Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4615
Peer-review started: April 29, 2020
First decision: May 21, 2020
Revised: June 1, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 6, 2020
Processing time: 151 Days and 8.9 Hours
Primary hepatic myelolipoma is a rare hepatic mesenchymal tumor mixed by adipose tissue and bone marrow, and there is a lack of general guidelines related to its epidemiology and clinical management.
A 26-year-old woman was admitted to our department complaining of a newly found tumor on her left lobe of the liver. The tumor was painless and not associated with any systemic or localized compressive symptoms. Serological tests disclosed a slight increase of gamma-glutamyl transpeptidase (70.0 U/L) and total bilirubin (19.2 μmol/L). Ultrasonography showed a mass about 5.0 cm × 5.0 cm in size that was located in the left lobe of the liver and displayed hyperechoic and well-encapsulated characteristics. Surgical resection was applied, and the following histopathological examination observed a variable proportion of myeloid tissues scattering throughout mature fibrotic adipose tissues, in which myeloid, erythroid, and megakaryocytic cells can be found in magnified view. The follow-up did not show any changes 6 mo after surgery.
This case highlights an extremely rare hepatic mesenchymal tumor, the primary hepatic myelolipoma, and discloses the common characteristics behind this disease and gives clinical recommendations.
Core Tip: Primary hepatic myelolipoma is an extremely rare benign tumor composed of mature adipose tissue and hematopoietic elements. We present herein a case of asymptomatic primary hepatic myelolipoma and include another 24 previously published cases in our analysis, to disclose the common clinical characteristics behind this disease and give clinical recommendations.
- Citation: Li KY, Wei AL, Li A. Primary hepatic myelolipoma: A case report and review of the literature. World J Clin Cases 2020; 8(19): 4615-4623
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4615.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4615
Myelolipomas are a kind of benign tumor first reported by Gierke[1] as early as 1905 and named by Oberling[2] 24 years later. Myelolipomas are mesenchymal tumors composed of adipose tissue and bone marrow-derived hemopoietic elements like myeloid, erythroid, and megakaryocytic cells. The majority of myelolipomas occur in the adrenal glands, but cases with extra-adrenal myelolipomas (EAMs) have also been reported in the presacral region, retroperitoneum, mesentery, etc. In general, EAMs are asymptomatic, but in some circumstances, they can produce severe complications including local compressive effects, rupture, and hemorrhage if not treated properly. Primary hepatic myelolipoma was first named in France in 1973[3]. We present herein a case of primary hepatic myelolipoma and consider the rarity of this benign hepatic tumor. We also collected the clinical data of almost all the primary hepatic myelolipoma (HM) cases that have been reported and compared them with our case in this paper.
A 26-year-old woman with a newly found tumor on the left lobe of her liver was admitted to our department.
The tumor was found in a medical examination 3 d ago without any pain or any systemic or localized compressive symptoms.
The patient had a free previous medical history.
When admitted, the patient’s temperature was 36.5 °C, heart rate was 81 bpm, respiratory rate was 16 breaths per minute, blood pressure was 121/70 mmHg, and oxygen saturation in room air was 99%. No abnormal signs were detected.
Serological tests disclosed a slight increase of gamma-glutamyl transpeptidase (70.0 U/L) and total bilirubin (19.2 μmol/L).
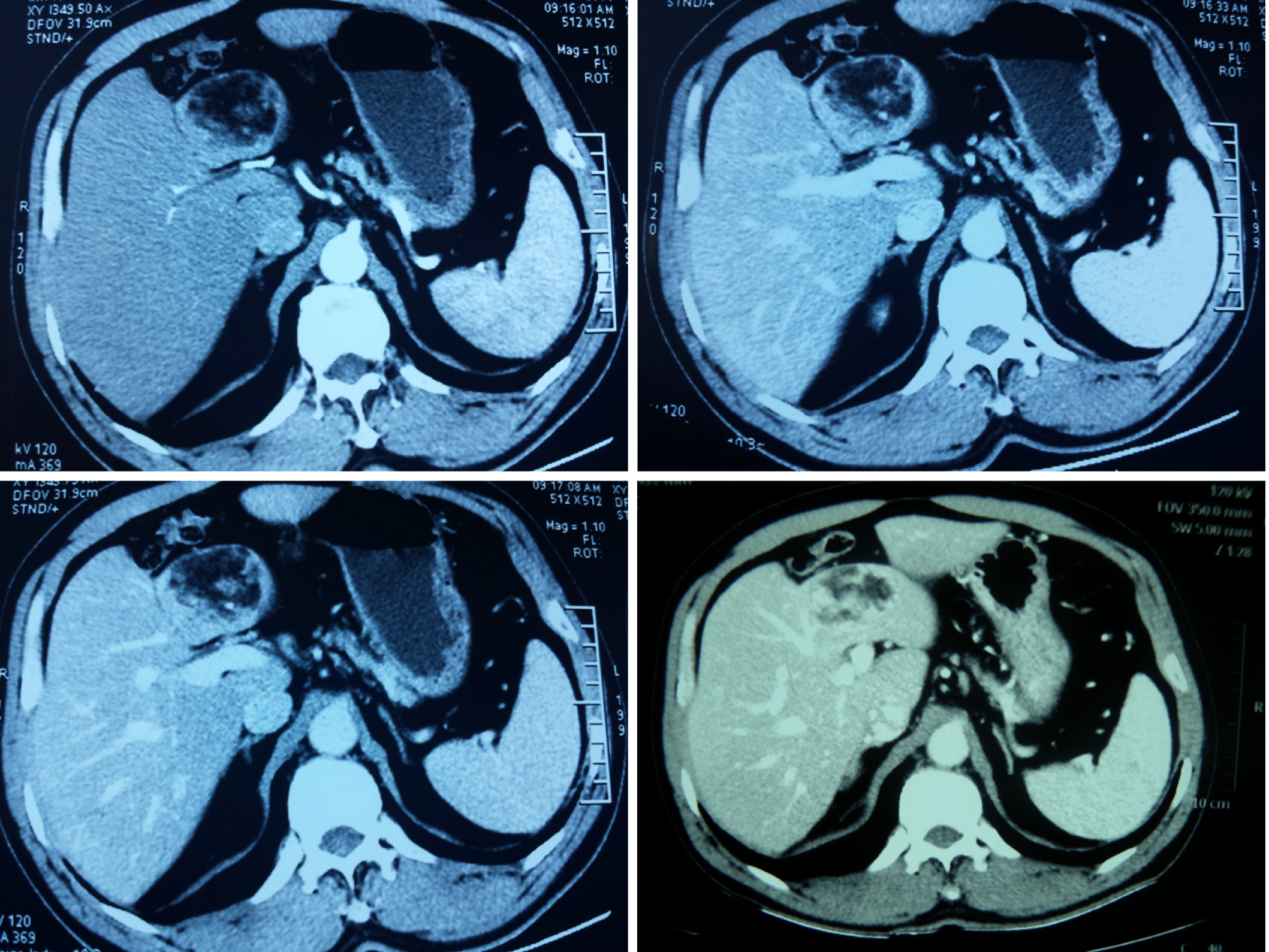
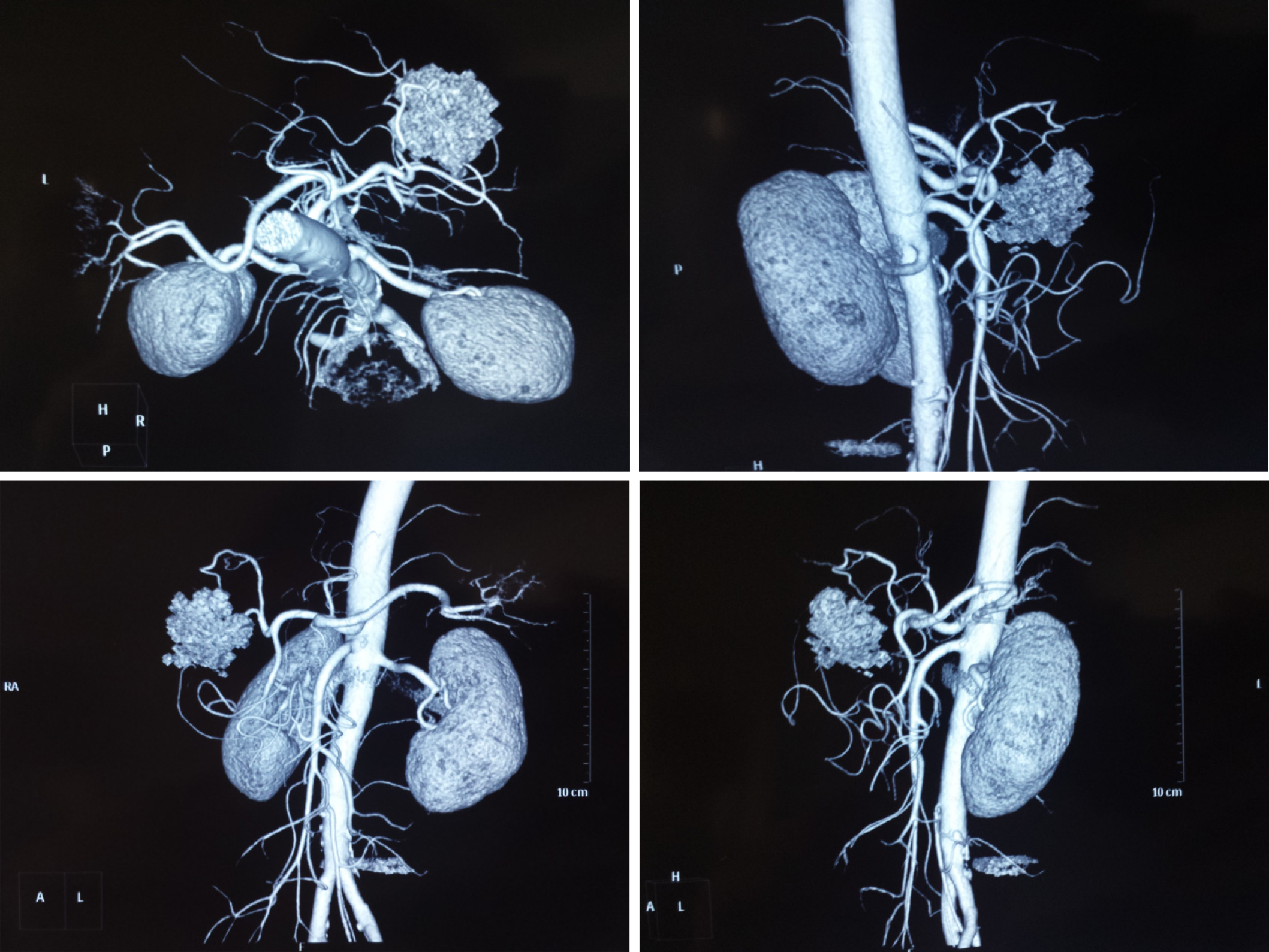
Ultrasonography showed a hyperechoic and well-encapsulated mass roughly 5.0 cm × 5.0 cm in size located in the left lobe of the liver. Computed tomography (CT) scans disclosed a quasi-circular mass measuring about 5.0 cm × 6.0 cm × 5.0 cm on the segment 4 of the liver, with mixed density. Under the contrast-enhanced CT scan, the density of this oval mass was mixed, with the edge and some parts intensified. Detailedly, as shown in Figure 1, in the early arterial phase, the tumor was not well intensified, while in the subsequent portal phase, the boundary of the whole tumor and some parts in the center get well-intensified, which lasted to the final phase. The last figure in Figure 1 shows a higher layer of the tumor that presented typical central enhancement. CT angiography revealed a mass in the liver with a rich blood supply that did not correlate to the main branches of the hepatic arteries (Figure 2).
The final diagnosis of the presented case was primary hepatic myelolipoma.
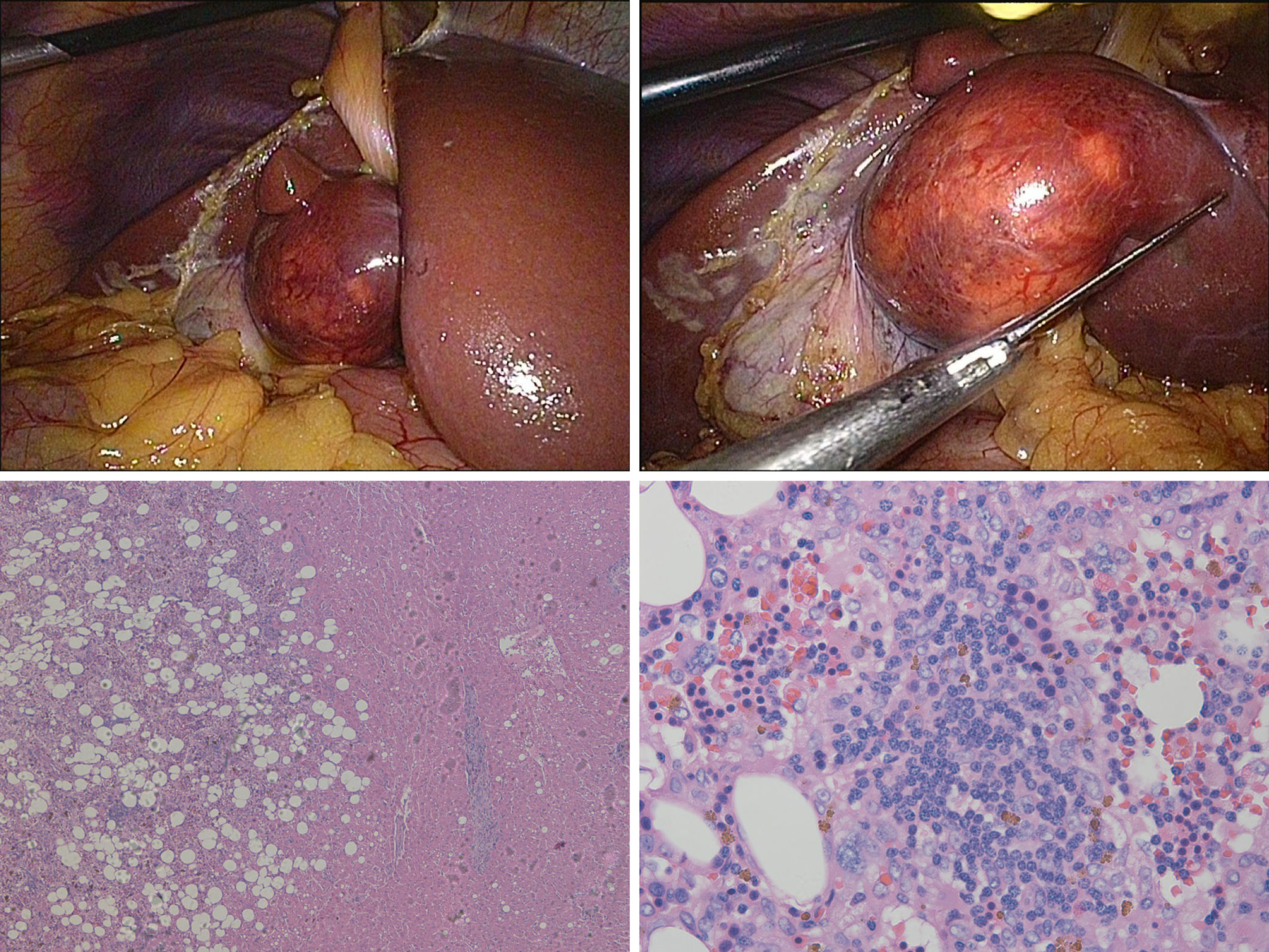
Considering the imaging characteristics of the tumor, we suspected this tumor to be benign and planned to perform a needle biopsy; however, after discussing with the patient, she asked for a full resection. A laparoscopic non-anatomic hepatectomy was performed to fully remove the tumor, and the resected tumor was transferred to our pathology department for further histopathological examination. The hepatic myelolipoma was made up of some yellow adipose tissue with clear blood vessels through the laparoscopic view. Histologically, most of the mass consisted of irregularly fibrotic adipose tissue, in which conspicuous islands of myeloid elements, including myeloid, erythroid, and megakaryocytic cells, were scattered (Figure 3).
Follow-up by CT scans and ultrasonography did not show any changes 6 mo after surgery.
We present an asymptomatic HM and review another 24 previously published cases to include in our analysis, in which we summarize the clinicopathological information including patients’ age, gender, clinical symptoms, tumor location, tumor size, imaging findings, liver functions, diagnostic methods, and treatments in Table 1[3-26]. According to our analysis, primary HM occurred in females more than males, with an incidence of 64.0% and 36.0%, respectively. The mean age at presentation was 54 (54.4 ± 12.32) years. Six patients were asymptomatic, and patients who presented with clinical manifestations had minimal symptoms. Based on the analysis of tumor sizes and locations, we found that primary HM often occurred within the right lobe (16/25), and over half of the patients had tumors less than 10 cm in diameter, with the largest reaching 17 cm. Imaging modalities including ultrasound, CT scans, or magnetic resonance imaging (MRI) scans were used to locate these masses and hepatic angiography was utilized to help determine their blood supply. Serologically, liver enzymes ranged from normal to slightly elevated. Moreover, almost all patients received invasive interventions like a needle biopsy or an operation followed by pathological examinations to confirm their diagnosis.
| Ref. | Age/gender | Symptoms | Location/size | Imaging findings | Liver function | Diagnosis | Treatment |
| Grosdidier[3], 1973 | 63/F | Slight hepatomegaly | RL/ 13 cm | NA | Normal | Resected specimen | Resection |
| Ishak[4], 1976 | 53/F | NA | LL/2 cm | NA | NA | Autopsy | Resection |
| Le Néel et al[5], 1984 | 69/F | RUQ pain | RL/4 cm | Echogenic, low density area, avascular zone | Normal | Resected specimen | Resection |
| Rubin et al[6], 1984 | 56/F | Abdominal pain, RUQ tenderness | RL/8 cm | Low density area, hypervascular mass with an avascular center | ↑GOT, GGT, LDH, ALP | Resected specimen | Resection |
| Mali et al[7], 1986 | 63/M | Hepatomegaly | RL/15 cm | Echogenic, low density area, hypervascular mass | ↑TNABIL, GGT | Needle biopsy | Observation |
| Kaurich et al[8], 1988 | 42/F | Asymptomatic | LL/6 cm | Echogenic, low density area | ↑GGT, ALP | Resected specimen | Resection |
| Nishizaki et al[9], 1989 | 56/F | RUQ pain | RL/5,5 cm | Echogenic, low density area, hypervascular mass | ↑GOT, GPT, ALP | Resected specimen | Resection |
| Moreno et al[10], 1991 | 40/M | Debilitation, RUQ pain, hepatomegaly | RL/15 cm | NA | ↑GPT | Resected specimen | Resection |
| Dai[11], 1993 | 49/F | RUQ pain | LL/5 cm | NA | NA | Resected specimen | Resection |
| Orlandi et al[12], 1994 | 53/F | RUQ pain | LL/5,5 cm | Echogenic | Normal | Resected specimen | Resection |
| Van Hoe et al[13], 1994 | 57/M | Hepatoegaly | LL/12 cm | Echogenic, low density area | ↑GGT | Resected specimen | Resection |
| Takizawa et al[14], 1995 | 66/F | NA | RL/15 cm | NA | NA | Resected specimen | Resection |
| Fauchery et al[15], 1998 | 63/F | Asymptomatic | LL/2 cm | NA | Normal | Resected specimen | Resection |
| Hoang et al[16], 1999 | 49/F | RUQ pain | RL/15 cm | Low density area | NA | Needle biopsy | Resection |
| Savoye-Collet et al[17], 2000 | 25/M | Asymptomatic | RL/17 cm | Echogenic, hyperintensity on T2 and hypointensity on T1 | ↑ALP | Resected specimen | Resection |
| Zhao et al[18], 2000 | 57/F | RUQ pain | LL/10.5 cm | Echogenic, low density area | Normal | Resected specimen | Resection |
| Tang et al[19], 2006 | 49/M | Asymptomatic | RL/14 cm | Echogenic, low density area (NA78HU) | NA | Resected specimen | Resection |
| Pavón et al[20], 2009 | 63/M | Asymptomatic | RL/4 cm | Echogenic, hyperintensity on T2 | ↑GPT, GGT | Resected specimen | Resection |
| Radhi et al[22], 2010 | 76/M | Urinary tract infection | RL/3.2 cm | Echogenic, low density mass | NA | Resected specimen | Resection |
| Chen et al[21], 2010 | 46/M | Abdominal distention | RL/3 cm | Low density areas | NA | Needle biopsy | Observation |
| Suárez-Peñaranda et al[23], 2014 | 57/F | Hematuria, urinary frequency, dysuria | RL/5 cm | Echogenic, hyperintensity on T2 | NA | Resected specimen | Resection |
| Menozzi et al[24], 2016 | 72/F | Dyspepsia | RL/3 cm | Echogenic, abundant fatty component | NA | Needle biopsy | NA |
| Xu et al[25], 2016 | 26/F | RUQ | RL/4.2 cm | Low-density mass, heterogeneously enhanced | ↑GGT | Resected specimen | Resection |
| Gallo et al[26], 2017 | 55/M | NA | NA | NA | NA | Biopsy | NA |
| Present case | 55/F | Asymptomatic | LL/5 cm | Echogenic, low density area, hypervascular mass | ↑GGT, TNABIL | Resected specimen | Resection |
EAMs are extremely rare benign tumors composed of mature adipose tissue and hematopoietic elements. Most EAMs are unintentionally discovered during surgery or autopsy, and manifest as a single lesion with a diameter ranging from 4 cm to 15 cm. EAMs are more common in people over 60 years old[27]. Due to the macroscopic adipose tissue within an adrenal myelolipoma, a preoperative diagnosis is relatively easy to make via a CT or MRI scan. However, it is difficult to diagnose an EAM based on imaging because of its variable location and other clinical characteristics. EAMs have been reported to occur in the retroperitoneum, presacral tissue, perirenal tissue, lung, and nasal cavity, but hepatic EAMs are extremely uncommon[28-31].
Although several hypotheses have been proposed as to the cause of myelolipoma, the etiology and exact pathogenesis are still unknown. Possible causes include the degeneration of adrenal tumors or adenomas, metaplasia of the early adrenal cortex mesenchymal stem cells, and translocation of differentiated bone marrow tissue during embryogenesis[32]. At present, a widely accepted theory is metaplasia of the reticuloendothelial cells of blood capillaries. However, Bishop et al[33] demonstrated a pattern of X-chromosome inactivation in the hematopoietic elements and fat of myelolipomas, indicating that the majority of these masses were clonal proliferation derived from a pluripotential stem cell[33]. The etiology of HM is also not fully understood but may be related to the translocation of adrenal glands or the metaplasia of hepatic cells. Furthermore, based on the data of the tumor sites that we collected, we boldly speculate that the most likely reason for HM to grow more in the right lobe is because of the close anatomic relationship to the right adrenal gland. However, further studies are needed to more thoroughly and accurately explain this phenomenon.
To diagnose primary HM before a pathological examination is difficult. Patients are usually asymptomatic or manifest mild symptoms like right upper quadrant discomfort, pain, and liver enlargement. Serological tests can be normal or show slight changes on some non-specific enzymes, like alkaline phosphatase, glutamic pyruvic transaminase, and gamma-glutamyl transpeptidase. Imaging can be helpful in diagnosing these tumors. Ultrasonography is able to show hyperechoic lumps with a clear boundary. CT scans may present quasi-circular, sometimes lobulated, masses with clear borders, partially or fully enveloped by membranes that were confirmed to be pseudocapsules aggregated by connective tissue under pathological examinations. These masses consist of variable low-density adipose tissue (-78-20 Hounsfield units) and medium density bone marrow tissue, and sometimes even present with enhancement on contrast-enhanced CT due to the calcification, infarction, or hemorrhage[34]. MRI scans can detect hyperintense signals from fat tissue on the T1-weighted sequences while T2-weighted sequences may detect intermediate to hyperintense signals due to the different concentrations of myeloid components[35]. For myelolipomas with a suspicion of malignancy, an 18-flurodeoxyglucose-PET scan is capable of tracking the malignant components by exposing increased uptake[36].
At present, although fat suppression imaging techniques contribute to the diagnosis, a needle biopsy followed by pathological examination remains the primary method to confirm the diagnosis. In our case, the primary HM was composed of irregularly mature fibrotic adipose tissue and some myeloid tissue with myeloid, erythroid, and megakaryocytic cells inside. Moreover, although both adrenal and extra-adrenal myelolipomas containing bone spicules have been reported, HM cases with reticular sinusoids or heterotopic ossification have never been shown[37]. The main differential diagnosis of a primary HM includes well-differentiated liposarcoma, hepatic angiomyolipomas, hepatic hamartoma, echinococcosis, extramedullary myeloid cell tumor, etc.
Due to the rare incidence, no general surgical guidelines are mentioned in the studied literature and most surgical excisions were performed because of the mass effect or the uncertainty regarding the nature of the tumor. For asymptomatic ECMs with a small size, if malignancy can be ruled out, the recommended clinical management is observation, according to the American Association of Clinical Endocrinologists/ American Association of Endocrine Surgeons Guideline (2009). We believe that this kind of clinical management for some HMs is acceptable; however, surgical intervention is warranted for those HMs with the larger size, because they may manifest symptoms due to a mass effect and increase the possibilities of spontaneous rupture or acute abdomen. Intralesional hemorrhage, infarctions, and suspicion of malignancy are also indications for surgery[38]. It is worth mentioning a case reporting the synchronous occurrence of a hepatic myelolipoma and two hepatocellular carcinomas in one patient[25]. Therefore, we have to emphasize that for those patients in whom malignancy cannot be ruled out, a preoperative biopsy is recommended[13]. Fortunately, the prognosis of primary HM is relatively good following appropriate resection and postoperative follow-up is not needed. Moreover, it is worth mentioning that, depending on the location of primary HMs, a laparoscopic hepatectomy, interventional radiology, or embolization can also be utilized to achieve a better prognosis. In the present case, we recommended needle biopsy instead of surgery at first, but the patient herself insisted on a full removal of this neoplasm from her body no matter benign or malignant. Our clinic team finally designed a laparoscopic non-anatomic hepatectomy to remove the tumor and keep the maximum volume of segment 4.
In summary, only a few cases of primary hepatic myelolipoma have been reported, and by reviewing all the 25 cases of primary HMs, we found that most of the patients were middle-aged women, asymptomatic, or with nonspecific clinical manifestations. The imaging characteristics of CT scans demonstrate low-density lesions localized in the right lobe with abundant blood supply and MRI scans demonstrate hyperintense signal on T1- and intermediate to hyperintense signal on T2-weighted sequences. Needle biopsy followed by observation is recommended, but resection should be considered if these masses meet surgical indications.
The authors appreciate the help of the pathologist for providing pathological data.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ramsay MA, Takemura N S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Gierke E. Unusual myeloid tissue in the adrenal gland. Beitr Pathol Anat. 1905;7:311-325. |
| 2. | Oberling C. The formation of myelolipomas [article in French]. Bull Assoc Fr Cancer. 1929;18:234-246. |
| 3. | Grosdidier J. Myelolipoma Hepatique. A propos d'une observation. Nouv Press Med. 1973;2:1777-1779. |
| 4. | Ishak K. Mesenchymal tumors of the liver. Okuda K, Peters RL, Hepatocellular Carcinoma. Wiley and Sons, York; 1976. |
| 5. | Le Néel JC, Gasquet C, Lenne Y, Moulin S, Heloury Y, Leborgne J. [Myelolipoma of the liver]. Gastroenterol Clin Biol. 1984;8:682-683. [PubMed] |
| 6. | Rubin E, Russinovich NA, Luna RF, Tishler JM, Wilkerson JA. Myelolipoma of the liver. Cancer. 1984;54:2043-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Mali SP, Gratama S, Mulder H. Myelolipoma of the liver. Rofo. 1986;144:610-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Kaurich JD, Coombs RJ, Zeiss J. Myelolipoma of the liver: CT features. J Comput Assist Tomogr. 1988;12:660-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Nishizaki T, Kanematsu T, Matsumata T, Yasunaga C, Kakizoe S, Sugimachi K. Myelolipoma of the liver. A case report. Cancer. 1989;63:930-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Moreno Gonzalez E, Seoane Gonzalez JB, Bercedo Martinez J, Santoyo Santoyo J, Gomez Sanz R, Vargas Castrijon J, Ballestin Carcavilla C, Garcia Mauriño ML, Colina Ruiz-Delgado F. Hepatic myelolipoma: new case and review of the literature. Hepatogastroenterology. 1991;38:60-63. [PubMed] |
| 11. | Dai QR. A case of spherical bulge of lesser curvature caused by hepatic myelolipoma. Zhongguo Shiyong Neike Zazhi. 1993;13:569 Available from: http://www.cnki.com.cn/Article/CJFDTotal-SYNK199309043.htm. |
| 12. | Orlandi A, Marino B, De Dona G, Cefaro A, Spagnoli LG. [Clinico-pathological considerations about a case of hepatic myelolipoma]. Ann Ital Chir. 1994;65:253-256. [PubMed] |
| 13. | Van Hoe L, Gryspeerdt S, Van Eycken P, Baert AL, Marchal G. Myelolipoma in a hepatocellular carcinoma: CT-pathologic correlation. AJR Am J Roentgenol. 1994;163:1111-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 14. | Takizawa H, Takikawa S, Koike M, Hirose S, Kusama A, Okamura N. [A case of hepatic myelolipoma]. Nihon Shokakibyo Gakkai Zasshi. 1995;92:1882-1885. [PubMed] |
| 15. | Fauchery A, Michel F, Rat P, Depret O, Kallee R, Justrabo E. [Isolated leiomyomatosis of the right renal vein associated with hepatic myelolipoma: diagnostic and therapeutic problems]. Prog Urol. 1998;8:398-403. [PubMed] |
| 16. | Hoang MP, Ordoñez NG. Myelolipoma of the Liver: Report of a Case and Literature Review. Int J Surg Pathol. 1999;7:251-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Savoye-Collet C, Goria O, Scotté M, Hemet J. MR imaging of hepatic myelolipoma. AJR Am J Roentgenol. 2000;174:574-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Zhao JJ, Liu SM, Hu JQ. A giant hepatic myelolipoma: a case report and literature review. Zhonghua Gandan Waike Zazhi. 2004;10:237. [DOI] [Full Text] |
| 19. | Tang F, Zhang PY. A case report of hepatic myelolipoma. Zhongguo Linchuang Yixue Yingxiang Zazhi. 2006;17:600. [DOI] [Full Text] |
| 20. | Pavón CJ, Llodra GP, Cuadrado JF, Artero SM, Rodríguez AC, Cao RB, Fernández FP. [Hepatic myelolipoma. Diagnosis and treatment]. Gastroenterol Hepatol. 2009;32:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Chen XX, Jiang XW, Huang W. A case report of multiple hepatic myelolipoma. Zhonghua Neike Zazhi. 2010;49:262. [DOI] [Full Text] |
| 22. | Radhi J. Hepatic myelolipoma. J Gastrointestin Liver Dis. 2010;19:106-107. [PubMed] |
| 23. | Suárez-Peñaranda JM, Bermúdez Naveira A, Fraga M, Aliste-Santos C, Cordeiro C, Muñoz-Barús JI. Unusual Forms of Adrenal and Extra-Adrenal Myelolipomas. Int J Surg Pathol. 2014;22:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Menozzi G, Maccabruni V, Marini G, Froio E, Garlassi E. Contrast-enhanced ultrasound (CEUS) appearance of hepatic myelolipoma. J Ultrasound. 2016;19:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Xu SY, Xie HY, Zhou L, Zheng SS, Wang WL. Synchronous occurrence of a hepatic myelolipoma and two hepatocellular carcinomas. World J Gastroenterol. 2016;22:9654-9660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Gallo M, Mineur L, Emptas H, Costes V, Ramos J. [Hepatic myelolipoma: A rare entity, case report and review of the literature]. Ann Pathol. 2017;37:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Hakim A, Rozeik C. Adrenal and extra-adrenal myelolipomas - a comparative case report. J Radiol Case Rep. 2014;8:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Sagan D, Zdunek M, Korobowicz E. Primary myelolipoma of the chest wall. Ann Thorac Surg. 2009;88:e39-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Huang W, Zhao Y, Yin X, Qi Q. Primary myelolipoma of the lung: a case of report and review of literature. Pol J Pathol. 2012;63:204-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Hajiran A, Morley C, Jansen R, Kandzari S, Bacaj P, Zaslau S, Cardinal J. Perirenal extra-adrenal myelolipoma. World J Clin Cases. 2014;2:279-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | George SA, Manipadam MT, Thomas R. Primary myelolipoma presenting as a nasal cavity polyp: a case report and review of the literature. J Med Case Rep. 2012;6:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Feng C, Jiang H, Ding Q, Wen H. Adrenal myelolipoma: a mingle of progenitor cells? Med Hypotheses. 2013;80:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Bishop E, Eble JN, Cheng L, Wang M, Chase DR, Orazi A, O'Malley DP. Adrenal myelolipomas show nonrandom X-chromosome inactivation in hematopoietic elements and fat: support for a clonal origin of myelolipomas. Am J Surg Pathol. 2006;30:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Park BK, Kim CK, Kim B, Kwon GY. Adrenal tumors with late enhancement on CT and MRI. Abdom Imaging. 2007;32:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Shenoy VG, Thota A, Shankar R, Desai MG. Adrenal myelolipoma: Controversies in its management. Indian J Urol. 2015;31:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Katabathina VS, Flaherty E, Kaza R, Ojili V, Chintapalli KN, Prasad SR. Adrenal collision tumors and their mimics: multimodality imaging findings. Cancer Imaging. 2013;13:602-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Mitsui Y, Yasumoto H, Hiraki M, Arichi N, Ishikawa N, Harada Y. Overexpression of bone morphogenetic protein 2 through aberrant canonical Wnt/β-catenin signaling pathway plays an important role in heterotopic ossification of adrenal myelolipoma. Can Urol Assoc J. 2014;8:e1047. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Gershuni VM, Bittner JG 4th, Moley JF, Brunt LM. Adrenal myelolipoma: operative indications and outcomes. J Laparoendosc Adv Surg Tech A. 2014;24:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |