Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3259
Peer-review started: March 4, 2020
First decision: April 14, 2020
Revised: June 2, 2020
Accepted: June 23, 2020
Article in press: June 23, 2020
Published online: August 6, 2020
Processing time: 154 Days and 21.8 Hours
Dydrogesterone has shown significant efficacy in treatment of irregular menstrual cycle due to abnormal uterine bleeding - ovulation dysfunction (AUB-O), but there were few relevant studies. This observational study was designed to evaluate the effectiveness of dydrogesterone for the treatment of Chinese patients with AUB-O.
To evaluate the effects of dydrogesterone on menstrual-cycle (MC) regularization and metabolism in the patients with AUB-O.
A prospective, non-interventional, single-arm, post-marketing observational study was conducted. Chinese women aged 16 years or above with AUB-O who had been prescribed dydrogesterone were enrolled. The patients were treated with dydrogesterone 10 mg from day 16 to day 25 of each cycle, consecutively for at least 3 cycles. The main outcome was defined as the percentage of patients whose MCs returned to normal (defined as 21 d < menstrual cycle ≤ 35 d) after three cycles of dydrogesterone treatment.
One hundred and fourteen women with AUB-O were enrolled in the present study. Of 89 patients who completed treatment, 72 (80.9%) achieved a regular MC at the end of the 3rd circle. The level of androgen, including testosterone and dehydroepiandrosterone sulfate, declined significantly (P = 0.01 and 0.031, respectively), whereas other hormone levels remained steady. During the treatment, 44/80 (55.0%) subjects in the per-protocol set had reported biphasic basal body temperature.
Dydrogesterone therapy was effective in achieving MC regularization for Chinese patients with AUB-O.
Core tip: The normal interval of menstrual-cycle is typically 21 to 35 d and duration of normal menstrual flow is generally 5 d. Abnormal uterine bleeding continues to pose significant physical and financial burdens on patients. In this study, we analyzed the data of 114 women with abnormal uterine bleeding - ovulation dysfunction (AUB-O) who were treated with dydrogesterone 10 mg from day 16 to day 25 of each cycle, consecutively for at least 3 cycles. It is suggested that dydrogesterone therapy was effective in achieving menstrual-cycle regularization for Chinese patients with AUB-O.
- Citation: Wang L, Guan HY, Xia HX, Chen XY, Zhang W. Dydrogesterone treatment for menstrual-cycle regularization in abnormal uterine bleeding – ovulation dysfunction patients. World J Clin Cases 2020; 8(15): 3259-3266
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3259.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3259
The normal interval of menstrual cycle (MC) is typically 21 to 35 d and duration of normal menstrual flow is generally 5 d[1]. Abnormal uterine bleeding, which is defined as bleeding from the uterine corpus that is abnormal in volume, regularity, and/or timing, continues to pose significant physical and financial burdens on patients[2,3]. Abnormal uterine bleeding - ovulatory dysfunction (AUB-O) is a subtype of AUB which has been newly classified by the International Federation of Gynecology and Obstetrics (FIGO), manifesting heavy and irregular uterine bleeding due to the absence of ovulation and formation of corpus luteum[4]. It is widely accepted that AUB-O is caused by the deficiency of progesterone and the unopposed secretion of estrogens, leading to a sustained endometrial proliferation without progesterone-withdrawal-induced shedding[5].
One strategy for the treatment of irregular menstrual cycle due to AUB-O is using progestins to schedule orderly withdrawal bleeding by stopping endometrial growth and transforming estrogen-primed endometrium. For this purpose, dydrogesterone has displayed remarkable advantages. Dydrogesterone is an orally active retro-progesterone, a stereoisomer of progesterone, which differs slightly in molecular structure from natural progesterone. In addition, its retrostructure endows dydrogesterone with a highly selective property binding to the progesterone receptor[6]. However, although increasing evidence showed the good efficacy of dydrogesterone in treating menstrual disorders[3,7-10], studies focusing its efficacy for AUB-O patients are limited, especially in China where synthetic progestins are commonly prescribed for hemostasis and menstrual cycles regulation. Thus, the present prospective, single center, observational study was designed to assess the effectiveness of dydrogesterone for menstrual cycle regularization in Chinese AUB-O patients.
We recruited a total of 114 female patients aged 16 years or above with menses, who were suffering from irregular MCs for at least 3 mo and diagnosed with AUB-O and treated with dydrogesterone for cycle regulation, between December 2013 and February 2015. All patients were administered oral dydrogesterone, 1 tablet (10 mg) twice daily from day 16 to 25 of each cycle, consecutively for at least 3 cycles. In this observational study, whether patients were treated with dydrogesterone was decided by the physicians based on their clinical experience.
Exclusion criteria included the use of oral contraceptives, sex hormone or glucocorticoid in the past 1 mo, the history of hyperprolactinemia, thyroid dysfunction or estrogen-deficiency-related symptoms. Patients who were pregnant, lactating, ineligible for the study according to physician’s discretion or presented with the contraindications listed in the Chinese label of dydrogesterone were also excluded. The study protocol was approved by the Ethics Committee, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China. Written informed consent was obtained from each participant. The study was registered in Clinical Trials.gov (ID: NCT02029144).
Patient visits were scheduled based on routine practice of the physicians. However, it would be preferable to have the following 3 visits for each patient: (1) Any day before day 16 of cycle 1; (2) Any day between cessation of withdrawal bleeding of cycle 1 and day 16 in cycle 2; and (3) Any day within 1 wk after cessation of withdrawal bleeding of cycle 3. All patients were required to fill up a diary card to capture the information pertaining to the study variables including date of last menstrual period, duration of menstrual cycle (in days), duration of menstrual bleeding (in days), date of medication and basal body temperature (BBT). The data of the subjects were collected between December 2013 and February 2015. Authors had access to information that could identify individual participants during data collection.
The primary objective was to observe the percentage of patients whose menstrual cycles returned to normal (defined as 21 d < MC ≤ 35 d) after three cycles of dydrogesterone treatment. Secondary objectives were to know the percentage of patients whose MCs returned to normal after the first and second cycles of dydrogesterone treatment; the percentage of patients reporting regular MC after three cycles of treatment with dydrogesterone in the two sub-groups of patients with a MC < 21 d and those with a MC > 35 d; the changes in estrogen hormone, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels before and after three cycles of dydrogesterone treatment; and the number of recovery of biphasic BBT during the period of drug administration.
Safety parameters included the changes of insulin, glucose and lipid levels and adverse events. Tabulation of adverse events (AEs) classified by relationship with the investigational product and whether the AE resulted in withdrawal was performed.
Since this was an observational, non-randomized, single-arm study, the sample size of 114 subjects was not based on statistical power considerations. However, available literature suggested that dydrogesterone was effective in regularizing cycle in 91.6% patients with irregular cycle. Based on a precision of 7%, about 74 subjects were required. In addition, considering the long duration of study with around 35% drop out, a total of 110 patients need to be enrolled in this study.
Statistical analysis was conducted using SAS (SAS Institute Inc., Cary, NC, United States). Continuous data were described using mean and standard deviation. Categorical data were reported in frequency and percentage with 95% confidence interval. Paired t test was used for within-group comparison of data before and after treatment, and McNemar χ2 test was carried out for within-group rate comparison before and after treatment. A two-sided P value ≤ 0.05 was regarded as statistically significant. Full analysis set (FAS) was determined according to the intention-to-treat (ITT) principle, including all subjects who were included in this study and who had taken at least dydrogesterone once. Per-protocol set (PPS) was a subset of FAS, including all subjects who fulfilled the trial protocol. Safety set (SS) included subjects who had taken at least dydrogesterone once and had received at least one post-administration safety evaluation.
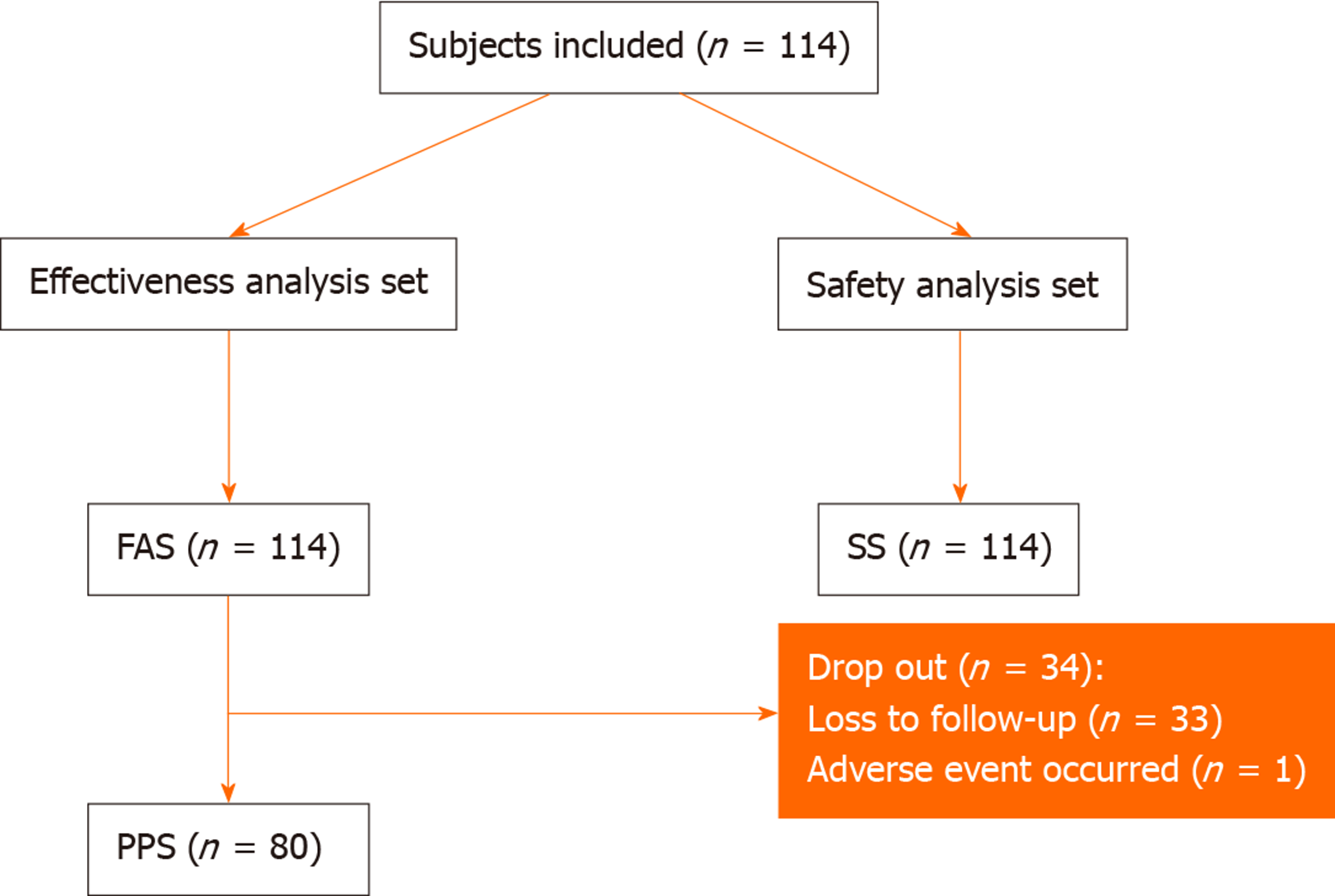
The flow chart of enrollment of patients is shown in Figure 1. A total of 114 patients were enrolled in this study, all of whom were included in the FAS and SS. Eighty (70.2%) patients finished the follow-up and were included in PPS. The main reason for withdrawal was loss to follow-up. The baseline and demographic characteristics of these patients are summarized in Table 1.
| Variables | n (missing) | Values, mean ± SD; n (%) |
| Age (yr) | 114 (0) | 24.5 ± 6.3 |
| Average days of menstrual cycles in the | 96 (18) | 63.7 ± 25.5 |
| Last three months | ||
| Average days of menstrual periods in the | 108 (6) | 7.1 ± 6.2 |
| Last three months | ||
| Urine pregnancy test (enrollment) | 114 (0) | |
| Negative | 1 (0.9) | |
| Positive | 0 (0.0) | |
| Not applicable | 5 (4.4) | |
| Deferred | 108 (94.7) | |
| Diagnosis of polycystic ovaries syndrome 114 (0) | ||
| Yes | 41 (36.0) | |
| No | 73 (64.0) | |
In FAS, 72 of 89 patients (80.9%) (18 with missing data) achieved regular MC at the end of three cycles of dydrogesterone treatment. For the patients in PPS, 62 of 65 patients (95.4%) (10 with missing data) achieved cycle regularization over the treatment period (Table 2). Seven subjects in FAS and 5 subjects in PPS who had a normal baseline menstrual cycle (defined as 21 d < MC ≤ 35 d) were not included for the statistical analysis.
| Variables | FAS analysis | PPS analysis | ||||
| n (missing)1 | Normal | Rate (95%CI) | n (missing)1 | Normal | Rate (95%CI) | |
| Percentage of patients who achieved regular MC after | 89 (18) | 72 | 80.9 (71.2-88.5) | 65 (10) | 62 | 95.4 (87.1-99.0) |
| Three cycles of dydrogesterone treatment | ||||||
| Subgroups | ||||||
| Patients with menstrual cycle > 35 d | 63 (25) | 61 | 96.8 (89.0-99.6) | 63 (1) | 61 | 96.8 (89.0-99.6) |
| Patients with menstrual period > 8 d | 8 (3) | 7 | 87.5 (47.3-99.7) | 8 (0) | 7 | 87.5 (47.3-99.7) |
| After cycle 1 treatment of dydrogesterone | 75 (32) | 71 | 94.7 (86.9-98.5) | 64 (11) | 61 | 95.3 (86.9-99.0) |
| After cycle 2 treatment of dydrogesterone | 65 (42) | 59 | 90.8 (81.0-96.5) | 64 (11) | 59 | 92.1 (82.7-97.4) |
After cycle one treatment, proportion of subjects with regular MC was 94.7% (71/75, 32 with missing data) in FAS and 95.3% (61/64, 11 with missing data) in PPS. The proportion was 90.8% (59/65, 42 with missing data) and 92.1% (59/64, 11 with missing data) at the end of two cycles of therapy in FAS and PPS, respectively (Table 2). Among the patients with a menstrual cycle > 35 d, 61 were evaluated as “effective”, accounting for 96.8% (Table 2) and the difference in the length of menstrual cycles before (68.7 ± 25.6 d) and after (29.1 ± 2.4 d) treatment was statistically significant (P < 0.0005) both in FAS and PPS. Similarly, 87.5% (7/8) of the patients whose menstrual period > 8 d achieved regular MC after dydrogesterone treatment (Table 2). Among those patients, the menstrual period averaged 19.4 ± 14.6 d before treatment and 6.0 ± 1.4 d after three cycles of dydrogesterone treatment, decreasing by 15.6 ± 17.8 d on average (P = 0.042). Interestingly, 44 (55.0%) subjects had biphasic BBT during the period of treatment (Table 3).
| Variables | FAS analysis | PPS analysis | ||||
| Baseline | End of treatment | P value | Baseline | End of treatment | P value | |
| FSH (mIU/mL) | 7.1 ± 3.0 | 6.9 ± 2.9 | 0.229 | 7.2 ± 3.0 | 6.9 ± 2.9 | 0.229 |
| LH (mIU/mL) | 10.4 ± 5.8 | 9.6 ± 6.0 | 0.095 | 10.8 ± 6.0 | 9.6 ± 6.0 | 0.095 |
| PRL (mIU/mL) | 11.3 ± 5.7 | 10.9 ± 4.3 | 0.449 | 11.4 ± 6.1 | 10.9 ± 4.3 | 0.449 |
| E2 (mIU/mL) | 59.7 ± 72.7 | 54.7 ± 98.0 | 0.809 | 51.9 ± 29.6 | 54.7 ± 98.0 | 0.809 |
| T (mIU/mL) | 0.58 ± 0.31 | 0.52 ± 0.19 | 0.010 | 0.59 ± 0.33 | 0.52 ± 0.19 | 0.010 |
| DHEA-S (mIU/mL) | 248.6 ± 114.7 | 231.3 ± 101.7 | 0.031 | 248.6 ± 110.8 | 231.3 ± 101.7 | 0.031 |
| Patients with biphasic BBT after three cycles of treatment (%, 95%CI) | 55 (21.2-45.1) | 55 (21.2-45.1) | ||||
| TC (mmol/L) | 4.30 ± 0.80 | 4.12 ± 0.86 | 0.17 | 4.24 ± 0.80 | 4.13 ± 0.86 | 0.17 |
| TG (mmol/L) | 1.01 ± 0.59 | 1.03 ± 0.47 | 0.29 | 0.97 ± 0.59 | 1.03 ± 0.47 | 0.29 |
| HDL (mmol/L) | 1.02 ± 0.24 | 0.99 ± 0.28 | 0.54 | 1.01 ± 0.24 | 0.99 ± 0.28 | 0.54 |
| LDL (mmol/L) | 2.74 ± 0.54 | 2.53 ± 0.54 | 0.001 | 2.71 ± 0.54 | 2.53 ± 0.54 | 0.001 |
| Lp (a1) | 1.04 ± 0.21 | 1.25 ± 0.09 | 0.78 | 1.00 ± 0.21 | 1.26 ± 0.09 | 0.78 |
| Lp (a2) | 0.78 ± 0.16 | 0.71 ± 0.06 | 1.00 | 0.79 ± 0.19 | 0.71 ± 0.06 | 1.00 |
| Insulin (µU/mL) | 11.3 ± 8.4 | 10.5 ± 7.0 | 0.045 | 11.7 ± 8.9 | 10.5 ± 7.0 | 0.045 |
| Glucose (mmol/L) | 4.9 ± 0.9 | 4.7 ± 0.6 | 0.42 | 4.9 ± 1.1 | 4.7 ± 0.6 | 0.42 |
Either in FAS analysis or in PPS analysis, the levels of sex hormones including FSH, LH, prolactin and estrogen 2, did not show statistically significant differences before and after treatment with dydrogesterone. In contrast, both androgen testosterone (T) and dehydroepiandrosterone sulfate (DHEA-S) significantly decreased (Table 3).
Among the 114 subjects included in the present study, three (2.6%) experienced three AEs, which were considered unrelated to the investigated drug. Interestingly, treatment with dydrogesterone demonstrated moderate beneficial effects on blood lipid and glucose metabolism (Table 3). Of note, significant reduction of low-density lipoprotein (P = 0.001) and insulin (P = 0.045) was detected.
The effectiveness and safety of dydrogesterone for the treatment of AUB-O in Chinese women was evaluated in this observational study. Overall, 95.4% of patients gained cycle regularization after dydrogesterone treatment in PPS. In the subgroup analysis, the recovery ratewas more than 87% whilst the median MC duration for patients with polymenorrhea or oligomenorrhea was 29.1 d at the end of therapy, within the normal cycle (21-35 d). These results are comparable to the results of previous studies where regularization of MCs restored by dydrogesterone was discovered in 86.8%-99.1% of patients and the mean MC duration after treatment was around 27.7-29.8 d[7,9-11].
It has been reported that the response induced by dydrogesterone was equal to or greater than other orally administered progestins on eliciting secretory phase activity including norethindrone, medroxyprogesterone acetate, norgestrel and progesterone[12]. A recent post-marketing observational investigation containing 996 subjects with irregular MCs by Podzolkova et al[9] demonstrated that 99.1% of patients achieved > 1 regular MC and 79.1% maintained > 6 regular MCs after treatment with dydrogesterone, suggesting that cycle regularization may sustain over time after dydrogesterone treatment[9].
Actually, the absence of clinically relevant androgenic, estrogenic, glucocorticoid or mineralocorticoid activities makes dydrogesterone more pronounced compared to other progestin regimens[13] such as medroxyprogesterone acetate (anti-gonadotropic and glucocorticoid effect), norethisterone (anti-gonadotropic, estrogenic and androgenic effect) and levonorgestrel (anti-gonadotropic and androgenic effect)[6,14,15]. Additionally, a previous research comparing the systematic biochemical character-ization of dydrogesterone and progesterone suggested no androgenic and mild anti-androgenic effects of dydrogesterone[16]. These are in line with our results that dydrogesterone did not affect the levels of FSH, LH, prolactin and estrogen 2 apart from reducing the concentration of androgen T and DHEA-S. The fact that 55% subjects presented biphasic BBT in the present study indicates that dydrogesterone at least does not inhibit ovulation and this is in agreement with the earlier investigation[17]. It is conceivable that dydrogesterone promoted ovulation function through regulating LH meanwhile restraining the estrogen T and DHEA-S.
Although a number of studies as above-mentioned have established the clear significant advantages of dydrogesterone for various menstrual disorders, there are limited data on the efficacy of dydrogesterone on AUB-O since the definition of AUB-O was recommended to describe uterine bleeding abnormalities by FIGO in 2011. For this point, the results of our study may be of a greater reference value for the application of dydrogesterone in patients with AUB-O.
In contrast with other progestins, dydrogesterone appears to be largely neutral in terms of biochemical markers and indirect clinical endpoints[18,19]. Dydrogesterone did not exert to undermine the beneficial effects of oestradiol on low density lipoproteins or insulin based on a previous prospective study[20], which is concordant with a randomized controlled research indicating that dydrogesterone beneficially influence the serum lipids when sequentially combined with oestradiol[21]. With respect to insulin sensitivity, Dansuk et al[22] found that dydrogesterone combining with 17 β-estradiol enhanced insulin sensitivity in postmenopausal women with 3 mo of treatment[22]. In addition, the safety of dydrogesterone has been confirmed in previous literature[7,9-11]. The associated AEs were also recorded throughout the treatment in the present study. Only three AEs occurred during the study, none of them was considered to be related to dydrogesterone.
We are very cautious about our results because of two major limitations of the present study. Firstly, as most observational studies, the present study was limited by the absence of placebo or control group, and the relative short follow-up on 3 cycles of treatment. In addition, lack of stratification by age or reproductive status and relatively small sample size may also weaken the statistical power of this exploratory analysis.
In summary, dydrogesterone therapy was effective in achieving MC regularization in Chinese patients with AUB-O. In addition, the neutral effects of dydrogesterone on sex hormones as well as the metabolic level reiterate its role in regulating irregular menstrual cycles. Moreover, the drug was well tolerated by patients. To further confirm our findings, more studies with a larger sample size, longer follow-up and generally accepted nomenclature of menstrual disorders would be highly desirable.
Abnormal uterine bleeding - ovulation dysfunction (AUB-O) is caused by the deficiency of progesterone and the unopposed secretion of estrogens, leading to a sustained endometrial proliferation without progesterone-withdrawal-induced shedding. Dydrogesterone has shown significant efficacy in the treatment of irregular menstrual cycle due to AUB-O.
Although increasing evidence showed the good efficacy of dydrogesterone in menstrual disorders, few studies are available focusing its efficacy for AUB-O patients, especially in China where synthetic progestins are commonly prescribed for hemostasis and menstrual cycle regulation.
Our study aimed to evaluate the effects of dydrogesterone on menstrual-cycle (MC) regularization and metabolism in the patients with AUB-O.
Women aged 16 years or above with AUB-O were treated with dydrogesterone orally 1 tablet (10 mg) twice daily from day 16 to 25 of each cycle and consecutively for at least 3 cycles, the primary objective was to observe the percentage of patients whose MCs returned to normal (defined as 21 d < MC ≤ 35 d) after treatment.
Seventy-two (80.9%) of the 89 patients achieved a regular MC at the end of the 3rd circle treatment, and the level of androgen, including testosterone and dehydro-epiandrosterone sulfate, declined significantly (P = 0.01 and 0.031, respectively).
Dydrogesterone therapy was effective in achieving MC regularization for Chinese patients with AUB-O.
This observational study suggested that dydrogesterone was effective and safe for the treatment of AUB-O in Chinese women. More studies with a larger sample size, longer follow-up and generally accepted nomenclature of menstrual disorders would be highly desirable.
The authors would like to thank all members for offering help in the study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernandez JM, Knittel T S-Editor: Zhang L L-Editor: MedE-Ma JY E-Editor: Liu JH
| 1. | Ely JW, Kennedy CM, Clark EC, Bowdler NC. Abnormal uterine bleeding: a management algorithm. J Am Board Fam Med. 2006;19:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Matteson KA, Raker CA, Clark MA, Frick KD. Abnormal uterine bleeding, health status, and usual source of medical care: analyses using the Medical Expenditures Panel Survey. J Womens Health (Larchmt). 2013;22:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Marret H, Fauconnier A, Chabbert-Buffet N, Cravello L, Golfier F, Gondry J, Agostini A, Bazot M, Brailly-Tabard S, Brun JL, De Raucourt E, Gervaise A, Gompel A, Graesslin O, Huchon C, Lucot JP, Plu-Bureau G, Roman H, Fernandez H; CNGOF Collège National des Gynécologues et Obstétriciens Français. Clinical practice guidelines on menorrhagia: management of abnormal uterine bleeding before menopause. Eur J Obstet Gynecol Reprod Biol. 2010;152:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 789] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 5. | Khrouf M, Terras K. Diagnosis and Management of Formerly Called "Dysfunctional Uterine Bleeding" According to PALM-COEIN FIGO Classification and the New Guidelines. J Obstet Gynaecol India. 2014;64:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH. Classification and pharmacology of progestins. Maturitas. 2003;46 Suppl 1:S7-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Trivedi N, Chauhan N, Vaidya V. Effectiveness and safety of dydrogesterone in regularization of menstrual cycle: a post-marketing study. Gynecol Endocrinol. 2016;32:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Karakus S, Kiran G, Ciralik H. Efficacy of micronised vaginal progesterone versus oral dydrogestrone in the treatment of irregular dysfunctional uterine bleeding: a pilot randomised controlled trial. Aust N Z J Obstet Gynaecol. 2009;49:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Podzolkova N, Tatarchuk T, Doshchanova A, Eshimbetova G, Pexman-Fieth C. Dydrogesterone treatment for menstrual-cycle regularization in routine clinical practice: a multicenter observational study. Gynecol Endocrinol. 2016;32:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Saldanha E, Tank D, Chainani M. Dydrogesterone in the management of dysfunctional uterine bleeding. Int J Gynecol Obstet India. 1997;1:36-39. |
| 11. | Naib JM, Siddiqui MI, Ajmal W. The role of dydrogesterone in the medical management of 100 cases of dysfunctional uterine bleeding (DUB) above 35 years of age. Journal of Postgraduate Medical Institute. 2011;17. |
| 12. | King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril. 1986;46:1062-1066. [RCA] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 81] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Schindler AE. Progestational effects of dydrogesterone in vitro, in vivo and on the human endometrium. Maturitas. 2009;65 Suppl 1:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Klijn JG, Pre-clinical and clinical treatment of breast cancer with antiprogestins. Human reproduction. 1994;9 Suppl 1:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Golbs S, Nikolov R, Zimmermann T. Pharmacology of nortestosterone derivatives. Menopause Rev. 2001;6:29-44. |
| 16. | Rižner TL, Brožič P, Doucette C, Turek-Etienne T, Müller-Vieira U, Sonneveld E, van der Burg B, Böcker C, Husen B. Selectivity and potency of the retroprogesterone dydrogesterone in vitro. Steroids. 2011;76:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Bishop PM, Borell U, Diczfalusy E, Tillinger KG. Effect of dydrogesterone on human endometrium and ovarian activity. Acta Endocrinol (Copenh). 1962;40:203-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Seeger H, Mueck AO. Effects of dydrogesterone on the vascular system. Gynecol Endocrinol. 2007;23 Suppl 1:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1995;273:199-208. [PubMed] |
| 20. | Crook D, Godsland IF, Hull J, Stevenson JC. Hormone replacement therapy with dydrogesterone and 17 beta-oestradiol: effects on serum lipoproteins and glucose tolerance during 24 month follow up. Br J Obstet Gynaecol. 1997;104:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Hänggi W, Lippuner K, Riesen W, Jaeger P, Birkhäuser MH. Long-term influence of different postmenopausal hormone replacement regimens on serum lipids and lipoprotein(a): a randomised study. Br J Obstet Gynaecol. 1997;104:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Dansuk R, Unal O, Karsidag YK, Turan C. Evaluation of the effects of various gestagens on insulin sensitivity, using homeostatic model assessment, in postmenopausal women on hormone replacement therapy. Gynecol Endocrinol. 2005;20:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |