Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3329
Peer-review started: April 7, 2020
First decision: June 8, 2020
Revised: June 21, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 6, 2020
Processing time: 119 Days and 5.1 Hours
A primary central nervous system lymphoma (PCNSL) presenting with massive hemorrhage is a rare occurrence that is difficult to distinguish from a high-grade glioblastoma. Comprehensive descriptions of the imaging characteristics of such tumors have not yet been reported. Herein, we reported a case of a PCNSL with massive hemorrhage by presenting the imaging features of computed tomography (CT) imaging and structural and perfusion magnetic resonance imaging (MRI).
A 48-year-old man presented with headache lasting for 10 d. CT of the brain showed a round, heterogeneous, high-density lesion with surrounding edema in the right temporal lobe. For further diagnosis, a series of MRI examinations of the brain were subsequently performed, and a hemorrhagic lesion with ring-like enhancement was determined. The whole lesion was relatively hypoperfused on arterial spin labeling images. Surgical resection of the lesion and histopathological examination confirmed that the lesion was a diffuse large B-cell lymphoma with massive hemorrhage.
PCNSLs with hemorrhage occur very rarely, and structural and perfusion MRI examinations are requested exceedingly rarely. This case provided insight into some characteristics of a hemorrhagic lymphoma on CT and MRI examinations. Perfusion MRI examination may be useful for the differential diagnosis of PCNSLs and other brain tumors.
Core tip: Primary central nervous system lymphoma (PCNSL) presenting with massive hemorrhage is a rare occurrence that is difficult to distinguish from a high-grade glioblastoma. Little is known about its imaging features, especially multimodal magnetic resonance imaging (MRI) findings. Here, we report on the computed tomography and MRI findings of PCNSL in a 48-year-old man. To the best of our knowledge, this is the first report with detailed computed tomography and MRI findings in hemorrhagic PCNSL and could provide useful information for the preoperative diagnosis.
- Citation: Wu YW, Zheng J, Liu LL, Cai JH, Yuan H, Ye J. Imaging of hemorrhagic primary central nervous system lymphoma: A case report. World J Clin Cases 2020; 8(15): 3329-3333
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3329.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3329
Primary central nervous system lymphoma (PCNSL) is a relatively rare tumor that accounts for approximately 2%-6% of all primary brain tumors. The majority of intracerebral lymphomas are non-Hodgkin’s lymphomas, and approximately 90% are diffuse large B-cell lymphomas[1,2]. PCNSL is less common in immunocompetent individuals. However, due to the increasing prevalence of human immunodeficiency virus infection and the growing number of organ transplantations, the incidence of PCNSL has been increasingly observed in both immunocompromised patients and the immunocompetent population over the last decades[3]. Mild to moderate edema and space-occupying effects can always be seen in PCNSL[4], but massive hemorrhage at presentation in PCNSL is extremely rare. The presence of hemorrhage is utilized to exclude primary cerebral lymphoma in the differential diagnosis[5].
The clinical treatment for PCNSLs is different from that of other tumors, and an early and accurate diagnosis is vital to improve the treatment outcomes. Herein, we present a particular case of PCNSL with multimodal magnetic resonance imaging (MRI) examinations. The purpose of this study was to provide insight into some MRI characteristics of hemorrhagic lymphomas and to facilitate the differentiation between PCNSLs and other brain tumors.
A 48-year-old man was admitted to a local hospital with a 10 d history of headache and dizziness, followed by aggravation of these symptoms for 2 d.
None.
The patient was previously in good condition and had no history of congenital or acquired immunodeficiency.
A series of routine examinations before the surgical operation were unremarkable, including: (1) Routine blood testing of serum levels of platelet, alpha fetoprotein, Carcinoembryonic antigen, CA199, neuron-specific enolase and progastrin releasing peptide; and (2) Cerebrospinal fluid analysis.
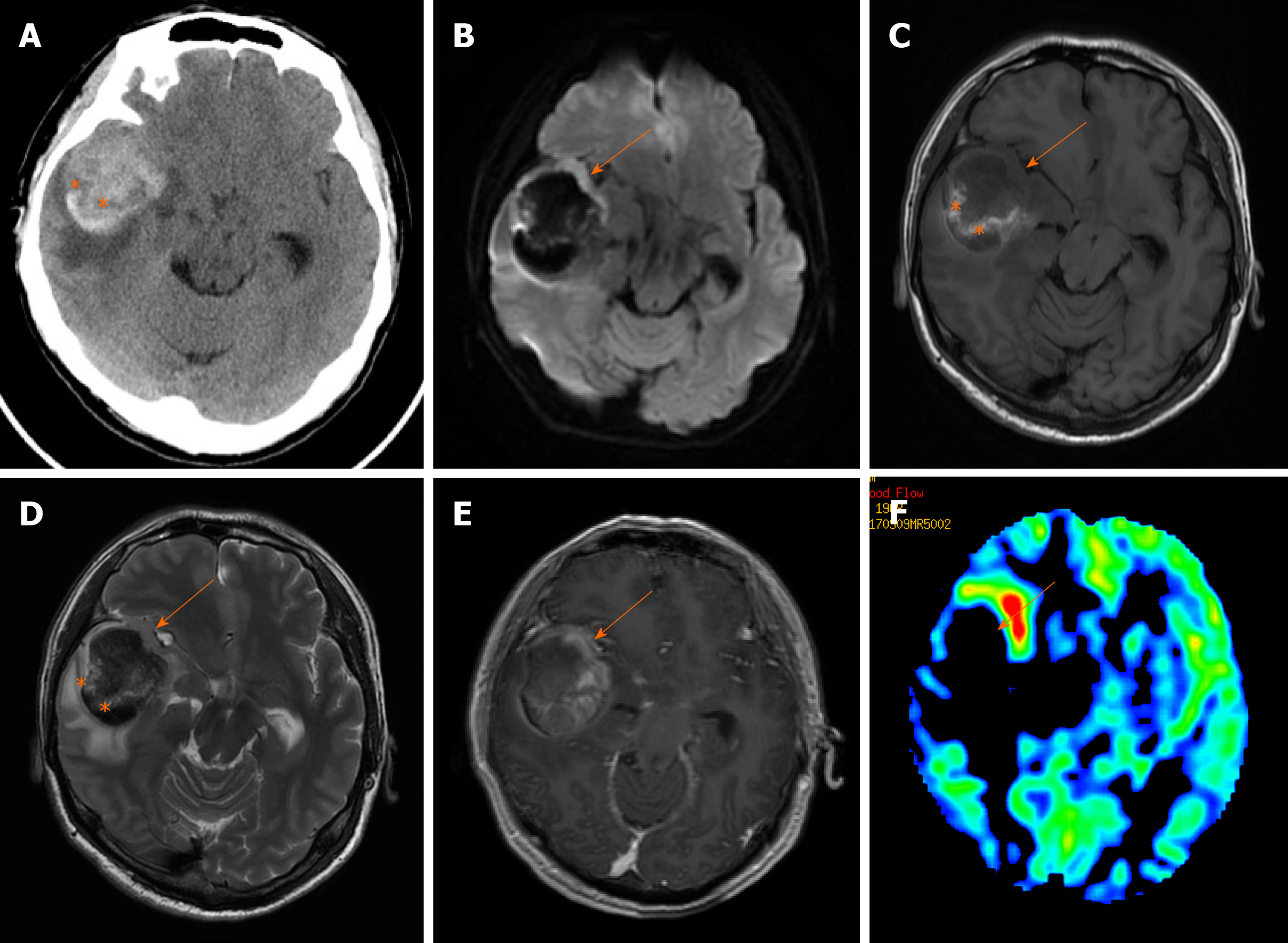
Non-contrast-enhanced computed tomography (CT) examination was performed immediately after admission, and a round and high-density lesion approximately 56 mm × 46 mm in size was found in the right temporal lobe surrounded by moderate edema (Figure 1A). It compressed the right basal ganglia and the right lateral ventricle, forcing a midline shift towards the left. For further diagnosis, CT angiography examination was performed, and the possibilities of vascular malformation and aneurysms were excluded. Multimodal MRI examinations of the brain were conducted on the same day. The gross appearance of the lesion was heterogeneous on MR images. The parenchyma of the lesion showed iso- to hypointensity on T1-weighted (T1W) and T2-weighted images, which can be interpreted as a relatively hyperintense signal on diffusion-weighted images (DWI). Contrast-enhanced T1W images showed the lesion with ring-like enhancement. The gross lesion displayed relatively low perfusion on arterial spin labeling (ASL) images (Figure 1).
According to the radiological findings, the initial imaging impression was glioblastoma combined with hemorrhage. Surgery was performed 3 d after admission to the hospital. The lesion was completely resected, and the hematoma (approximately 30 mL) was also removed. The parenchyma of the lesion was 30 mm × 25 mm × 20 mm in size; it looked like rotten fish, was yellow-gray in color and accompanied by some blood vessels.
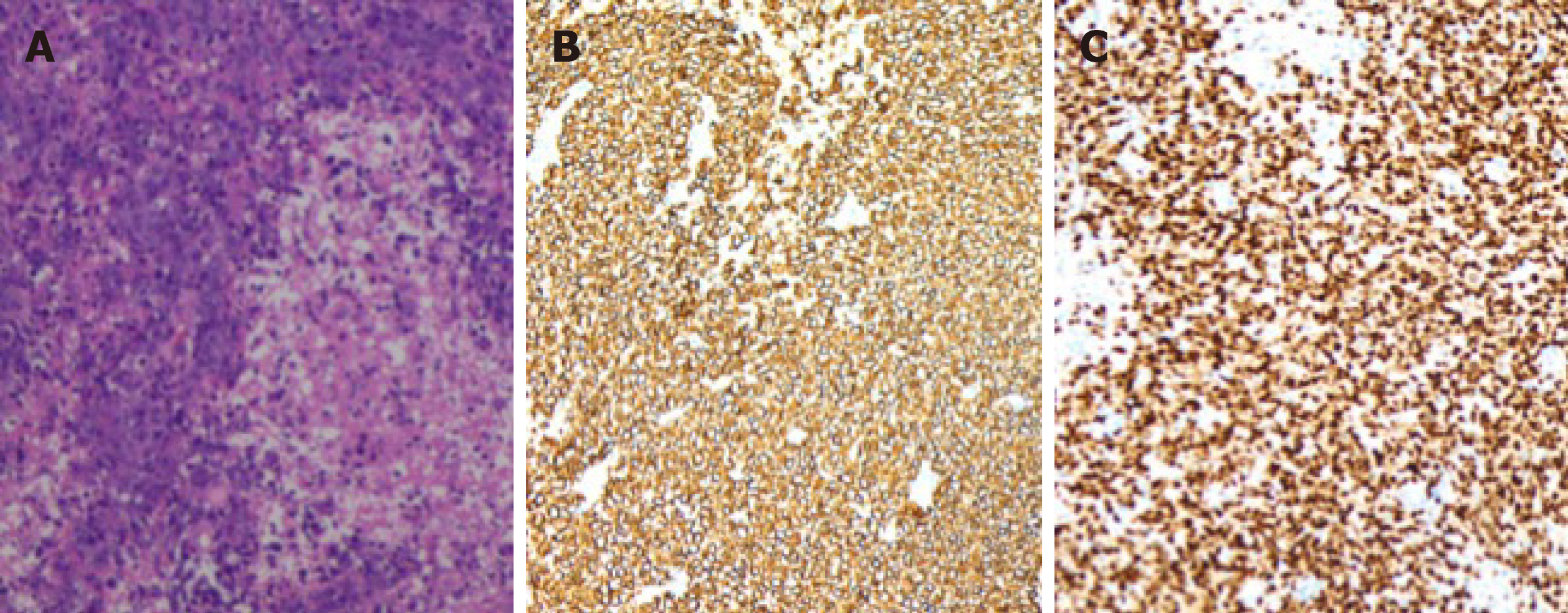
Histopathological examination revealed that the nuclei of the tumor cells were round or elliptic, with prominent nucleoli, diffuse arrangement and necrotic tissue formed in the lesion. In the immunohistochemical stains, cells were positive for B-cell markers (CD20 and Pax-5; Figure 2), and the Ki-67 index was 70%. The lesion was identified as a diffuse large B-cell lymphoma with acute hemorrhage.
The lesion was completely resected.
The patient was treated with chemotherapy following surgery. He had no imaging findings of recurrence at 6 and 12 mo after treatment.
Even though PCNSLs are relatively rare, and represent 1%-2% of all primary CNS malignancies, their incidence has risen over recent years[3]. In immunocompetent populations, the median age of PCNSL occurrence is 53–years-old to 57-years-old, with a male to female ratio of 1.5:1[6]. PCNSLs can occur in the brain parenchyma, meninges, eyes or spinal cord. Approximately 70% are restricted to the supratentorial brain[2]. The most common presentation of a PCNSL is a single intracranial mass. The clinical manifestations of PCNSLs are similar to those of other intracranial tumors, including high intracranial pressure and focal neurological deficits. To date, six cases of lymphoma with hemorrhage have been reported. Massive bleeding hemorrhage at the first presentation of lymphoma has been reported in only three cases thus far, and this is the only case with detailed structural and perfusion MRI examinations.
In this case, the structural MR images revealed that the parenchyma of the lesion was consistent with a previous study on PCNSLs. PCNSLs always present with a defined margin and are surrounded by mild to moderate edema[2,7]. They appear as homogeneous, iso- to high-density lesions on CT images and as iso- to hypointense lesions relative to the gray matter in T1W and T2-weighted images due to the hypercellularity of the lymphomatous deposits. Approximately 85% of lesions exhibit homogeneous enhancement both on CT and MRI following contrast administration[8]. Ring-like enhancement is rarely seen unless necrosis occurs in the center of the mass, which can always be seen in acquired immunodeficiency syndrome-related PCNSLs[1,2]. On perfusion images, the lesions show relatively lower perfusion as a whole. The proliferation pattern of lymphomas includes vasocentric growth, and vessels in lymphomas are few and small. Compared with the fulminant neovascularization of glioblastomas, the blood flow of PCNSLs is relatively lower[9].
Recently, some advanced imaging techniques, such as ASL perfusion imaging and DWI, which respectively reflect tumor vascularity and cellularity, have been successfully applied for the differential diagnosis between lymphomas and other brain tumors[10]. The majority of PCNSLs demonstrate relatively lower perfusion in ASL images and higher intensity on DWI images than other brain tumors[7,11], which is consistent with our findings. As previous studies have shown, ASL and DWI are potential diagnostic tools for differentiating PCNSL from glioblastoma due to their differing hemodynamics and tumor density[12,13].
Hemorrhage is very rarely observed in untreated CNS lymphoma[4]. The mechanism of the occurrence of primary lymphoma with hemorrhage remains unclear, and it could be potentially explained by high immunoreactivity for a vascular endothelial growth factor may account for it[5,14,15]. Another explanation is that fragile vessels traversing necrotic areas or tumor invasion of large vessels lead to the breakdown of the vessel wall, resulting in bleeding[16].
Accurate diagnosis of lymphoma is essential in clinical practice and is related to therapeutic decision-making and the patients’ prognosis, whereas the diagnosis of atypical PCNSLs is difficult. We presented a special case of a PCNSL with acute massive hemorrhage. The case provided detailed information on CT and MRI examinations for reference, which may be useful for the differentiation between PCNSLs and other brain tumors.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Srinivasamurthy B S-Editor: Zhang L L-Editor: Filipodia E-Editor: Xing YX
| 1. | Haldorsen IS, Kråkenes J, Krossnes BK, Mella O, Espeland A. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989-2003. AJNR Am J Neuroradiol. 2009;30:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Nabavizadeh SA, Vossough A, Hajmomenian M, Assadsangabi R, Mohan S. Neuroimaging in Central Nervous System Lymphoma. Hematol Oncol Clin North Am. 2016;30:799-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, Guy G, Lapierre F. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg. 2000;92:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 357] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Jenkins CN, Colquhoun IR. Characterization of primary intracranial lymphoma by computed tomography: an analysis of 36 cases and a review of the literature with particular reference to calcification haemorrhage and cyst formation. Clin Radiol. 1998;53:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Kimura N, Ishibashi M, Masuda T, Morishige M, Abe T, Fujiki M, Kashima K, Kumamoto T. Primary central nervous system lymphoma with cortical laminar hemorrhage. J Neurol Sci. 2009;287:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Bessell EM, Hoang-Xuan K, Ferreri AJ, Reni M. Primary central nervous system lymphoma: biological aspects and controversies in management. Eur J Cancer. 2007;43:1141-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Partovi S, Karimi S, Lyo JK, Esmaeili A, Tan J, Deangelis LM. Multimodality imaging of primary CNS lymphoma in immunocompetent patients. Br J Radiol. 2014;87:20130684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Küker W, Nägele T, Korfel A, Heckl S, Thiel E, Bamberg M, Weller M, Herrlinger U. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005;72:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Furtner J, Schöpf V, Preusser M, Asenbaum U, Woitek R, Wöhrer A, Hainfellner JA, Wolfsberger S, Prayer D. Non-invasive assessment of intratumoral vascularity using arterial spin labeling: A comparison to susceptibility-weighted imaging for the differentiation of primary cerebral lymphoma and glioblastoma. Eur J Radiol. 2014;83:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Suh CH, Kim HS, Jung SC, Park JE, Choi CG, Kim SJ. MRI as a diagnostic biomarker for differentiating primary central nervous system lymphoma from glioblastoma: A systematic review and meta-analysis. J Magn Reson Imaging. 2019;50:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Yamashita K, Yoshiura T, Hiwatashi A, Togao O, Yoshimoto K, Suzuki SO, Abe K, Kikuchi K, Maruoka Y, Mizoguchi M, Iwaki T, Honda H. Differentiating primary CNS lymphoma from glioblastoma multiforme: assessment using arterial spin labeling, diffusion-weighted imaging, and ¹⁸F-fluorodeoxyglucose positron emission tomography. Neuroradiology. 2013;55:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Di N, Cheng W, Chen H, Zhai F, Liu Y, Mu X, Chu Z, Lu N, Liu X, Wang B. Utility of arterial spin labelling MRI for discriminating atypical high-grade glioma from primary central nervous system lymphoma. Clin Radiol. 2019;74:165.e1-165.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Lin X, Lee M, Buck O, Woo KM, Zhang Z, Hatzoglou V, Omuro A, Arevalo-Perez J, Thomas AA, Huse J, Peck K, Holodny AI, Young RJ. Diagnostic Accuracy of T1-Weighted Dynamic Contrast-Enhanced-MRI and DWI-ADC for Differentiation of Glioblastoma and Primary CNS Lymphoma. AJNR Am J Neuroradiol. 2017;38:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Rubenstein J, Fischbein N, Aldape K, Burton E, Shuman M. Hemorrhage and VEGF expression in a case of primary CNS lymphoma. J Neurooncol. 2002;58:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Kim IY, Jung S, Jung TY, Kang SS, Choi C. Primary central nervous system lymphoma presenting as an acute massive intracerebral hemorrhage: case report with immunohistochemical study. Surg Neurol. 2008;70:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Yuguang L, Meng L, Shugan Z, Yuquan J, Gang L, Xingang L, Chengyuan W. Intracranial tumoural haemorrhage--a report of 58 cases. J Clin Neurosci. 2002;9:637-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |