Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3305
Peer-review started: April 22, 2020
First decision: May 1, 2020
Revised: May 15, 2020
Accepted: July 16, 2020
Article in press: July 16, 2020
Published online: August 6, 2020
Processing time: 106 Days and 5 Hours
Patients with critical coronavirus disease 2019 (COVID-19), characterized by respiratory failure requiring mechanical ventilation (MV), are at high risk of mortality. An effective and practical MV weaning protocol is needed for these fragile cases.
Here, we present two critical COVID-19 patients who presented with fever, cough and fatigue. COVID-19 diagnosis was confirmed based on blood cell counts, chest computed tomography (CT) imaging, and nuclei acid test results. To address the patients’ respiratory failure, they first received noninvasive ventilation (NIV). When their condition did not improve after 2 h of NIV, each patient was advanced to MV [tidal volume (Vt), 6 mL/kg ideal body weight (IBW); 8-10 cmH2O of positive end-expiratory pressure; respiratory rate, 20 breaths/min; and 40%-80% FiO2] with prone positioning for 12 h/day for the first 5 d of MV. Extensive infection control measures were conducted to minimize morbidity, and pharmacotherapy consisting of an antiviral, immune-enhancer, and thrombosis prophylactic was administered in both cases. Upon resolution of lung changes evidenced by CT, the patients were sequentially weaned using a weaning screening test, spontaneous breathing test, and airbag leak test. After withdrawal of MV, the patients were transitioned through NIV and high-flow nasal cannula oxygen support. Both patients recovered well.
A MV protocol attentive to intubation/extubation timing, prone positioning early in MV, infection control, and sequential withdrawal of respiratory support, may be an effective regimen for patients with critical COVID-19.
Core tip: An effective and practical weaning protocol is of the utmost importance to coronavirus disease 2019 (COVID-19) patients with respiratory failure requiring mechanical ventilation. Here, we present two patients with critical COVID-19. Such patients can achieve good outcomes following a sequential weaning protocol that is attentive to the timing of intubation and extubation, early prone positioning, infection control, and sequential advancement and withdrawal of invasive ventilation.
- Citation: Peng M, Ren D, Liu YF, Meng X, Wu M, Chen RL, Yu BJ, Tao LC, Chen L, Lai ZQ. Two mechanically ventilated cases of COVID-19 successfully managed with a sequential ventilation weaning protocol: Two case reports. World J Clin Cases 2020; 8(15): 3305-3313
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3305.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3305
The viral pathogen severe acute respiratory syndrome-related novel coronavirus (SARS-CoV-2, also known as 2019 novel coronavirus or 2019-nCoV), which has been determined to have originated in Wuhan, China, has infected patients around the globe and produced the ongoing grave pandemic[1,2]. Human-to-human SARS-CoV-2 transmission between people in close contact has been known to occur since the middle of December 2019[3,4]. By late February 2020, there were tens of thousands of cases of coronavirus disease 2019 (COVID-19), the disease caused by infection with SARS-CoV-2, and several thousand deaths due to COVID-19 had been reported in China alone, in addition to thousands of COVID-19 cases in other countries[5]. Giving the immense spread of COVID-19, there is an urgent need for treatment options to support these patients.
Mortality due to COVID-19 is associated predominantly with respiratory failure requiring invasive mechanical ventilation (MV). In a small study of 36 patients diagnosed with COVID-19 and admitted to the intensive care unit (ICU) in a Wuhan hospital between January 1 to January 28, 2020, 17 (47.2%) received MV, of which 4 were switched to extracorporeal membrane oxygenation (ECMO), and the overall mortality rate for the sample was 4.3%[6]. An effective and practical weaning protocol is of the utmost importance for mitigating mortality, minimizing MV duration, and reducing the need for conversion to ECMO. Here, we present two cases of COVID-19 who received MV and were managed successfully with a sequential weaning protocol.
Two critically ill patients with a median age of 60.5 years are described in this report. Patient 1 was a 65 year-old woman who was admitted on January 20, 2020 after 16 d of persistent cough, 4 d of fever, and 1 d of chest tightness. Patient 2 was a 56-year-old man who was admitted on February 3, 2020 after 4 d of persistent cough, 4 d of fever and 11 h of dyspnea.
Patient 1 experienced progressive aggravation of her cough without productive sputum, since its initial onset 16 d prior to her admission. 4 d before being admitted, she had sought care in a community health center, at which time she had a fever of 38.5°Cwithout chills, sweating, abdominal pain, dyspnea, or any unusual changes in urination frequency or urgency. At the health center, she was presumed to have a common cold and was prescribed medications for cold symptoms. Three days later, 1 d before being admitted, she developed chest tightness, fatigue, and loss of appetite.
Patient 2 had a fever and cough 4 d before admission, accompanied by fatigue and poor appetite. He experienced dyspnea 11 h before being admitted. He was transferred to our hospital from Nanshan District People’s Hospital, where a nucleic acid test following nasopharyngeal swabs was performed and confirmed that he was infected with SARS-CoV-2.
Both patients had a preexisting medical condition. At the time of admission, Patient 1 had a 15-year history of type 2 diabetes mellitus and Patient 2 had a 30-year history of hypertension. Neither patient was taking any medications.
Only Patient 1 had a Wuhan contact history. Neither patient had a family history of a disease cluster.
At the time of admission, Patient 1 had normal body temperature (36.3°C) with a borderline normal/high respiratory rate (RR) (22 breaths/min), and a normal heart rate (69 beats/min) with normal blood pressure (138/79 mmHg). At the time of admission, Patient 2 had a body temperature consistent with a low-grade fever (38°C), an elevated RR (30 breaths/min), and a normal heart rate (84 beats/min) with normal blood pressure (124/76 mmHg). Pulmonary crepitations were heard in both patients. Physical examination did not reveal any other abnormal findings.
Laboratory test results for both patients are reported in Table 1. Notably, both patients were found to have normal or lower than normal white blood cell (WBC) and lymphocyte counts, and the renal and liver function index tests were unremarkable in both cases. Real-time reverse-transcriptase polymerase chain reaction nucleic acid testing performed with respiratory specimens (nasopharyngeal swabs, sputum, or bronchoalveolar lavage) confirmed that both patients were infected with SARS-CoV-2. These patients experienced respiratory failure with a low partial pressure of oxygen (PaO2)/inspired fraction of oxygen (FiO2) ratio (P/F ratio) (136, 68 mmHg, respectively) prompting transfer to the ICU on January 24 for Patient 1 and on February 3 for Patient 2.
| Blood analysis variable | Patient 1 | Patient 2 |
| WBC count, × 109/L | 5.89 | 6.51 |
| Neutrophil count, × 109/L | 5.35 | 5.36 |
| Lymphocyte count, × 109/L | 0.42 | 0.93 |
| Lactate, mmol/L | 2.7 | 2.6 |
| Creatinine, µmol/L | 54.1 | 94.6 |
| Urea, mmol/L | 4.48 | 8.48 |
| Albumin, g/L | 34.6 | 35.5 |
| Alanine aminotransferase, U/L | 15.7 | 34.8 |
| Total bilirubin, µmol/L | 8.4 | 12.2 |
| Nucleic acid result | + | + |
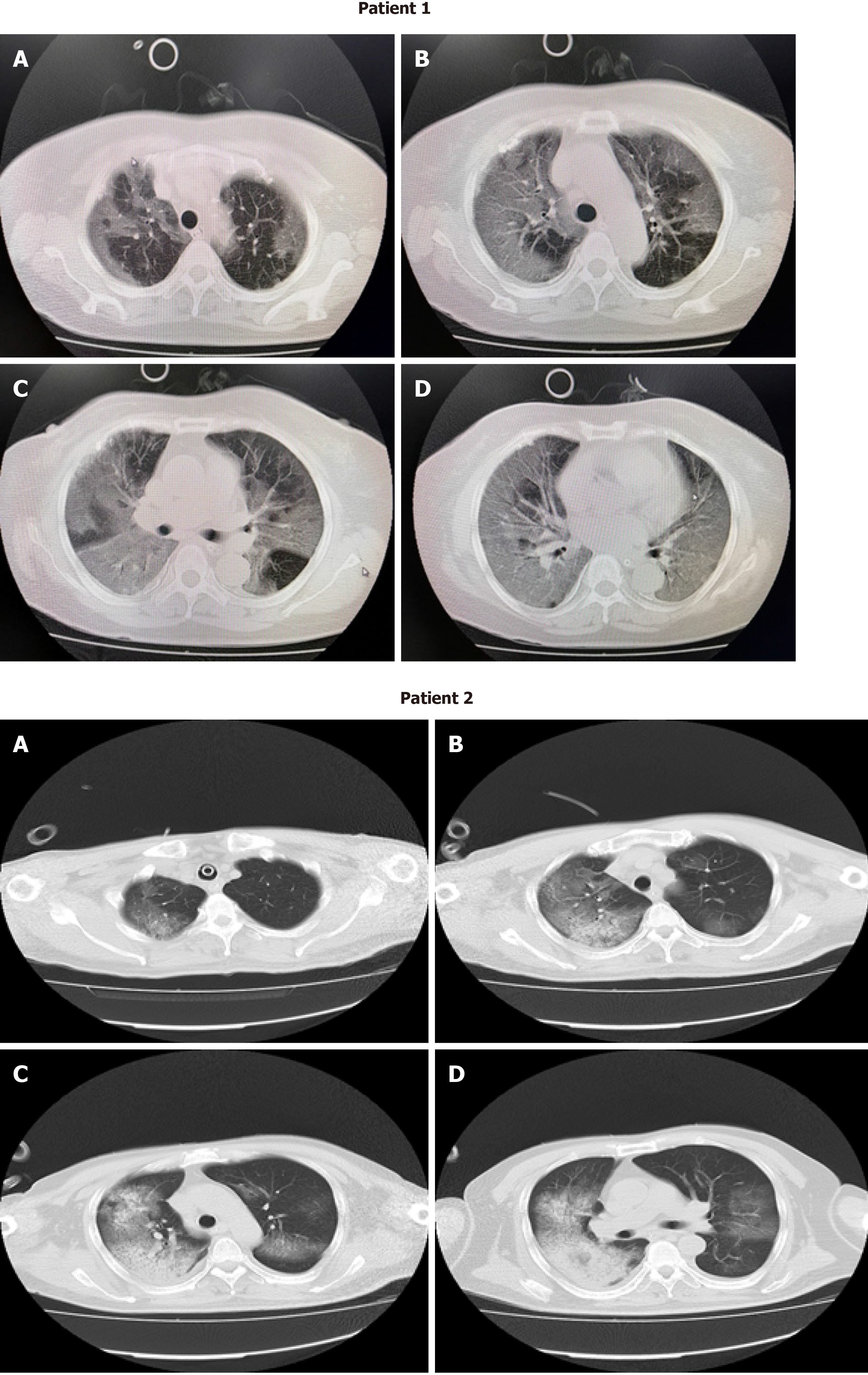
Pretreatment computed tomography (CT) imaging examinations of these two patients revealed bilateral patchy shadows or ground-glass opacity in the lungs (Figure 1).
Suspected diagnoses of COVID-19 were made based on clinical symptoms. WBC count, lymphocyte count, and chest CT findings were consistent with COVID-19. Finally, nuclei acid test results confirmed the COVID-19 diagnoses. Following confirmation of COVID-19, both patients suffered respiratory failure requiring MV and their disease was re-classified from serious to critical. Both patients were further diagnosed with acute respiratory distress syndrome (ARDS) secondary to COVID-19.
Both patients received noninvasive ventilation (NIV) with a 60% FiO2 and inspired/expiratory positive airway pressure (IPAP/EPAP) levels of 8-12 cmH2O and 6 cmH2O, respectively. Both patients remained in respiratory distress with persistently low P/F ratios for 2 h (Table 2). Consequently, each patient was advanced to invasive MV with a tidal volume (Vt) of 6 mL/kg ideal body weight (IBW) and 8-10 cmH2O of positive end-expiratory pressure (PEEP), RR of 20 breaths/min and 40%-80% FiO2. For the first 5 d of MV, each patient lay in the prone position 12 h/d (9 pm to 9 am).
| Time point | PaO2/FiO2 ratio (mmHg) | |
| Patient 1 | Patient 2 | |
| Admission to ICU | 136 | 68 |
| After 2 h of NIV | 141 | 116 |
| Day 1 of MV | 153 | 190 |
| Day 2 of MV | 227 | 176 |
| Day 3 of MV | 308 | 258 |
| Day 4 of MV | 318 | 269 |
| Day 5 of MV | 334 | 258 |
| Day 6 of MV | 340 | 316 |
| Day 7 of MV | 318 | 362 |
| Day 8 of MV | + | + |
In addition, both patients were treated with a pharmacotherapy regimen consisting of an antiviral treatment [oral lopinavir/ritonavir tablets (500 mg every 12 h) together with atomized inhaled α-interferon (5.0 MU every 12 h)] and an immune-enhancer (1.6 mg thymosin α1 daily via subcutaneous injection). Low-molecular-weight heparin (4000 IU) was injected subcutaneously to prevent thrombosis.
Enteral nutrition was administered according to weight (25 kcal/kg). Hence, at 60 kg, Patient 1 received 1500 kcal/d in 1500 mL. At 70 kg, Patient 2 received 1750 kcal/d in 1750 mL. To reduce reflux aspiration risk, feeding was supplied via a nasal jejunal tube at a rate of 90-100 mL/h while each patient lay in the supine position and at a rate of 40-50 mL/h while each patient lay in the prone position. To enable closely monitored airway management, each patient received 1:1 nursing care. The patients were kept sedated at Richmond Agitation-Sedation Scale levels of -4 and -2 while in the prone and supine position, respectively. Strict hand hygiene was maintained to prevent secondary nosocomial infection.
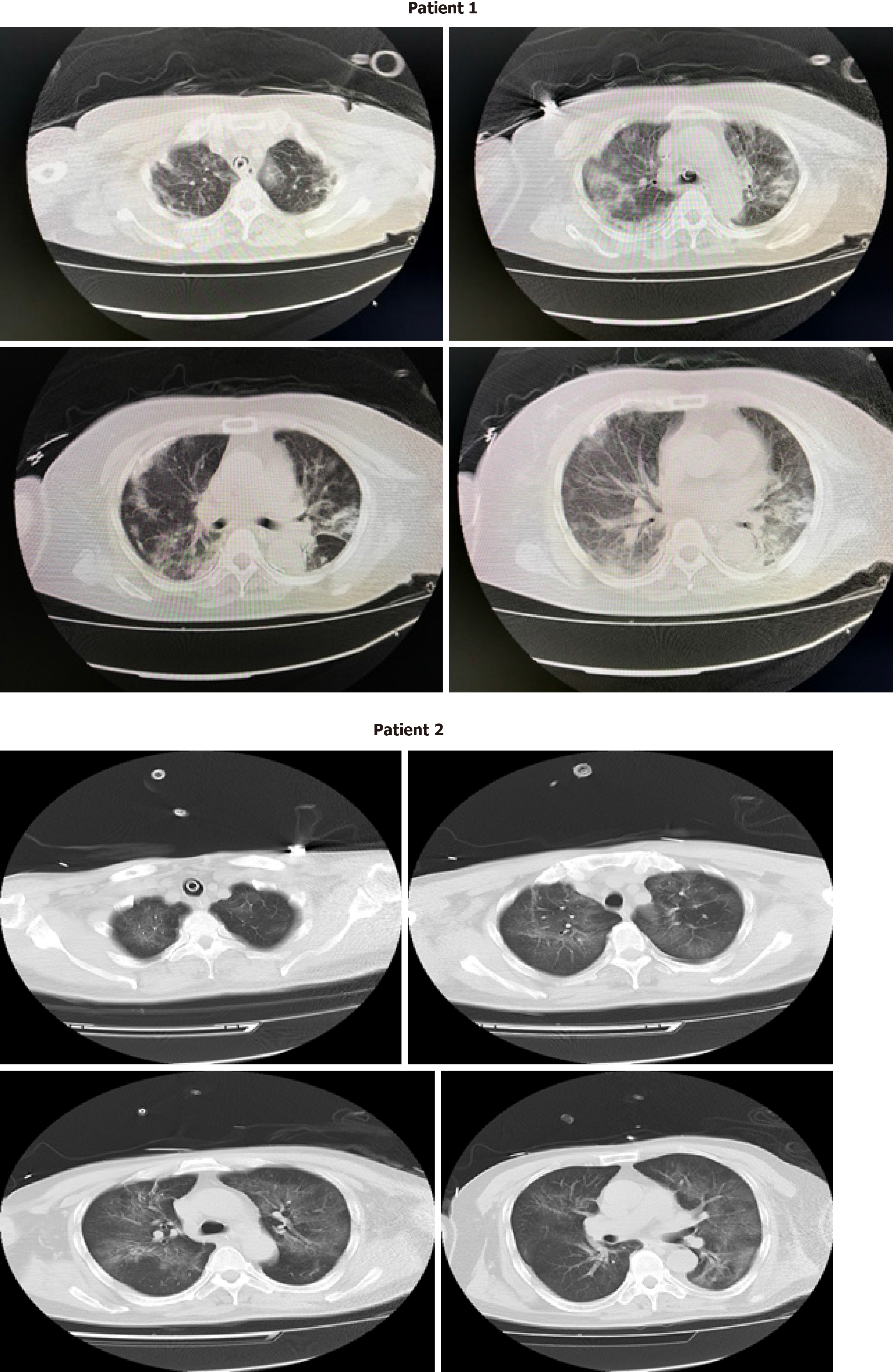
As shown in Table 2, each patient’s P/F ratio improved gradually during the course of MV with prone positioning, which was particularly notable after approximately 4-5 d. When the patients’ P/F ratios remained above 250 consistently, we stopped placing the patients in the prone position, and reduced their sedation level to improve their alertness. Each patient received a follow-up CT scan, which showed absorption of most of the imaging signs of lung pathology (Figure 2), as well as nucleic acid SARS-CoV-2 testing, which was negative in both cases. Supported by the improvements observed on CT and no detectable viral load, we felt comfortable proceeding with an extubation plan.
In preparation for extubation, the patients were subjected to a sequential MV weaning protocol that commenced with weaning screening, during which each patient was first transitioned to pressure support ventilation (PSV) (pressure support 8-10 cmH2O, PEEP 5-8 cmH2O, FiO2 40%). Each patient was alert and breathing smoothing with a P/F ratio over 250 and stable hemodynamics while on PSV and thus was advanced to a 3 min 5 cmH2O PSV, spontaneous breathing test, wherein both patients breathed smoothly, without remarkable changes in RR, Vt, oxygen saturation, heart rate or blood pressure. Then, following 30 min of spontaneous breathing with a smooth airbag leak test, each patient was extubated and placed on NIV (IPAP 8-12 cmH2O, EPAP 6 cmH2O, and FiO2 40%). After 24 h on NIV with a stable RR, oxygen saturation and cardiovascular condition, each patient was transferred to high-flow nasal cannula oxygen (HFNC) (flow rate, 40-50 L/min; FiO2 40%-50%) and remained stable. As shown in Table 2, Patient 1 received 8 d of MV and Patient 2 received 7 d of MV (median 7.5 d). Neither patient received ECMO.
While on HFNC, our patients continued to exhibit clinically smooth breathing without a fever or any other COVID-19 symptoms. These observations together with the aforementioned resolution of lung changes in both cases (Figure 2) led us to transfer these patients from the ICU to the common ward. The hospital discharge criteria for recovered COVID-19 patients in our hospital, followed in both of these cases, are as follows: a normal body temperature for > 3 d, clinically significant alleviation of respiratory symptoms, majority resolution of radiological lung changes, and two consecutive negative respiratory sample nucleic acid tests performed at least 24 h apart. At the writing of this report, both patients have been discharged with a good prognosis.
The SARS-CoV-2 virus has sustained human-to-human community transmission within regions and can be transferred rapidly between regions and countries[7]. As of May 12, 2020, the WHO Dashboard reports a total of 4098018 confirmed COVID-19 cases globally (including 84451 in China and 1298287 in the USA), which have caused 283271 deaths. Following the SARS-CoV outbreak in 2002 and the Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in 2012, the emergence of SARS-CoV-2 and the associated worldwide COVID-19 pandemic[8] marked the third introduction of a highly pathogenic coronavirus into the human population this century. Like SARS-CoV, the novel coronavirus SARS-CoV-2 relies on binding to angiotensin-converting enzyme 2 and spreads mainly through the respiratory tract.
As critical COVID-19 is characterized by respiratory failure requiring MV, there is a need for a practical weaning protocol for this patient population. A comprehensive MV weaning protocol with checklists across four dimensions (spontaneous breathing, extubation, prophylactic non-invasive positive pressure ventilation, and post-extubation monitoring), may prevent respiratory failure following extubation which would require reintubation, and reduce mortality[9]. In accordance with this recommendation, we summarize our weaning procedure and associated experience with two critical COVID-19 patients, including intubation timing, use of the prone position, infection control, and sedation titration. Together with our weaning procedures, we share the pharmacotherapy that has become standard practice for critical COVID-19 patients in our hospital.
Intubation timing is of vital importance. Within China, some 3.2% of patients with COVID-19 have required MV. Timely intubation prevents potentially lethal oxygen debt worsening[10]. Pre-MV NIV duration should be limited to allow respiratory muscle rest, particularly when patients exhibit a persistent oxygen debt despite NIV. Thus, when the respiratory condition of these 2 patients did not improve after 2 h of NIV (IPAP 8–12 cmH2O, EPAP 6 cmH2O, FiO2 60%), they were transferred to MV (Vt 6 mL/kg IBW, PEEP 8-10 cmH2O, RR 20 breaths/min, FiO2 40%-80%).
We place patients in the prone position 12 h a day during the first 5 d of MV. Prone positioning is thought to be a beneficial strategy for patients with ARDS as it allows for greater chest wall expansion than the supine position, thereby improving alveolar recruitment and the ventilation/perfusion ratio. These benefits are thought to improve survival outcomes owing to improved oxygenation and reduced lung injury[11,12].
Infection control must be maintained during the weaning process. Mortality due to ventilator-associated pneumonia (VAP) can extend ICU and hospital stays. Thus, non-pharmacological and pharmacological infection-prevention measures should be employed to reduce the risk of VAP[13]. Accordingly, we are attentive to factors such as jejunal tube placement, enteral feeding rate, sedation control, strict hand hygiene, and close monitoring for signs of potential VAP.
Pharmacotherapy during the provision of respiratory support should include an antiviral, an immune enhancer, and thrombosis prophylaxis, with the goal of minimizing the duration of MV. Notwithstanding, MV weaning is a delicate process that should be undertaken with a careful phased plan, including a weaning screening test, a spontaneous breathing test, and an extubation screening test. Moreover, after extubation, we returned our patients to NIV and then HFNC sequentially and smoothly.
Our patients responded well to the sequential MV withdrawal protocol presented which includes careful attention to timing, use of the prone position early in the course of MV, infection control and monitoring, and sequential transition from IMV to NIV to HFNC. Based on our observations and the good outcomes of our patients, we recommend that this sequential respiratory support protocol be considered for patients with critical COVID-19.
We acknowledge the writing guidance provided by Prof. Kun-Mei Ji of Shenzhen University. We are grateful to the physicians and nurses at the Third People’s Hospital of Shenzhen who participated in clinical examinations and sample collection.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Carvalho WB, Ierardi E S-Editor: Wang DM L-Editor: Webster JR E-Editor: Xing YX
| 1. | Kruse RL. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 2. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4624] [Article Influence: 924.8] [Reference Citation Analysis (0)] |
| 3. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9310] [Article Influence: 1862.0] [Reference Citation Analysis (0)] |
| 4. | Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6483] [Cited by in RCA: 5419] [Article Influence: 1083.8] [Reference Citation Analysis (0)] |
| 5. | Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 356] [Article Influence: 71.2] [Reference Citation Analysis (1)] |
| 6. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14751] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 7. | He F, Deng Y, Li W. Coronavirus disease 2019: What we know? J Med Virol. 2020;92:719-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 435] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 8. | Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1854] [Cited by in RCA: 2017] [Article Influence: 403.4] [Reference Citation Analysis (0)] |
| 9. | Nitta K, Okamoto K, Imamura H, Mochizuki K, Takayama H, Kamijo H, Okada M, Takeshige K, Kashima Y, Satou T. A comprehensive protocol for ventilator weaning and extubation: a prospective observational study. J Intensive Care. 2019;7:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, Deshpande R, Zhang L, Meng J, Tong C, Liu H, Xiong L. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan's Experience. Anesthesiology. 2020;132:1317-1332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 396] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 11. | Mezidi M, Guérin C. Effects of patient positioning on respiratory mechanics in mechanically ventilated ICU patients. Ann Transl Med. 2018;6:384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Gordon A, Rabold E, Thirumala R, Husain AA, Patel S, Cheema T. Prone Positioning in ARDS. Crit Care Nurs Q. 2019;42:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Oliveira J, Zagalo C, Cavaco-Silva P. Prevention of ventilator-associated pneumonia. Rev Port Pneumol. 2014;20:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |