Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3365
Peer-review started: April 7, 2020
First decision: June 13, 2020
Revised: July 8, 2020
Accepted: July 15, 2020
Article in press: July 15, 2020
Published online: August 6, 2020
Processing time: 121 Days and 8 Hours
Gastrointestinal stromal tumors are lesions that originate from digestive tract walls. Several laparoscopic techniques, including local resections, wedge resections and partial gastrectomies, have been used successfully. However, there are no reports on laparoscopic segmental gastrectomy for gastrointestinal stromal tumors.
We present our analysis of 17 patients who were admitted to our hospital from January 2014 to December 2018. All tumors were located in the corpus and antrum of the stomach, close to the lesser curvature of the stomach. The tumors originated from the anterior wall in nine cases and from the posterior wall of the stomach in eight cases. Laparoscopic segmental gastrectomy and end-to-end anastomosis between the proximal and the distal residual stomach were used in all patients. The mean operative time was 112.4 min. The mean length of hospital stay was 4.6 d. Mean operative blood loss was 73.5 mL. There were no leaks, no postoperative bleeding nor need for reintervention. Mean postoperative follow-up was 35.4 mo. The Visick grading index showed fewer gastrointestinal symptoms 3 mo after surgery. Two patients (11.8%) had reflux esophagitis and gastritis.
Laparoscopic segmental gastrectomy may be a new function-preserving gastrectomy that is feasible for treatment of gastrointestinal stromal tumors that grow in the middle third of the stomach and on the lesser stomach curvature.
Core tip: Local resection, wedge resection and partial gastrectomy have been successfully applied to the treatment of gastrointestinal stromal tumors. However, the application of laparoscopic segmental gastroscopy in the treatment of gastrointestinal stromal tumors has not been reported. In this study, we analyzed a series of cases from 2014 to 2018 in our single center of applied laparoscopic segmental gastrectomy for gastrointestinal stromal tumors. We describe the preoperative and intraoperative conditions of all patients as well as the postoperative complications.
- Citation: Ren YX, He M, Ye PC, Wei SJ. Total laparoscopic segmental gastrectomy for gastrointestinal stromal tumors: A case report. World J Clin Cases 2020; 8(15): 3365-3371
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3365.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3365
Gastrointestinal stromal tumors (GISTs), once known as gastrointestinal leiomyosarcomas, represent about 1% of gastrointestinal malignancies but are the most common of the mesenchymal tumors. They tend to occur in individuals older than 60 years[1,2]. The annual incidence is ten cases per million inhabitants[2,3]. They have a characteristic morphology, are generally positive for CD117 (c-kit) and are primarily caused by activating mutations in the KIT or PDGFRA genes[4,5]. The main treatment is surgical excision combined with imatinib therapy. However, several laparoscopic techniques for GISTs, including local resections, wedge resections and partial gastrectomies, have been used successfully. No current consensus exists on the therapy of GISTs concomitant with performance of a laparoscopic segmental gastrectomy procedure.
The superiority of segmental gastrectomy over conventional D2 (extended lymph node dissection) gastrectomy for early gastric cancer in terms of postoperative quality of life seems apparent[6-10]. Because of the characteristics of GISTs, segmental resection of the intestine and stomach is accepted. Thus, an aggressive and more extensive surgery to remove unaffected tissue is unnecessary.
We describe our single-center experience on laparoscopic segmental gastrectomy for GISTs that were growing in the middle third of the stomach and on the lesser stomach curvature. We used mechanical stapling devices for end-to-end anastomosis between the proximal residual stomach and the distal residual stomach.
The study includes 17 patients with GIST that were operated on from January 2014 to December 2018 in the Department of General Surgery of our hospital. Patients with metastatic disease at the time of resection and patients operated without histologic confirmation of GISTs were excluded from the study. All tumors were located in the corpus and antrum of the stomach, close to the lesser curvature of the stomach. The tumors originated from the anterior wall in nine cases and from the posterior wall of the stomach in eight cases. Table 1 presents the preoperative demographic and clinical characteristics of the case series. This report is a retrospective, noncomparative review of confirmed GIST cases performed in a single center.
| Subjects | Age in yr | Sex | Weight in kg | Height in m | BMI in kg/m2 | Anterior wall | Posterior wall |
| 1 | 43 | M | 62 | 1.68 | 22.0 | Yes | |
| 2 | 50 | F | 54 | 1.56 | 22.2 | Yes | |
| 3 | 62 | M | 68 | 1.65 | 25.0 | Yes | |
| 4 | 58 | M | 74 | 1.72 | 25.0 | Yes | |
| 5 | 35 | F | 50 | 1.56 | 20.6 | Yes | |
| 6 | 70 | F | 59 | 1.53 | 25.2 | Yes | |
| 7 | 47 | F | 56 | 1.54 | 23.6 | Yes | |
| 8 | 55 | M | 64 | 1.70 | 22.2 | Yes | |
| 9 | 73 | M | 68 | 1.68 | 24.1 | Yes | |
| 10 | 61 | F | 55 | 1.49 | 24.8 | Yes | |
| 11 | 59 | F | 62 | 1.63 | 23.3 | Yes | |
| 12 | 57 | F | 59 | 1.55 | 24.6 | Yes | |
| 13 | 63 | F | 48 | 1.50 | 21.3 | Yes | |
| 14 | 40 | M | 85 | 1.75 | 27.8 | Yes | |
| 15 | 52 | M | 70 | 1.70 | 24.2 | Yes | |
| 16 | 49 | F | 50 | 1.54 | 21.1 | Yes | |
| 17 | 60 | M | 75 | 1.68 | 26.6 | Yes |
Based on the history of all patients and preoperative examination, 17 patients were diagnosed with GISTs.
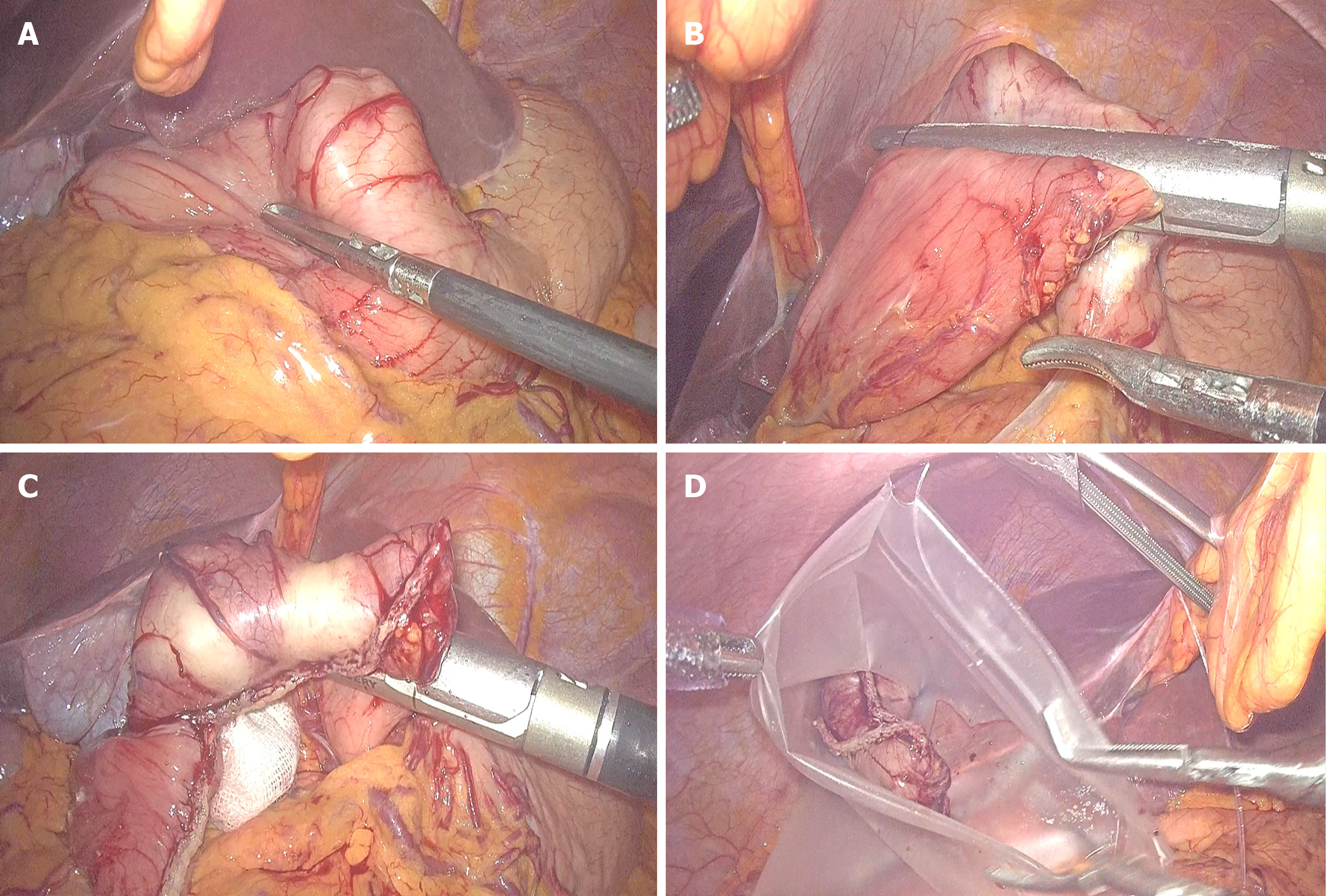
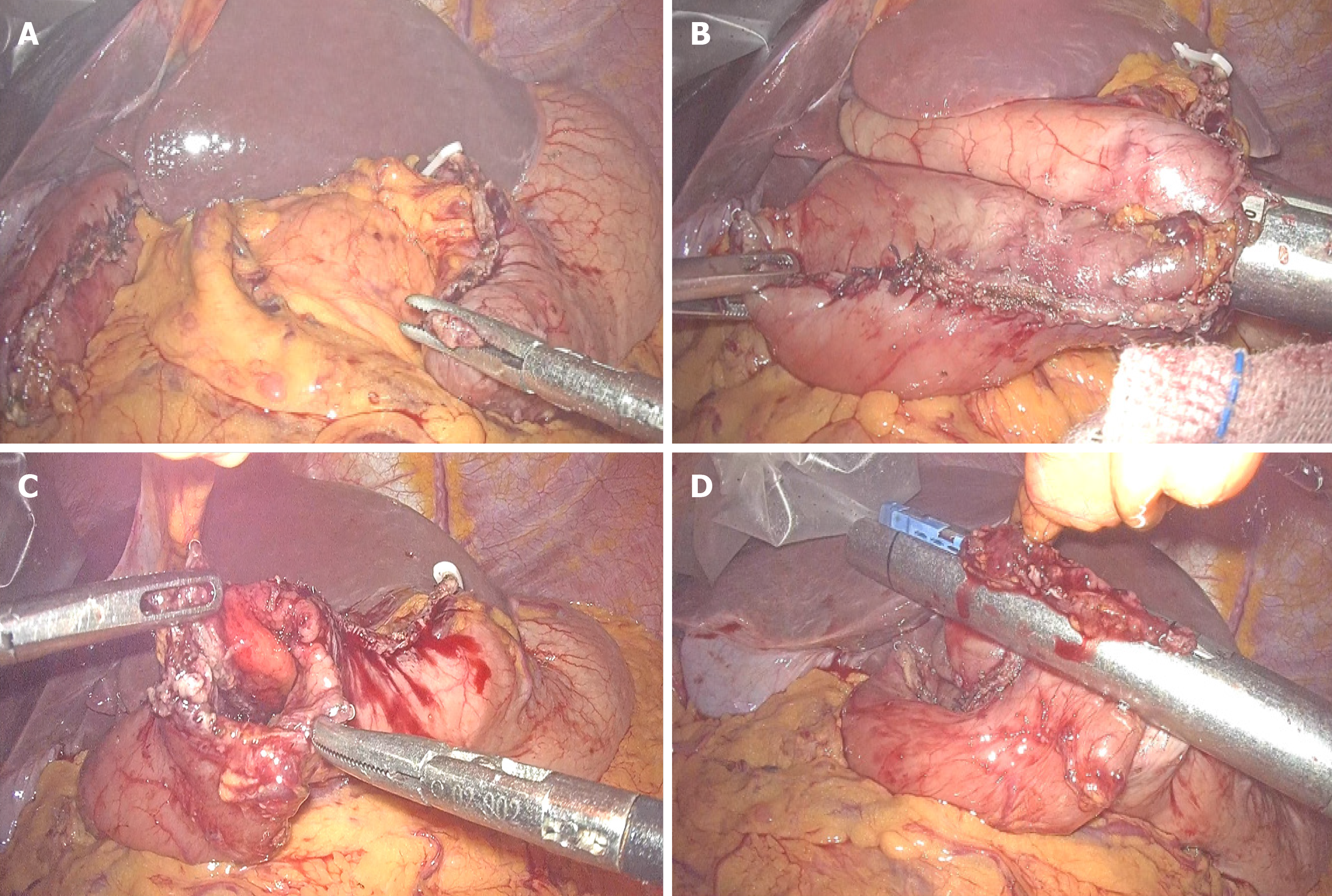
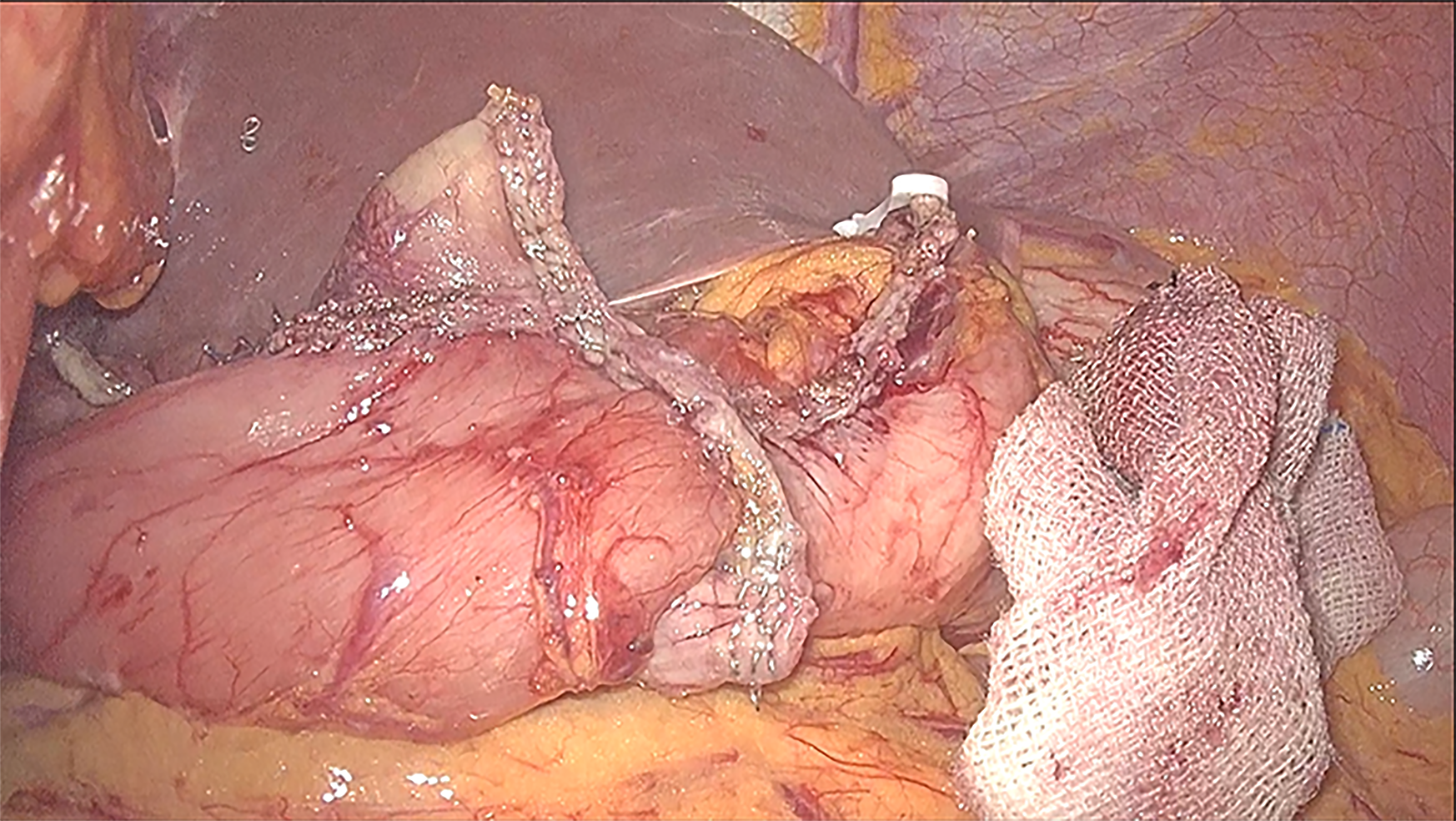
Because these tumors are on the lesser stomach curvature, all patients with primary gastric GISTs were initially prepared for laparoscopic segmental gastrectomy using mechanical stapling devices for end-to-end anastomosis between the proximal residual stomach and the distal residual stomach. Laparoscopic segmental gastrectomy was performed with a standard technique. After general anesthesia with endotracheal intubation, the pneumoperitoneum was established via umbilical puncture with a Veress needle. A 1 cm transverse skin incision was made on the umbilicus, and a 10 mm lengthened trocar was implanted as the observation hole. Under the guidance of a laparoscope, a 10 mm trocar was implanted in the midline of the left upper abdomen clavicle as the main operation hole. Additionally, 10 mm and 5 mm trocars were respectively implanted in the corresponding location of the right side of the abdominal wall and above the main surgical opening as auxiliary operation holes. A 10 mm trocar was also implanted below the xiphoid to place the liver retractor and expose the operative area when necessary. The resection line was made close to the tumor margins between differentiated and undifferentiated-type histology. The greater omentum was preserved, and the gastrocolic ligament corresponding to the resection area of the stomach was dissected after separation at least 3 cm away from the gastroepiploic vessels. After transection of the stomach, the surgical margins were subjected to intraoperative histological examination by frozen section analysis. Reconstruction was performed using mechanical stapling devices for end-to-end anastomosis. Special care was taken to avoid tumor capsule rupture during specimen handling and extraction from the cavity. The specimen was retrieved using a special pouch (Endocatch; Covidien, United States). Figure 1 shows the process of tumor resection. The procedure of end-to-end anastomosis is presented in Figure 2. Figure 3 shows the gastric sac after segmental resection.
There were no conversions to open technique or other procedures. The mean [standard deviation (SD)] operative time was 112.4 (32.4) min (range 60-180 min). Mean (SD) operative blood loss was 73.5 (33.1) mL (range 30-150 mL).The mean (SD) length of hospital stay was 4.6 (0.8) d (range 3-6 d). There were no instances of anastomotic leakage, hemorrhage, gastric stasis, wound infection or operative death.
The mean (SD) follow-up time was 35.4 (13.0) mo (range 6-60 mo). The postoperative course is summarized in Table 2. In the 3 mo after surgery, postoperative weight recovery was significantly improved in all patients (before vs after: 62.3 ± 9.8 vs 61.1 ± 9.6, P < 0.05). The incidence of reflux esophagitis and gastritis after surgery was less frequent in the laparoscopic segmental gastrectomy patients. Reflux gastritis[11] (segmental gastrectomy vs distal gastrectomy: 11.8 vs 63.8%, P < 0.05 ).
| Postoperative complications | n (%) |
| Gastric stasis | 0 |
| Umping syndrome | 0 |
| Reflux gastritisa | 2 (11.8) |
| Reflux esophagitis | 2 (11.8) |
The number of meals, single meal intake and Visick quality of life assessment are summarized in Table 3. Three mo after surgery all patients had a single meal intake that was more than half of the preoperative dose and ate fewer meals. The Visick grading index assessment was performed according to the degree of gastrointestinal symptoms in patients 3 mo after surgery. No recurrence or death was observed in all patients during a median follow-up period of 35.4 mo.
| Post-operative meal | n (%) |
| The number of meals > 5 times | 0 |
| Compared with preoperative, single meal intake > 50% | 17 (100) |
| Visick quality of life assessment | |
| Grade I | 12 (70.6) |
| Grade II | 5 (29.4) |
| Grade III | 0 (0.0) |
| Grade IV | 0 (0.0) |
GISTs are infamous for their complexity in management from diagnosis to treatment. Tumors are usually discovered in patients who present with abdominal pain, anemia, stomach upset, early satiety or other signs of gastrointestinal bleeding. Thus, GISTs are often diagnosed incidentally[12]. Surgery remains the gold standard for the management of GISTs. According guidelines from the European Society for Medical Oncology, lesions larger than 2 cm should be removed[13]. A primary gastric GIST should be resected by wedge resection or a larger resection that includes subtotal gastrectomy and total gastrectomy.
As for tumors that grow in the middle third of the stomach and on the lesser stomach curvature, removal by wedge resection can injure the cardia and pylorus, causing postoperative gastric stenosis and even residual gastric obstruction. Therefore, laparoscopic primary GIST resection for these tumors should be considered by surgeons with advanced laparoscopic skills for subtotal gastrectomy or total gastrectomy.
However, GISTs are benign tumors with a tendency toward malignant transformation. Postoperative tumor-free survival is usually longer making eating and quality of life important considerations after surgery. Unfortunately, radical resection with retention of gastric function is often at odds with improved survival and quality of life.
In this study, we reported laparoscopic segmental gastrectomy with residual gastric end-to-end anastomosis in 17 patients with GISTs. Segmental gastrectomy has not been previously used to treat GISTs, since it was first reported by Wangensteen et al[14] as an operation for peptic ulcer in 1952. Yang et al[11] reported that the incidence of biliary reflux was 60.9% with uncut Roux-en-Y and 90.3% with Billroth II reconstruction 1 year after distal gastrectomy. The incidence of reflux gastritis was similar for both techniques (70.8% for Roux-en-Y and 63.8% for Billroth II).
In our series, the incidence of reflux esophagitis and gastritis after surgery was infrequent, occurring in only one patient. Meanwhile, not only the blood supply but also the venous drainage of the pyloric region was better with our procedure, and no postoperative gastric stasis was observed. The number of meals, single meal intakes and the Visick assessment of quality of life 3 mo after surgery suggests that all patients had better recovery of gastrointestinal function.
Laparoscopic segmental gastrectomy for primary gastric GISTs seems to be feasible and safe. However, further studies including a larger series of patients and longer follow-up periods are needed to fully evaluate the advantages of laparoscopic segmental gastrectomy.
We would like to acknowledge the assistance of John T. Cathey in language.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hilmi I S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX
| 1. | Rubin JL, Sanon M, Taylor DC, Coombs J, Bollu V, Sirulnik L. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. Int J Gen Med. 2011;4:121-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Mucciarini C, Rossi G, Bertolini F, Valli R, Cirilli C, Rashid I, Marcheselli L, Luppi G, Federico M. Incidence and clinicopathologic features of gastrointestinal stromal tumors. A population-based study. BMC Cancer. 2007;7:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Kingham TP, DeMatteo RP. Multidisciplinary treatment of gastrointestinal stromal tumors. Surg Clin North Am. 2009;89:217-233, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA, Demetri GD, Fletcher CD, Fletcher JA. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118-8121. [PubMed] |
| 5. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1720] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 6. | Furukawa H, Hiratsuka M, Imaoka S, Ishikawa O, Kabuto T, Sasaki Y, Kameyama M, Ohigashi H, Nakano H, Yasuda T, Murata K. Phase II study of limited surgery for early gastric cancer: segmental gastric resection. Ann Surg Oncol. 1999;6:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Ishikawa K, Arita T, Ninomiya S, Bandoh T, Shiraishi N, Kitano S. Outcome of segmental gastrectomy versus distal gastrectomy for early gastric cancer. World J Surg. 2007;31:2204-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Shimoyama S, Seto Y, Yasuda H, Mafune K, Kaminishi M. Concepts, rationale, and current outcomes of less invasive surgical strategies for early gastric cancer: data from a quarter-century of experience in a single institution. World J Surg. 2005;29:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Morita S, Katai H, Saka M, Fukagawa T, Sano T, Sasako M. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 2008;95:1131-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 10. | Poveda A, García Del Muro X, López-Guerrero JA, Cubedo R, Martínez V, Romero I, Serrano C, Valverde C, Martín-Broto J; GEIS (Grupo Español de Investigación en Sarcomas/Spanish Group for Sarcoma Research). GEIS guidelines for gastrointestinal sarcomas (GIST). Cancer Treat Rev. 2017;55:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 11. | Yang D, He L, Tong WH, Jia ZF, Su TR, Wang Q. Randomized controlled trial of uncut Roux-en-Y vs Billroth II reconstruction after distal gastrectomy for gastric cancer: Which technique is better for avoiding biliary reflux and gastritis? World J Gastroenterol. 2017;23:6350-6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 12. | Patil DT, Rubin BP. Gastrointestinal stromal tumor: advances in diagnosis and management. Arch Pathol Lab Med. 2011;135:1298-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY; ESMO Guidelines Working Group. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 14. | Wangensteen OH. Segmental gastric resection for peptic ulcer; method permitting restoration of anatomic continuity. J Am Med Assoc. 1952;149:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |