Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3334
Peer-review started: March 30, 2020
First decision: April 24, 2020
Revised: May 27, 2020
Accepted: July 14, 2020
Article in press: July 14, 2020
Published online: August 6, 2020
Processing time: 132 Days and 6.8 Hours
Type A insulin resistance syndrome is a rare disorder caused by mutations in the gene encoding the insulin receptor. Its coexistence with ovarian serous papillary cystadenofibroma is even rarer.
A 14-year-old girl developed type A insulin resistance syndrome and showed high fasting insulin, glucose, and hemoglobin A1c (HbA1c) levels. The girl suffered from ovarian serous papillary cystadenofibroma. The laboratory results were as follows: fasting insulin was 2624.90 pmol/L and HbA1c was 8.5%. A heterozygous missense mutation on exon 20 of the insulin receptor gene (c.3601C>T, Arg1201Trp) was observed. The histopathological diagnosis was a cystic lesion that extended to the upper right uterus, indicating a right ovarian serous papillary cystadefibroma accompanied by focal interstitial hyperplasia. The patient was treated with metformin for over 6 mo. Additionally, laparoscopic resection (bilateral) of the ovarian lesion and laparoscopic intestinal adhesiolysis were performed under general anesthesia. Diet therapy combined with exercise was then initiated. The patient had an uneventful recovery. The patient also showed improved blood glucose control, with reduced levels of fasting insulin (857.84 pmol/L) and HbA1c (7.0%).
Insulin resistance may play a significant role in the induction of tumors. It is important to investigate further the association between insulin resistance and tumors and the underlying mechanism.
Core tip: Type A insulin resistance syndrome (TAIRS) is a rare disorder that results from mutations in the gene encoding the insulin receptor. We present a rare case of TAIRS coexisting with an ovarian serous papillary cystadenofibroma in a 14-year-old girl caused by gene mutation. This case demonstrates that metformin has partial efficacy in the treatment of TAIRS. Furthermore, insulin may play a critical role in the development and progression of ovarian cystadenofibroma.
- Citation: Yan FF, Huang BK, Chen YL, Zhuang YZ, You XY, Liu CQ, Li XJ. Coexistence of ovarian serous papillary cystadenofibroma and type A insulin resistance syndrome in a 14-year-old girl: A case report. World J Clin Cases 2020; 8(15): 3334-3340
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3334.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3334
Type A insulin resistance syndrome (TAIRS), which is similar to Rabson-Mendenhall syndrome and Leprechaunism syndrome, is a rare disorder caused by mutations in the gene encoding the insulin receptor[1]. It is usually diagnosed in lean adolescent girls accompanied by hirsutism, oligomenorrhea, acne, and acanthosis nigricans. In contrast to Rabson-Mendenhall syndrome and Leprechaunism syndrome, which are characterized by several phenotypes including intrauterine and postnatal growth retardation, dysmorphic features, and early mortality, TAIRS is relatively mild. Ovarian serous papillary cystadenofibroma is also an infrequent disorder that contains both epithelial and fibrous stromal components and is mainly found in women aged from 15 years to 65 years. Here, we present an unusual case of TAIRS coexisting with an ovarian cystadenofibroma in a 14-year-old girl. To the best of our knowledge, this is the first case to report such a combination.
A 14-year-old girl was transferred to our outpatient department with symptoms including hirsutism and acanthosis nigricans, which had been noted since the age of 10 years. Her birth weight was 2.75 kg at a gestational age of 40 wk. Secondary sex characteristics and accelerated growth occurred from the age of 13 but without menarche.
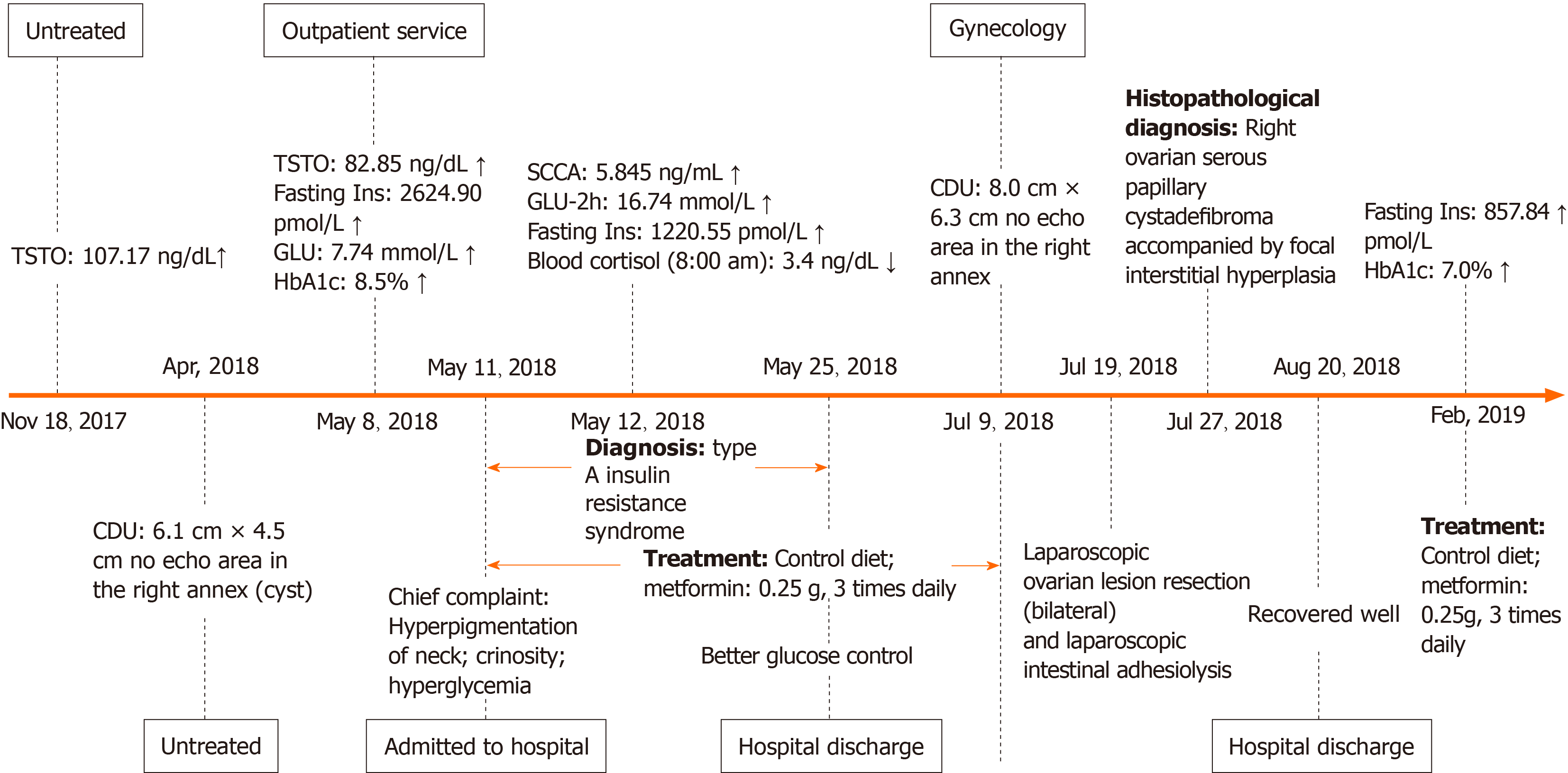
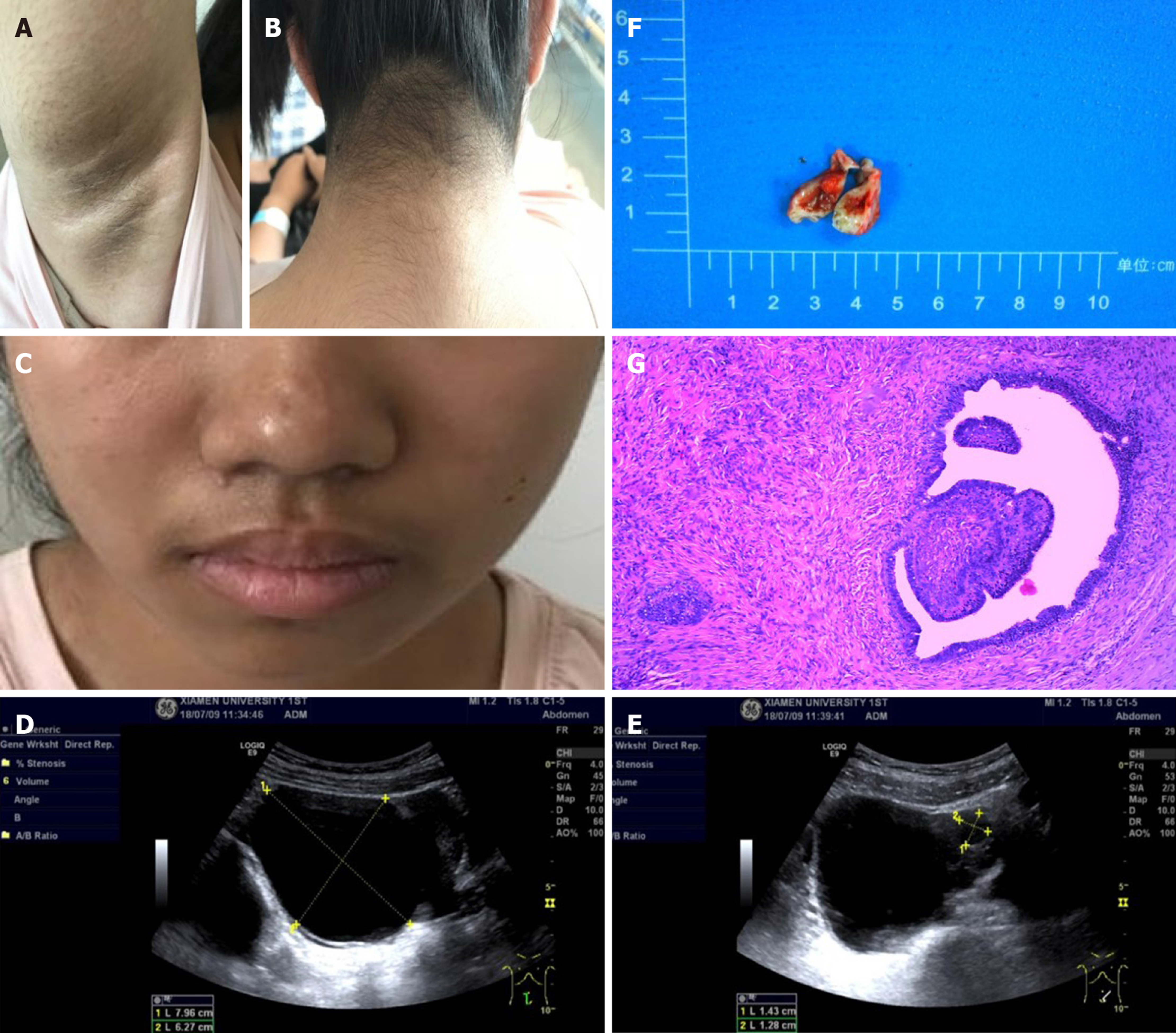
Physical examination revealed that her body mass index was 18.1 kg/m2. The Ferriman-Gallwey score was 19 (average 5). Clinical symptoms, laboratory indices, and treatment of the patient during 2018-2019 are shown in Figure 1. Evidence of acanthosis nigricans was observed in the neck and axillae (Figure 2A-C). Examination of the genitalia disclosed a mild clitoromegaly.
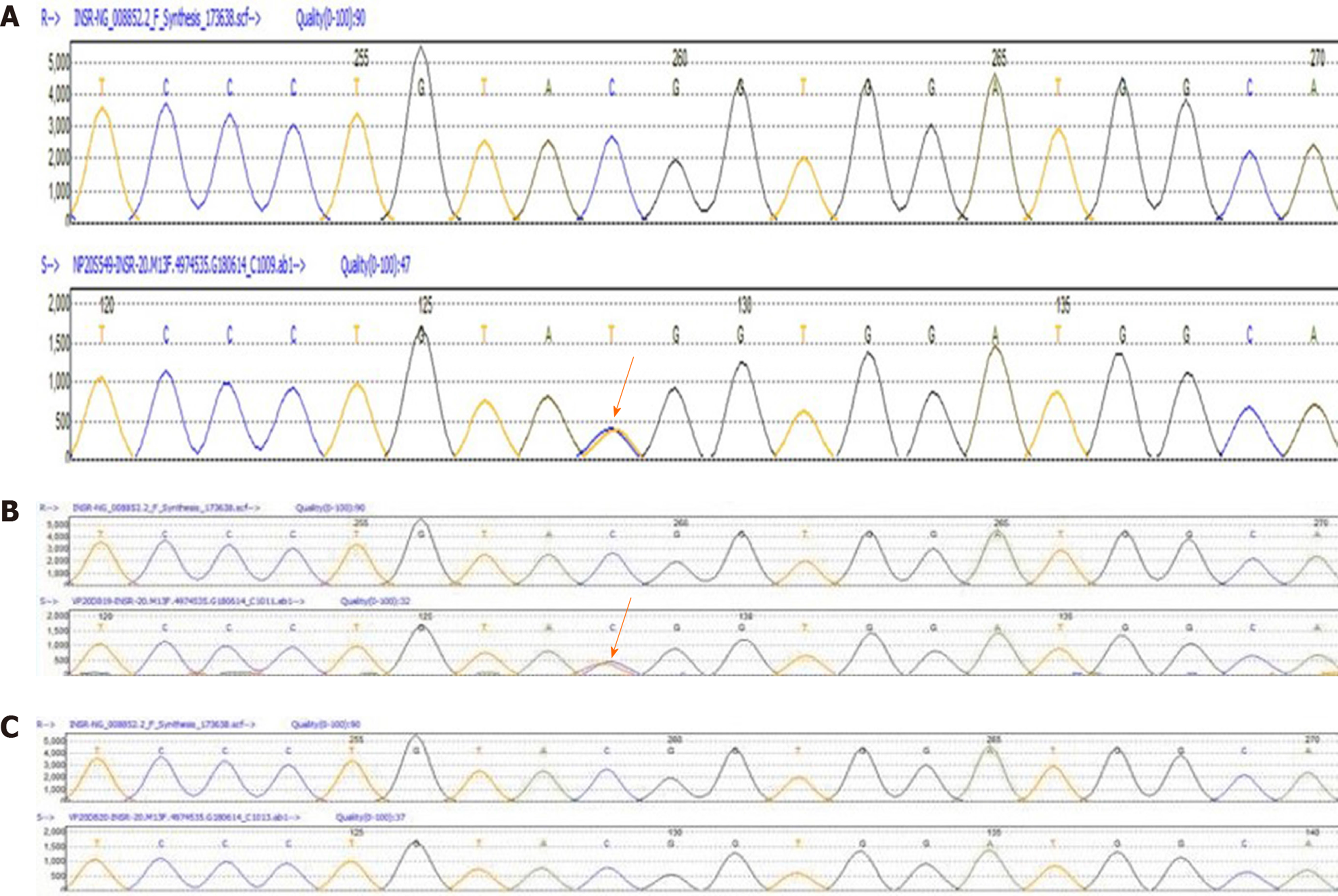
A 75-g oral glucose tolerance test (OGTT) showed that plasma glucose was 5.7, 10.8, 13.8, and 16.7 mmol/L at 0, 0.5-, 1-, and 2-h, respectively. Serum insulin concentrations were 1220.6, 2594.9, 3649.9, and 6958.3 pmol/L, respectively. Hemoglobin A1c (HbA1c) level was 8.3%. Hormone tests showed an elevated testosterone level at 82.85 ng/mL, (reference range 14-76 ng/mL). Molecular analysis showed a heterozygous missense mutation on exon 20 of the insulin receptor gene (Arg1201Trp) (Figure 3A). Furthermore, the patient’s phenotype matched the common genotypes of the mutation occurring in the insulin receptor gene. Chromosome test revealed a 46, XX karyotype.
No masses were identified on abdominal palpation. Pelvic sonography showed an 8.0 cm × 6.3 cm cystic lesion that extended to the upper right uterus, which appeared to originate from the ovary (Figure 2D). In addition, the cystic lesion included a larger quasi-circular anechoic area (1.4 cm × 1.2 cm) (Figure 2E). There was no free or circumscribed fluid in the pelvis. Patient was suspected to have an ovarian tumor.
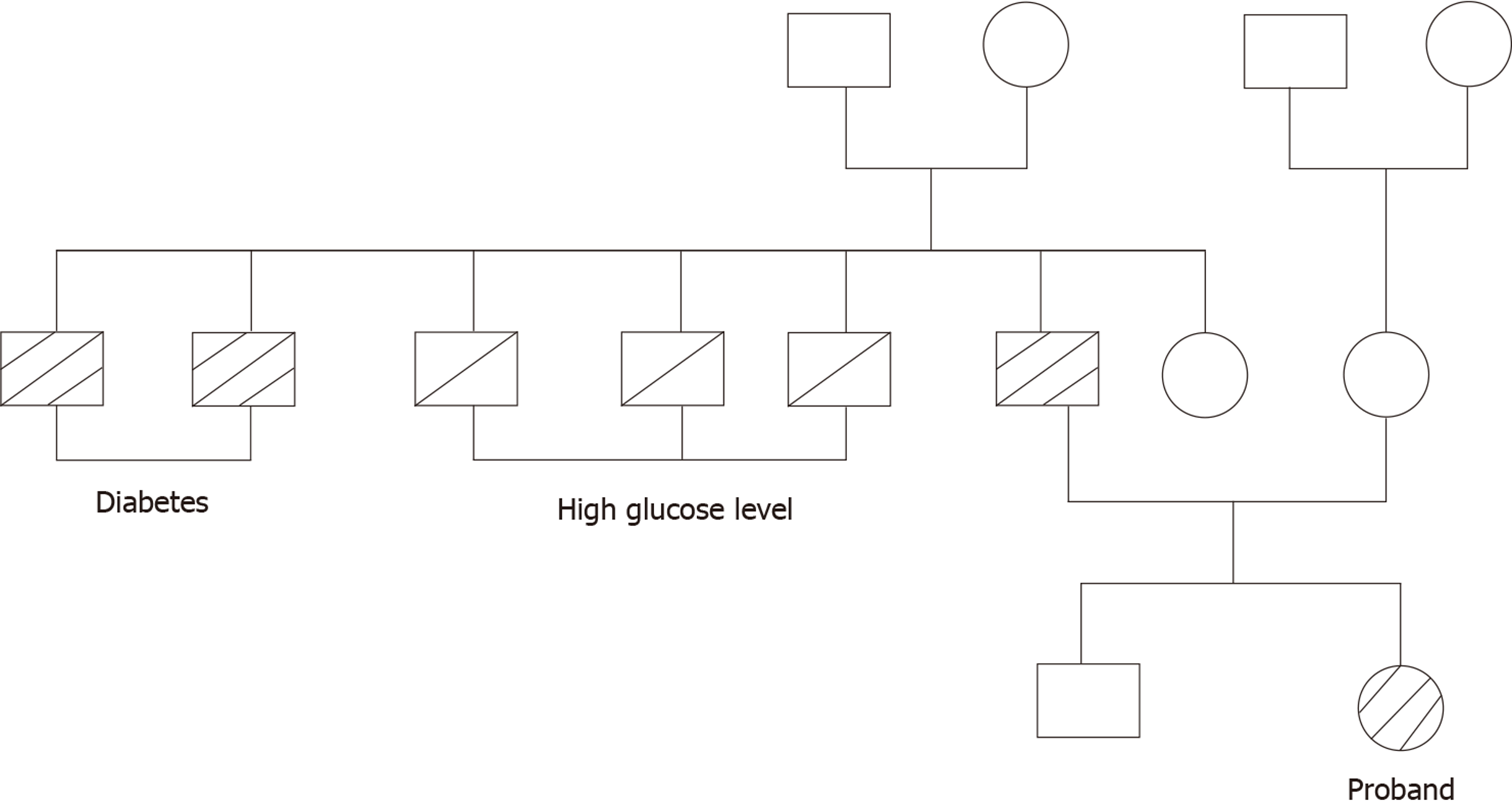
Her father was diagnosed with type 2 diabetes by the 75-g OGTT (fasting glucose, 14.3 mmol/L and postprandial, 26.7 mmol/L). He did not show any common clinical features of TAIRS. His blood glucose was well controlled with metformin 500 mg three times a day. Her mother’s glucose level was normal by 75-g OGTT (Table 1). Fasting plasma glucose and fasting insulin level in her younger brother were 4.27 mmol/L and 212.67 pmol/L, respectively. The family history of diabetes is shown in Figure 4. Her father underwent genetic analyses and showed a heterozygous missense mutation on exon 20 of the insulin receptor gene (Arg1201Trp) (Figure 3B). Her mother was normal in the genetic analyses (Figure 3C).
| 0 min | 30 min | 60 min | 120 min | |
| Father | ||||
| Glucose in mmol/L | 14.33 | 19.88 | 26.72 | 28.62 |
| Insulin in pmol/L | 148.35 | 176.44 | 325.94 | 315.81 |
| Mother | ||||
| Glucose in mmol/L | 5.38 | 8.04 | 8.73 | 6.30 |
| Insulin in pmol/L | 45.82 | 269.79 | 372.97 | 279.81 |
Based on her clinical features, blood glucose, and insulin level, the patient was diagnosed with TAIRS. Final histopathological diagnosis of postsurgical tissue showed a right ovarian serous papillary cystadenofibroma accompanied by focal interstitial hyperplasia (Figure 2F and G).
The patient took metformin for over 6 mo to treat TAIRS. Furthermore, laparoscopic resection (bilateral) of the ovarian lesion and laparoscopic intestinal adhesiolysis were performed under general anesthesia.
Fasting insulin level decreased to 857.84 pmol/L and HbA1c level dropped to 7.0%. Menstruation was not recorded during or after treatment. The patient had an uneventful recovery. Outpatient follow-up indicated that the patient was following the doctor’s advice by controlling her diet, taking her daily medicine, and exercising.
In this study, we report the first case of TAIRS coexisting with an ovarian serous papillary cystadenofibroma. TAIRS is one of three severe insulin resistance syndromes and is relatively mild. The patient showed hyperandrogenism, hyperinsulinemia, acanthosisnigricans, hirsutism, and diabetes mellitus but without the dysmorphic characteristic of leprechaunism or Rabson-Mendenhall syndrome. Moreover, genetic screening identified a single heterozygous mutation involving the tyrosine kinase domain in the β subunit of the insulin receptor gene. To date, more than 100 disease-causing mutations have been identified[2], and the phenotype usually varies in patients with different gene mutations[3]. The Arg1201Trp mutation that was identified in our patient has previously been reported in patients with severe syndromes of insulin resistance, such as leprechaunism and Rabson-Mendenhall syndrome[3-6], but has not been found in patient with TAIRS. Thus, the phenotype attributed to the mutation of the same gene locus can also be inconsistent.
There is a lack of large-scale, long-term, randomized controlled studies focusing on the treatment and prognosis of TAIRS to guide treatment choice in clinical practice. Treatment of TAIRS is usually empirical and lacks evidence. Many medications, including metformin, thiazolidinediones, acarbose, and leptin, have been used in patients with severe insulin resistance syndrome[7-10]. In China, metformin is the only oral antidiabetic agent approved for use in patients under the age of 14 years. In our case, the patient was treated with metformin for 6 mo, and fasting serum insulin level decreased from 1220.55 pmol/L to 857.84 pmol/L, and HbA1c level decreased by 1.3%. This indicated that metformin had a partial effect and can improve insulin sensitivity to a certain degree, as reported previously[11]. Despite acceptable control during adolescence, long-term metabolic control has been reported to be poor and diabetes complications are frequent in TAIRS patients[10,12]. Therefore, other than metformin, several future-proof treatments such as metreleptin, sodium–glucose cotransporter 2 inhibitor, and incretin[10,13] should be introduced during childhood.
Ovarian cystadenofibroma accounts for 1.7% of all benign ovarian tumors[14] that originate in the epithelium, which are classified as serous, endometrioid, mucinous, clear cell, and mixed categories. Ovarian cystadenofibroma is usually asymptomatic and is found incidentally, as in our case. A cystadenofibroma may show a solitary cyst or a multiloculated cystic mass, with solid nodules or papillary projections, and almost half of cases demonstrate increased vascularity on ultrasonography[15]. Clinically, because ovarian cystadenofibroma is a multicystic mass with solid components, preoperative differential diagnosis is important to distinguish it from malignant neoplasms. A computed tomography scan also provides limited information in the evaluation of this tumor. A frozen section diagnosis may be helpful in these cases and may help to avoid an extensive surgical procedure. The patient in this study had two rarely diagnosed conditions. It is unknown whether there is a correlation between these two rare diseases. Insulin can activate both the insulin receptor and insulin-like growth factor receptor, particularly at high concentrations, to promote tumor cell proliferation and migration, which possibly plays a critical role in tumor development and progression[16,17]. Previous studies suggest that exogenous insulin exposure is significantly associated with an increased incidence of cancer[18]. Although a high insulin level may be related to ovarian serous papillary cystadenofibroma onset and/or progression, the mechanism remains unclear. Further study is required to determine the mechanistic details associated with the connection between TAIRS and ovarian cystadenofibroma.
In this report, we highlight the rare coexistence of TAIRS and an ovarian serous papillary cystadenofibroma. However, this study also had some limitations. First, there was an over 1-mo delaying in treating TAIRS as the patient underwent surgery. Second, the patient’s ovarian cysts were surgically treated more than 1 year after diagnosis.
We report the first case of TAIRS coexisting with an ovarian serous papillary cystadenofibroma in a 14-year-old girl. We found that metformin had partial efficacy in TAIRS and improved insulin sensitivity to a certain extent. With respect to the ovarian serous papillary cystadenofibroma, cystectomy should be performed after puberty to prevent the risk of tumor development in the ovaries. The clinic-opathological results support the notions that insulin may play a critical role in tumor development and progression.
We would like to thank all of the patient, doctors, nurses, and technicians involved at our center for their dedication to the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishibashi S S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Liu JH
| 1. | Parker VE, Semple RK. Genetics in endocrinology: genetic forms of severe insulin resistance: what endocrinologists should know. Eur J Endocrinol. 2013;169:R71-R80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Ardon O, Procter M, Tvrdik T, Longo N, Mao R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol Genet Metab Rep. 2014;1:71-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Longo N, Wang Y, Smith SA, Langley SD, DiMeglio LA, Giannella-Neto D. Genotype-phenotype correlation in inherited severe insulin resistance. Hum Mol Genet. 2002;11:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Takasawa K, Tsuji-Hosokawa A, Takishima S, Wada Y, Nagasaki K, Dateki S, Numakura C, Hijikata A, Shirai T, Kashimada K, Morio T. Clinical characteristics of adolescent cases with Type A insulin resistance syndrome caused by heterozygous mutations in the β-subunit of the insulin receptor (INSR) gene. J Diabetes. 2019;11:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Whitehead JP, Soos MA, Jackson R, Tasic V, Kocova M, O'Rahilly S. Multiple molecular mechanisms of insulin receptor dysfunction in a patient with Donohue syndrome. Diabetes. 1998;47:1362-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Tsuji-Hosokawa A, Takasawa K, Nomura R, Miyakawa Y, Numakura C, Hijikata A, Shirai T, Ogawa Y, Kashimada K, Morio T. Molecular mechanisms of insulin resistance in 2 cases of primary insulin receptor defect-associated diseases. Pediatr Diabetes. 2017;18:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Semple RK, Williams RM, Dunger DB. What is the best management strategy for patients with severe insulin resistance? Clin Endocrinol (Oxf). 2010;73:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Hattori Y, Satoh H, Namatame T, Hattori S, Kasai K. A patient with extreme insulin resistance syndrome treated with pioglitazone. Diabetes Care. 2006;29:2328-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Cochran E, Young JR, Sebring N, DePaoli A, Oral EA, Gorden P. Efficacy of recombinant methionyl human leptin therapy for the extreme insulin resistance of the Rabson-Mendenhall syndrome. J Clin Endocrinol Metab. 2004;89:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Brown RJ, Cochran E, Gorden P. Metreleptin improves blood glucose in patients with insulin receptor mutations. J Clin Endocrinol Metab. 2013;98:E1749-E1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Moreira RO, Zagury RL, Nascimento TS, Zagury L. Multidrug therapy in a patient with Rabson-Mendenhall syndrome. Diabetologia. 2010;53:2454-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, Taylor S, Gorden P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore). 2004;83:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kawana Y, Imai J, Sawada S, Yamada T, Katagiri H. Sodium-Glucose Cotransporter 2 Inhibitor Improves Complications of Lipodystrophy: A Case Report. Ann Intern Med. 2017;166:450-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Cho SM, Byun JY, Rha SE, Jung SE, Park GS, Kim BK, Kim B, Cho KS, Jung NY, Kim SH, Lee JM. CT and MRI findings of cystadenofibromas of the ovary. Eur Radiol. 2004;14:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Alcázar JL, Errasti T, Mínguez JA, Galán MJ, García-Manero M, Ceamanos C. Sonographic features of ovarian cystadenofibromas: spectrum of findings. J Ultrasound Med. 2001;20:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Rostoker R, Abelson S, Bitton-Worms K, Genkin I, Ben-Shmuel S, Dakwar M, Orr ZS, Caspi A, Tzukerman M, LeRoith D. Highly specific role of the insulin receptor in breast cancer progression. Endocr Relat Cancer. 2015;22:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Singh P, Alex JM, Bast F. Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer. Med Oncol. 2014;31:805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 18. | Karlstad O, Starup-Linde J, Vestergaard P, Hjellvik V, Bazelier MT, Schmidt MK, Andersen M, Auvinen A, Haukka J, Furu K, de Vries F, De Bruin ML. Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr Drug Saf. 2013;8:333-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |