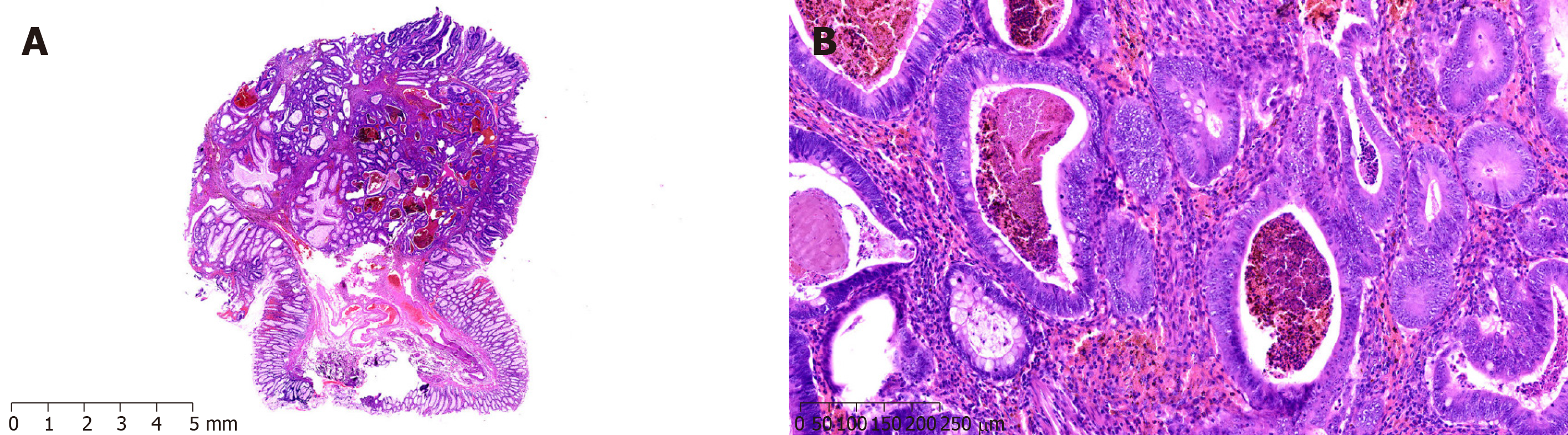
A 29-year-old man was admitted to the hospital because of intermittent abdominal pain, and abdominal distension combined with skin and mucous dark spots for 20 years, as well as abdominal pain for another month. Examination showed melanosis of the lips and fingertips; BMI was 21.2 kg/m2. Previously, he underwent open distal gastrectomy due to congenital choledochal cyst (Billroth II). The patient had a history of PJS for 20 years, and his elder sister suffered from PJS, too. Abdominal CT showed multiple nodules of soft tissue with various sizes in the gastric cavity, with maximum diameters of 6.2-5.4 cm. These symptoms were consistent with PJS, but without ruling out malignant change; upper gastrointestinal angiography showed large filling defects of the residual stomach, considering occupation and involvement of the anastomosis; gastroscopy and enteroscopy revealed multiple polyps in the stomach, intestine, and colon (Figure 3). ESD was performed, and postoperative pathology suggested a highly heterogeneous epithelium for some gastric polyps, hyperplasia, carcinogenesis, and invading mucosal muscle layer; other gastric and intestinal polyps were all P-J-like (Figure 3). During the operation, a tumor with a diameter of 6 cm could be reached near the gastro-jejunal anastomosis of the posterior wall of the great curvature of the residual stomach. There were many small polyps around the anastomosis, with diameters of 1-2 cm; multiple polypoid masses were observed in the small intestine and colon, with a maximum diameter of 3 cm. Subtotal gastrectomy (Roux-en-Y anastomosis) and small intestinal polypectomy were performed. After operation, the patient was discharged smoothly. Pathology revealed that most of the PJ polyps in the stomach turned into highly differentiated adenocarcinomas, and the tumor invaded the deep muscle layer. There were multiple P-J polyps in the surrounding mucosa and small intestine. The patient recovered well after surgery and received eight cycles of XELOX chemotherapy. The follow-up time was 20 months, with no clear signs of tumor recurrence.
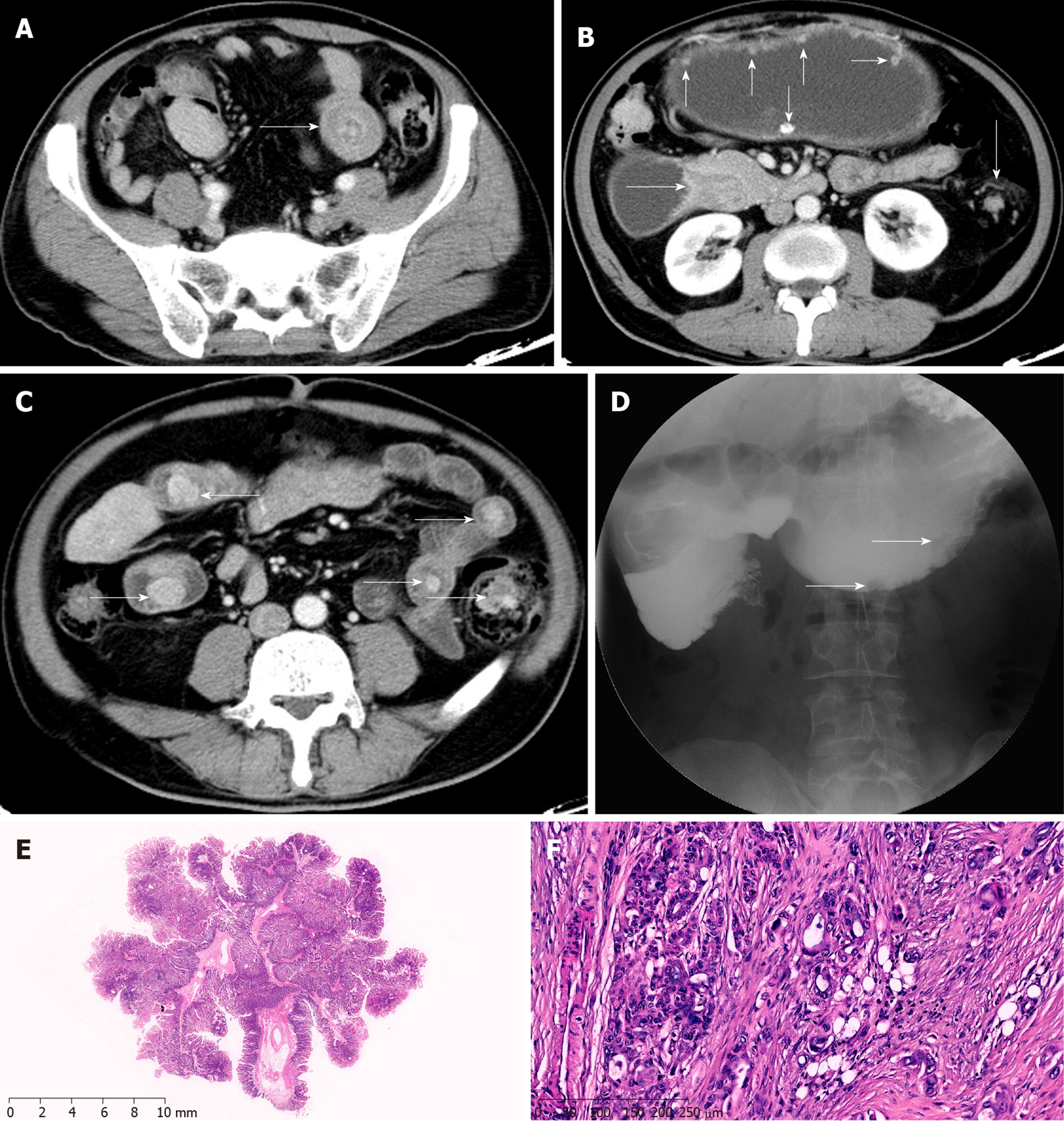
A 67-year-old man was admitted to our hospital with a BMI of 24.2 kg/m2, mainly for reduced flatus and defecation, combined with nausea and vomiting for 1 mo. Abdominal CT showed multiple nodules in the stomach and intestine, and polypoid lesions were considered. There were concentric circle-like changes in small intestinal tube, and intussusception was considered. The gastric wall in the pylorus of the gastric antrum had thickened, and pyloric obstruction was considered. Upper gastrointestinal angiography showed multiple polyps in the abdominal cavity and lower duodenal obstruction, and polyps were considered (Figure 4). During the operation, there were also ascites in the abdominal cavity, and the antral and duodenal masses were hard, invading the pancreatic head. Intussusception occurred in the distal part of the small intestine about 130 cm from the tuber ligament, with a length of 30 cm. Several polyps were found in the cavity of the small intestine, with a diameter of about 3 cm, and advanced-stage cancer was considered. As a result, radical resection could not be performed, and enterolysis, gastro-jejunal anastomosis after Roux-en-Y gastric bypass, intussusception reduction, and small intestinal polypectomy were performed. Postoperative pathology showed that adenocarcinomas in the omentum majus, nodular adenocarcinomas in the small intestinal mesentery, and adenocarcinomas in the ileal mesentery were infiltrating. There were six small intestinal polyps, one of which showed adenocarcinoma infiltration with a clean basal margin; the remaining five were P-J polyps (Figure 4). The patient received eight cycles of XELOX chemotherapy. Tumor progression was reflected by hepatic metastasis, and abdominal lymph node metastasis occurred 6 months after surgery. Cachexia occurred, and the patient finally died at another hospital.
A 29-year-old female patient was admitted to our hospital with a BMI of 17.6 kg/m2, mainly for colon polyps detected 7 mo ago with intermittent abdominal pain and distension. His father died of colon cancer due to PJS. No abnormality was observed by gastroscopy. Colonoscopy showed a colonic spleen curve polyp with dimensions of 6 cm × 4 cm, whose surface was uneven and congested. Considering a single polyp, the possibility of malignancy was not excluded, and there were no intestinal obstruction or intussusception. So, colonic polyps were treated with ESD. Postoperative pathology revealed P-J polyps, with a small number of high-grade intraepithelial neoplasias and clean margins (Figure 5). Forty-two months after surgery, regular follow-up was conducted by gastroenterological endoscopy. No new polyps were found, and her recovery was smooth.
A 51-year-old male patient was admitted to our hospital because of intermittent defecation for 2 mo. He had interruption of flatus and defecation, accompanied by abdominal distention 2 mo ago, without abdominal pain or fever; he received conservative treatment and recovered. There was no significant weight loss, and the BMI was 22.5 kg/m2. His younger brother suffered from PJS. Gastroscopy showed flat polyps at 0.2 cm from the bottom of the stomach, and multiple verrucous uplifts in the gastric antrum, slightly concave in the middle. He was diagnosed with gastric polyps; ESD for endoscopic gastric polyps was performed. Postoperative pathology suggested a polyp of the gastric fundus, and there was moderate intestinal metaplasia in the gastric antrum mucosa. Colonoscopy showed a 0.6 cm × 0.3 cm flat uplift in the transverse colon and two pedicle polyps in the sigmoid colon, with an erosive surface, and 0.2-0.4 cm flat polyps could be seen in the rectum. Considering the sigmoid colon polyps observed, cancer was not excluded and there were transverse colonic and rectal polyps. Therefore, colonic polyps were treated by ESD. Postoperative pathology showed that the sigmoid colon mucosa presented a P-J polypoid structure, and the epithelium partly presented high grade intraepithelial neoplasia, with clean margins (Figure 6). There were inflammatory polyps in the transverse colon. Forty-seven months after surgery, regular review by gastroscopy and colonoscopy was conducted. No new polyps were found, and his recovery was smooth.
The criteria for PJS diagnosis mainly include the following three aspects[4]: (1) Histologically, there are three or more P-J polyps; (2) A family history of PJS; and (3) Typical and significant pigmentation of the mucosa, which is mainly located on the lips, nose, fingertips, buccal mucosa, and anal area. Patients who meet two of the above criteria can be diagnosed with PJS.
Clinical manifestations are mainly intestinal obstruction caused by polyps, intussusception (mainly affecting the small intestine), repeated irregular abdominal pain, diarrhea, and gastrointestinal bleeding-based symptoms, with lack of significantly specific symptoms. Endoscopy and barium meal examination reveal polyps in the gastrointestinal tract, and pathological biopsy and gene detection of polyps remain the gold standards for PJS diagnosis[5]. With the development of endoscopy, capsule endoscopy and balloon enteroscopy play important roles in the diagnosis and treatment of small intestinal polyps, with the advantage of no pain or wounds. Currently, they have become the main methods for diagnosing and treating intestinal diseases[5], and are more sensitive for small polyps that cannot be found in barium meal examination. With the number increase and enlargement of polyps, intussusception often occurs, as well as multiple overlaps or double intussusception. Studies have confirmed that patients with PJS are more likely to show gastrointestinal tumors and malignancy outside the gastrointestinal tract compared with general patients. A study of 133 patients with PJS from 54 families showed that PJS patients had a higher risk of cancer[6], which directly caused death. The results show that[6] the median age for first diagnosed cancer is about 45 years old. With the coming age, the cumulative cancer risk rate of PJS patients significantly increases from 20% to 76%, especially higher in young female patients. Another research[7] analyzed 336 PJS patients and showed that 52 patients (15.5%) were diagnosed with cancer, and the cancer relative risk and the cumulative risk were 63.8 and 55%, respectively. The median age for first diagnosing malignant tumor was about 41 years old. In our study, there were 46 patients diagnosed with PJS. Among these patients, five developed a malignant tumor secondary to PJS, and the cancer risk rate was 10.9% and median age at first diagnosis was 43.6 years (range, 29-67 years). Our result is consistent with the above studies[6,7]. Interestingly, gene mutations in PJS patients also increase cancer risk rate. Mehenni et al[8] reported 149 patients with PJS, among which 31 with malignant changes developed STK11/LKB1 mutations, and confirmed the colorectal junction as a high incidence site for PJS polyps. Resta et al[9] reported 119 cases of PJS, including 36 patients with polyp malignancy and 21 with STK11/LKB1 mutations, and found a high incidence of tumors in the gastrointestinal tract (8.26%), followed by the pancreas (7.19%) and breast (6.17%). Another Italian multicenter research reported that[9] there were 29 patients with gene mutations among 119 patients. The overall cumulative cancer risk rates were 20%, 43%, 71%, and 89% at the age of 40, 50, 60, and 65 years old, respectively. Therefore, we need to perform the gene detection and it can be a promising way[10]. Meanwhile, for young female patients with PJS, long-term combined chronic abdominal pain, diarrhea, and gastrointestinal bleeding with no improvement after conservative treatment, malignant tumors should be taken into consideration and positive measures be taken.
There is no effective treatment for PJS, but related animal studies have shown that[11] use of cyclooxygenase-2 inhibitors in the treatment of STK11 mutant mice and PJS patients can significantly reduce the risk of cancer; therefore, COX-2 may be a potential therapeutic target for the treatment of PJS, which deserves further investigation. However, there is no need to treat skin mucosa pigmentation in general, and some patients can undergo laser plastic surgery for esthetics. Controversies remain regarding the treatment of polyps. Scholars believe that[12] P-J polyps are precancerous lesions which have the transformation pathway of hamartoma-adenoma-adenocarcinoma. Some scholars found that[13] there is another pathway of PJS polyp cancerization, namely, adenomatous polypoid to adenocarcinoma, and extra attention should be paid. Endoscopic resection or surgical removal of alimentary polyps is performed as early as possible. Meanwhile, others propose that[14] P-J polyps have a risk of malignancy, and routine endoscopic observation is necessary. For PJS patients with stomach, duodenum, small intestine, and colorectal polyps, we suggest that pedicle or latipolyps, with a size of 1-3 cm, should be resected under endoscopy as early as possible, at least once a year. With disease progression, if polyps are greater than 3 cm in diameter with or without pedicle, induce abdominal pain, gastrointestinal bleeding, intussusception, or intestinal obstruction, or show a clear cancerous pathology, active radical treatment should be performed as soon as possible. The risk of PJS increases due to hamartoma polyps and related gastrointestinal complications induced by polyps. Therefore, the most effective treatment for patients with PJS is timely monitoring of digestive tract polyps and possibly malignant polyps; in addition, active and effective endoscopy can reduce the risk of polyp malignancy as well as the occurrence of polyp related complications. Furthermore, endoscopic examination can detect early malignant tumors. In PJS patients, endoscopy guided surgery is the most effective and practical way of treatment. In individuals with secondary malignant lesions, radical surgery should be performed as soon as possible, and endoscopic examination is used for further resection of polyps. Postoperative adjuvant chemotherapy significantly improves patient prognosis. In individuals unable to achieve radical resection because of metastases to distant regions, the purpose of surgical treatment is to remove the clinical symptoms instead of radical resection. Large isolated polyps can be removed. While some polyps are concentrated in the intestine, some of the bowel can be removed, keeping the intestinal length as much as possible to reduce the incidence of short bowel syndrome and improve postoperative quality of life.
Regular follow-up is also critical in PJS patients[15,16], including gastrointestinal endoscopy, abdominal pelvic CT, B-ultrasound or MRI, and low-dose chest CT. Breast and gynecologic examinations should be performed in females[14], while male patients should undergo reproductive system examination and assessed for CA125, CA199, and other tumor markers[17]. Several studies[18] found that de nove pathway can cause malignant transformation of the extra-gastrointestinal organs. Hence, it is necessary to pay attention to polyp malignancy and the occurrence of external malignant tumors of the gastrointestinal tract.
This study had some limitations. It assessed a rare disease, whose incidence is low; in addition, this was a retrospective and single center study. Furthermore, the sample size was small, with no long-term follow-up data. Therefore, more clinical cases and long-term follow-up are required to confirm these findings.
To summarize, in patients with PJS suffering from chronic abdominal pain, diarrhea, and gastrointestinal bleeding, when conservative treatment fails, attention should be paid to malignant changes to secondary PJS. Endoscopy combined with CT and gastrointestinal imaging is of great significance for PJS diagnosis and treatment, while pathological diagnosis and gene detection are the gold standards for early diagnosis. Endoscopic resection of some gastrointestinal polyps is feasible. When malignant polyps cannot be resected under an endoscope, radical surgical treatment should be considered. In patients not able to achieve radical resection, the main surgical purpose is to relieve the clinical symptoms and improve prognosis with adjuvant chemotherapy after surgery. Therefore, we need to pay attention to the prevention in PJS patients. For PJS patients, early recognition, diagnosis, and effective treatment can significantly improve the efficacy of PJS treatment, with an important guiding value for accurate disease treatment. For females, we also need to pay attention to the conditions of the uterus, ovary, and cervix.
Peutz-Jeghers syndrome (PJS) is a rare inherited disease, which can result in gastrointestinal and other organs malignant tumors. However, we do not have consistent diagnosis, treatment, and follow-up plans.
The research motivation is that many scholars do not realize the risk of PJS associated malignancy. Meanwhile, there are also disputes about diagnosing and treating for PJS.
Through evaluating the clinical symptoms, diagnosis, and treatment, we aimed to present our methods for treating malignant change secondary to PJS.
We reviewed 46 PJS patients from 2014 to 2017. Among these patients, five developed malignant tumors. Hence, we collected their clinical, imaging, and pathological data for further analysis. In addition, we followed these five patients after they were discharged from hospital.
The PJS patients were first diagnosed with malignant tumor at about 43.6 years old. Among them, three patients received operations and pathology confirmed adenocarcinoma. One patient underwent genetic testing, which showed an STK11 mutation. Three patients received XELOX chemotherapy, while one patient die from tumor progression after 6 months. The other two patients received endoscopic resection; postoperative pathology confirmed high grade intraepithelial neoplasia. The surviving patients had no recurrence.
If PJS patients are suspected to have secondary malignant changes, endoscopy and imageological examination are often needed to evaluate the tumor. For patients suited for endoscopic resection, we can perform the ESD. Otherwise, radical operation should be considered. After surgery, we need to conduct pathological examination and gene detection. For advanced tumor, patients need to receive adjuvant chemotherapy. Meanwhile, we also need to pay attention to the prevention in PJS patients.
PJS patients do not always have typical symptoms. Compared with common people, they have higher tumor susceptibility. Therefore, they need to regularly check-up the body and take measures to prevent associated tumor complications and improve prognosis.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eren T, Chiang JM S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Li X
| 1. | Ishida H, Tajima Y, Gonda T, Kumamoto K, Ishibashi K, Iwama T. Update on our investigation of malignant tumors associated with Peutz-Jeghers syndrome in Japan. Surg Today. 2016;46:1231-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Friedl W, Møller P, Hes FJ, Järvinen H, Mecklin JP, Nagengast FM, Parc Y, Phillips RK, Hyer W, Ponz de Leon M, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen JT, Clark SK, Hodgson SV. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 3. | Chiang JM, Chen TC. A Peutz-Jeghers syndrome family associated with sinonasal adenocarcinoma: 28 years follow up report. Fam Cancer. 2017;16:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Mozaffari HR, Rezaei F, Sharifi R, Mirbahari SG. Seven-Year Follow-Up of Peutz-Jeghers Syndrome. Case Rep Dent. 2016;2016:6052181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Ohmiya N, Nakamura M, Takenaka H, Morishima K, Yamamura T, Ishihara M, Miyahara R, Kawashima H, Itoh A, Hirooka Y, Watanabe O, Ando T, Goto H. Management of small-bowel polyps in Peutz-Jeghers syndrome by using enteroclysis, double-balloon enteroscopy, and videocapsule endoscopy. Gastrointest Endosc. 2010;72:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | van Lier MG, Westerman AM, Wagner A, Looman CW, Wilson JH, de Rooij FW, Lemmens VE, Kuipers EJ, Mathus-Vliegen EM, van Leerdam ME. High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut. 2011;60:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Chen HY, Jin XW, Li BR, Zhu M, Li J, Mao GP, Zhang YF, Ning SB. Cancer risk in patients with Peutz-Jeghers syndrome: A retrospective cohort study of 336 cases. Tumour Biol. 2017;39:1010428317705131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Mehenni H, Blouin JL, Radhakrishna U, Bhardwaj SS, Bhardwaj K, Dixit VB, Richards KF, Bermejo-Fenoll A, Leal AS, Raval RC, Antonarakis SE. Peutz-Jeghers syndrome: confirmation of linkage to chromosome 19p13.3 and identification of a potential second locus, on 19q13.4. Am J Hum Genet. 1997;61:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Resta N, Pierannunzio D, Lenato GM, Stella A, Capocaccia R, Bagnulo R, Lastella P, Susca FC, Bozzao C, Loconte DC, Sabbà C, Urso E, Sala P, Fornasarig M, Grammatico P, Piepoli A, Host C, Turchetti D, Viel A, Memo L, Giunti L, Stigliano V, Varesco L, Bertario L, Genuardi M, Lucci Cordisco E, Tibiletti MG, Di Gregorio C, Andriulli A, Ponz de Leon M; AIFEG. Cancer risk associated with STK11/LKB1 germline mutations in Peutz-Jeghers syndrome patients: results of an Italian multicenter study. Dig Liver Dis. 2013;45:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Shen N, Li D, Zhu Y, Xie H, Lu Y. Early genetic testing of STK11 is important for management and genetic counseling for Peutz-Jeghers syndrome. Dig Liver Dis. 2019;51:1353-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Udd L, Katajisto P, Rossi DJ, Lepistö A, Lahesmaa AM, Ylikorkala A, Järvinen HJ, Ristimäki AP, Mäkelä TP. Suppression of Peutz-Jeghers polyposis by inhibition of cyclooxygenase-2. Gastroenterology. 2004;127:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Wang R, Qi X, Liu X, Guo X. Peutz-Jeghers syndrome: Four cases in one family. Intractable Rare Dis Res. 2016;5:42-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Kang SH, Cheng GH, Li GL, Zhao XR, Zhang SH, An AQ, Wu TY, Kang LC. Analysis for the process of pathology from adenomatous polyps to cancerization of polyps in Peutz-Jeghers syndrome. Chin J Clinicans. 2015;9:3498-3502. |
| 14. | Sharma M, Singh R, Grover AS. Peutz-jeghers syndrome with synchronous adenocarcinoma arising from ileal polyps. Indian J Surg. 2015;77:100-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Zhang LJ, Su Z, Liu X, Wang L, Zhang Q. Peutz-Jeghers syndrome with early onset of pre-adolescent gynecomastia: a predigree case report and clinical and molecular genetic analysis. Am J Transl Res. 2017;9:2639-2644. [PubMed] |
| 16. | Jiang YL, Zhao ZY, Li BR, Li J, Jin XW, Yu ED, Xu XD, Ning SB. Early screening the small bowel is key to protect Peutz-Jeghers syndrome patients from surgery: a novel mutation c.243delG in STK11 gene. BMC Gastroenterol. 2019;19:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Zhou F, Lv B, Dong L, Wan F, Qin J, Huang L. Multiple genital tract tumors and mucinous adenocarcinoma of colon in a woman with Peutz-Jeghers syndrome: a case report and review of literatures. Int J Clin Exp Pathol. 2014;7:4448-4453. [PubMed] |
| 18. | Taguchi T, Suita S, Taguchi S, Tanaka S. Peutz-Jeghers syndrome in children: high recurrence rate in short-term follow-up. Asian J Surg. 2003;26:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |