Published online Aug 6, 2020. doi: 10.12998/wjcc.v8.i15.3177
Peer-review started: April 26, 2020
First decision: July 3, 2020
Revised: July 10, 2020
Accepted: July 23, 2020
Article in press: July 23, 2020
Published online: August 6, 2020
Processing time: 101 Days and 14 Hours
In December 2019 a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 was identified and the disease associated was named coronavirus disease 2019 (COVID-19). Fever, cough, myalgia, fatigue associated to dyspnea represent most common clinical symptoms of the disease. The reference standard for diagnosis of severe acute respiratory syndrome coronavirus 2 infection is real time reverse-transcription polymerase chain reaction test applied on respiratory tract specimens. Despite of lower specificity, chest computed tomography (CT), as reported in manifold scientific studies, showed high sensitivity, therefore it may help in the early detection, management and follow-up of COVID-19 pneumonia. Patients affected by COVID-19 pneumonia usually showed on chest CT some typical features, such as: Bilateral ground glass opacities characterized by multilobe involvement with posterior and peripheral distribution; parenchymal consolidations with or without air bronchogram; interlobular septal thickening; crazy paving pattern, represented by interlobular and intralobular septal thickening surrounded by ground-glass opacities; subsegmental pulmonary vessels enlargement (> 3 mm). Halo sign, reversed halo sign, cavitation and pleural or pericardial effusion represent some of atypical findings of COVID-19 pneumonia. On the other hand lymphadenopathy’s and bronchiectasis’ frequency is unclear, indeed conflicting data emerged in literature. Radiologists play a key role in recognition of high suspicious findings of COVID-19 on chest CT, both typical and atypical ones. Thus, the aim of this review is to illustrate typical and atypical CT findings of COVID-19.
Core tip: This review makes an effort to summarize typical and atypical chest computed tomography features in coronavirus disease 2019 (COVID-19) pneumonia associated to the novel coronavirus, severe acute respiratory syndrome coronavirus 19. The content of the article will prove to be helpful for readers to recognize the high suspicious chest computed tomography findings in COVID-19 pneumonia.
- Citation: Caruso D, Polidori T, Guido G, Nicolai M, Bracci B, Cremona A, Zerunian M, Polici M, Pucciarelli F, Rucci C, Dominicis CD, Girolamo MD, Argento G, Sergi D, Laghi A. Typical and atypical COVID-19 computed tomography findings. World J Clin Cases 2020; 8(15): 3177-3187
- URL: https://www.wjgnet.com/2307-8960/full/v8/i15/3177.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i15.3177
In December 2019, several cases of pneumonia of unknown origin have emerged in Wuhan city, Hubei Province of China, manifesting as respiratory symptoms like fever, cough and dyspnea[1-4]. A novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified and the disease associated was consequently named coronavirus disease 2019 (COVID-19). In a few weeks, the World Health Organization declared COVID-19 as pandemic with more than 2 million cases, with United States, Spain and Italy mostly affected.
Clinical symptoms are characterized by fever, cough, myalgia, fatigue associated to dyspnea. According to the latest guidelines, real-time reverse transcription polymerase chain reaction applied on respiratory tract specimens is considered the reference standard for the diagnosis of SARS-CoV-2[5]. On the other hand, chest computed tomography (CT) may be extremely helpful for the diagnosis, management and follow-up of COVID-19 due to the high sensitivity as described by Ai et al[6] and Caruso et al[7] where both studies reported a sensitivity of 97%.
The typical pattern is characterized by bilateral posterior ground glass opacities (GGOs)[8,9], misdiagnosed at chest X-ray, as described by Yoon et al[10], where a considerable proportion of patients with COVID-19 pneumonia had normal chest radiographs. In a recent study[7], GGO with bilateral and peripheral distribution characterized by multilobe involvement were described as the typical CT features in patients affected by COVID-19 pneumonia, associated to subsegmental pulmonary vessels enlargement. Despite the typical pattern, other CT features were observed with a relative frequency; thus, the aim of this review is to illustrate typical and atypical CT findings of COVID-19.
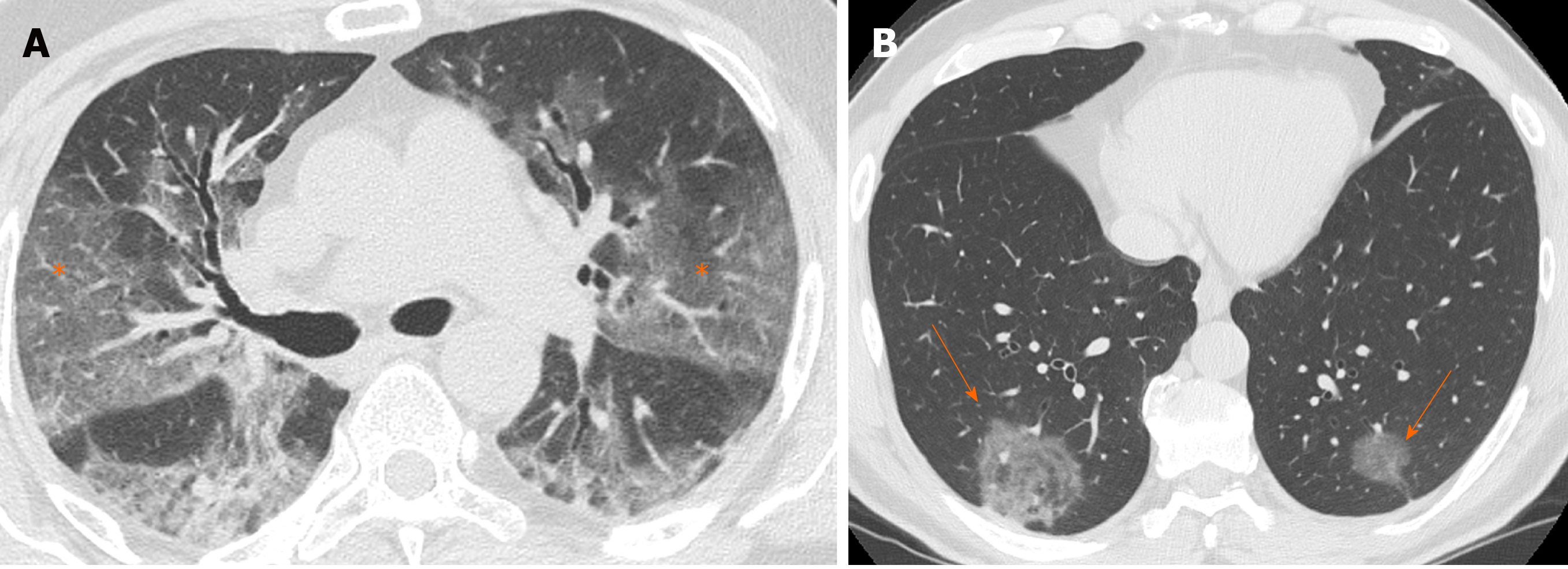
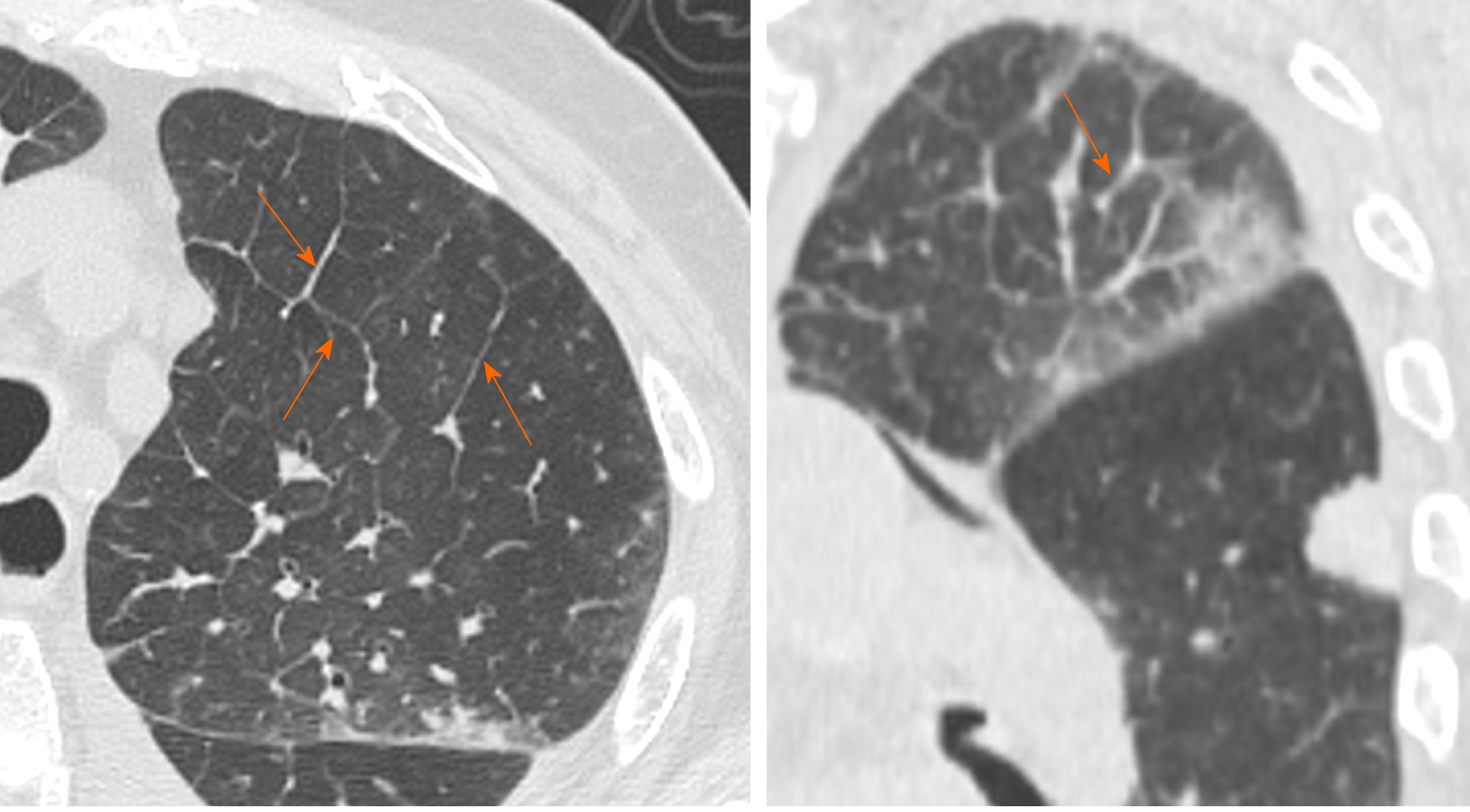
GGO is characterized by an increased area of attenuation of the lung, with recognizable bronchial and vascular structures, remaining less opaque than consolidation, in which bronchovascular margins are obscured[11] (Figure 1). Histologically, GGO represents an area of alveolar septal inflammation and intra-alveolar cellular desquamation with a small amount of granulation tissue in the terminal air spaces[12]. GGO can be observed in three typical patterns: Crazy paving, rounded or linear.
In a systematic review of 919 patients in China, Salehi et al[13] reported GGOs in 88% of COVID-19 patients, while Bai et al[14] reported GGOs in only 46% of the study population. In a longitudinal study performed by Wang et al[15], GGO was described as the predominant pattern of abnormalities after symptom, while consolidation was the second most seen feature in the first 11 d. When combined together, ground-glass opacity and consolidation constituted about 83% to 85% of all CT findings found in the early stage of the disease. CT examination may be effective in the baseline evaluation of patients affected by COVID-19, especially in those with symptoms of dyspnea and respiratory distress and it can be considered as an early sign of the damage to the alveolar epithelium caused by the virus[16].
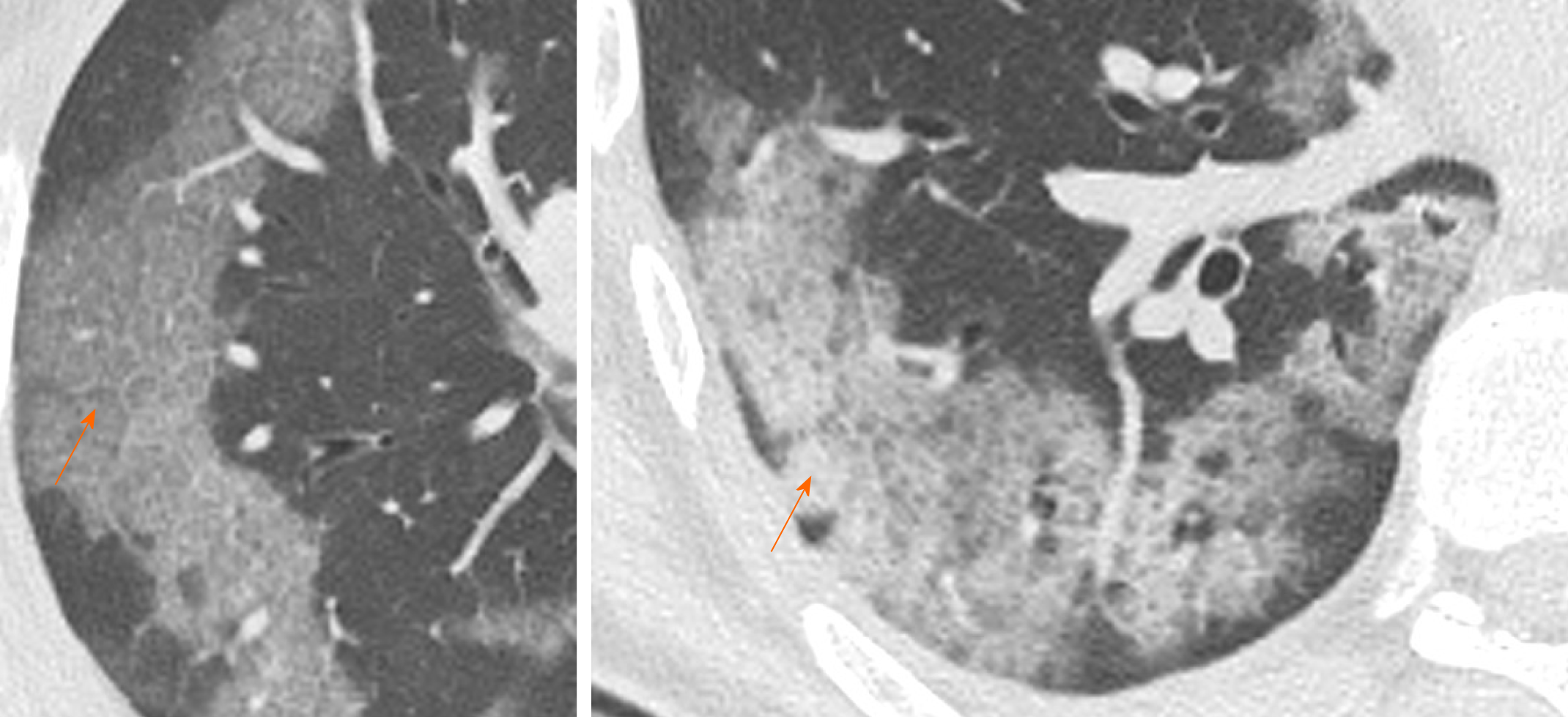
Crazy paving is characterized by GGOs associated with interlobular and intralobular septal thickening (Figure 2). These CT findings are non-specific since they can be found in numerous pathologies, such as non-specific alveolar proteinosis, acute respiratory distress syndrome and other interstitial pneumonia[17-19].
In a recent study, Guan et al[20] estimated that the incidence of the crazy-paving pattern in Chest CT of COVID-19 patients is approximately 89% (42/47 of the patients examined by them), with predominant subpleural distribution (93.6%). Similarly, Chen et al[21] reported a high incidence of crazy-paving pattern (48.8% of cases), distributed mainly to the peripheral areas of the lung. Therefore, based on their analysis, it emerges how the crazy paving pattern is a frequent feature in pulmonary involvement from COVID-19, with typical peripheral distribution. This finding is probably caused by SARS-CoV-2 tropism for terminal bronchioles and surrounding lung parenchyma.
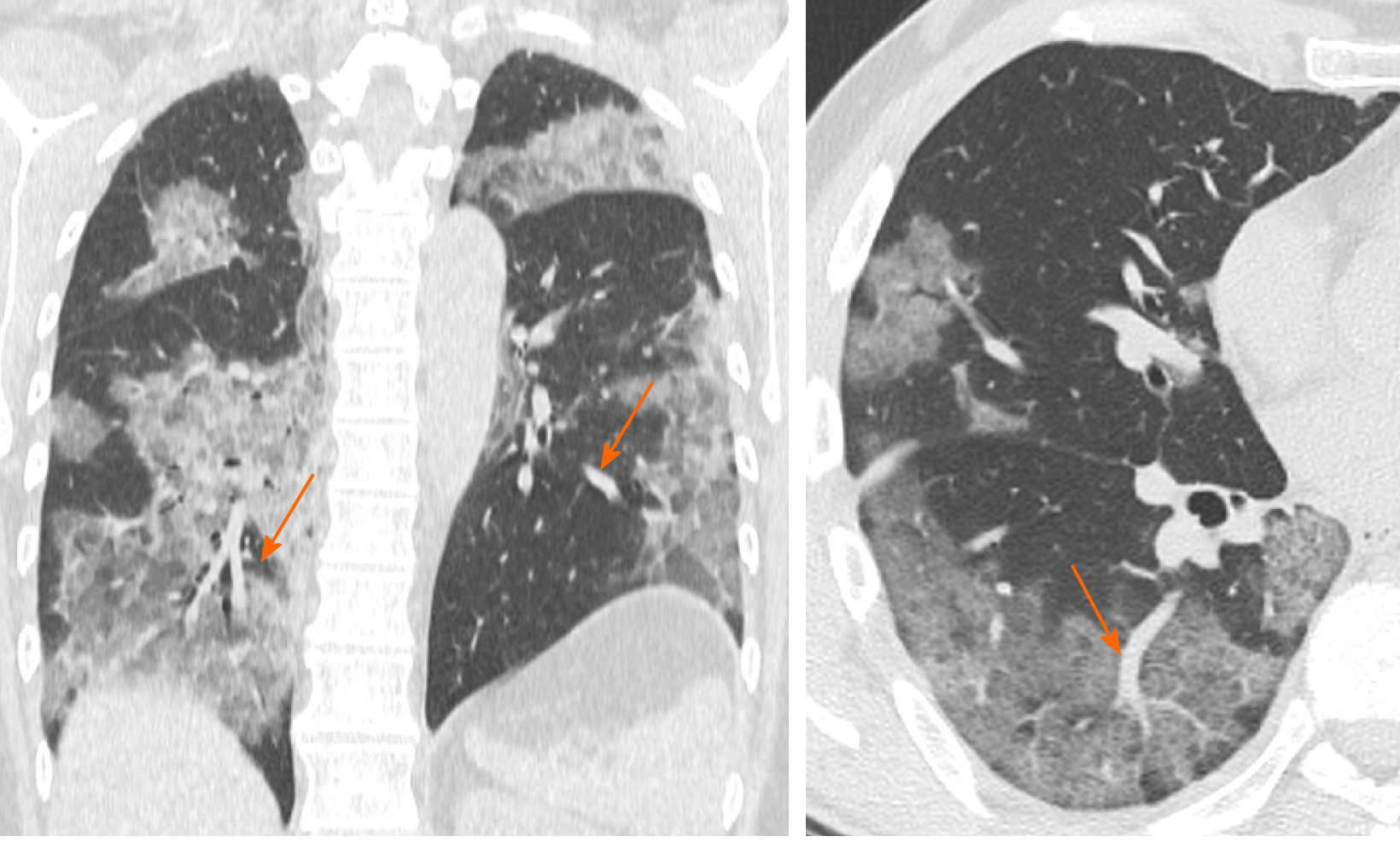
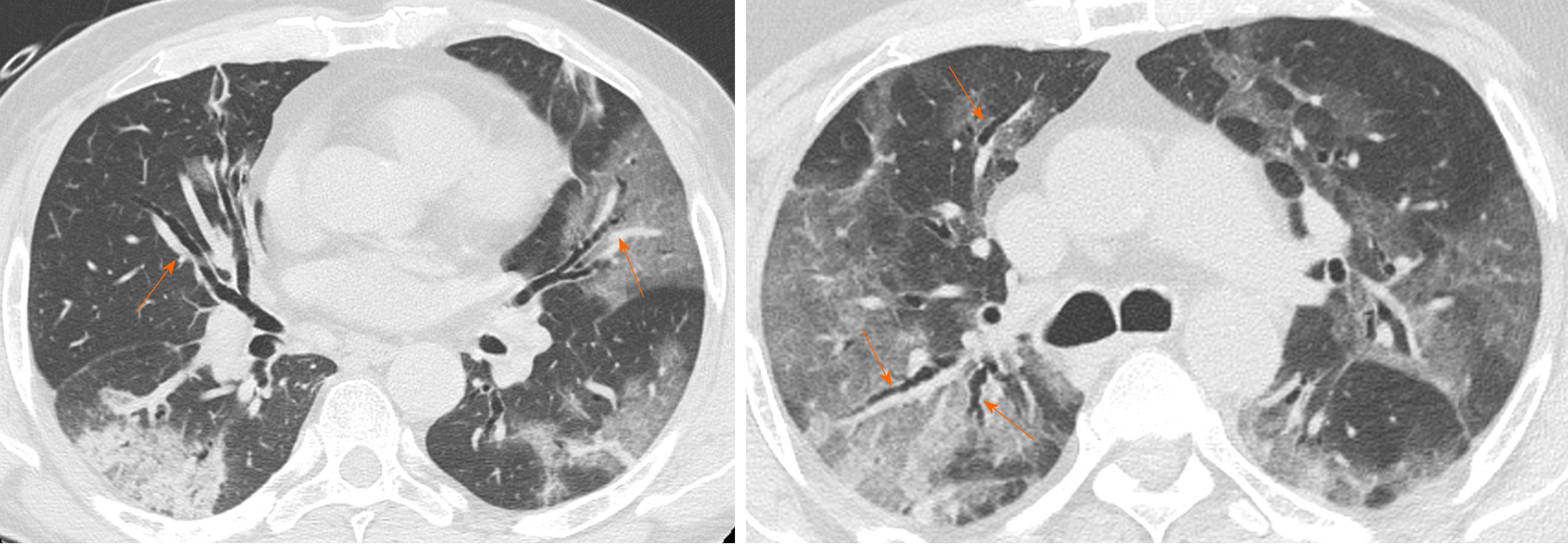
Vascular enlargement is defined as the hypertrophy of the subsegmental pulmonary vessels increased in size (> 3 mm) particularly in areas with more pronounced interstitial impairment[7,22] (Figure 3). Albarello et al[22] firstly described subsegmental vessels increasing in the baseline CT, with an additional increase from 5% to 14% at the sixth day during follow up. Bai et al[14] described subsegmental vascular enlargement in 59% of patients, whereas Caruso et al[7] reported 89% of patients with CT findings of subsegmental vascular enlargement.
This new radiological evidence suggested a different pattern of lung involvement compared to those observed in other known severe coronavirus infections (Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome), where pulmonary vessel vasoconstriction was possibly related to the presence of vasoactive substance within the lesions[23]. Vascular injury consisted in edema of pulmonary vessel’s wall and fibrous thrombi with or without pulmonary infarction.
In COVID-19 these findings might be attributed to damage and swelling of the capillary wall caused by pro-inflammatory factors[24]. Indeed, a systemic vasculitis was mostly observed in autopsies and this could be caused by autoantibodies against pulmonary epithelial and endothelial cells[25]. Coronavirus binds to the zinc peptidase angiotensin converting enzyme, a surface molecule that is localized on the endothelial cells of arteries, and the suppression of angiotensin converting enzyme expression during SARS-CoV-2 infection has been proposed to play a role in pathologic changes in the lungs[26].
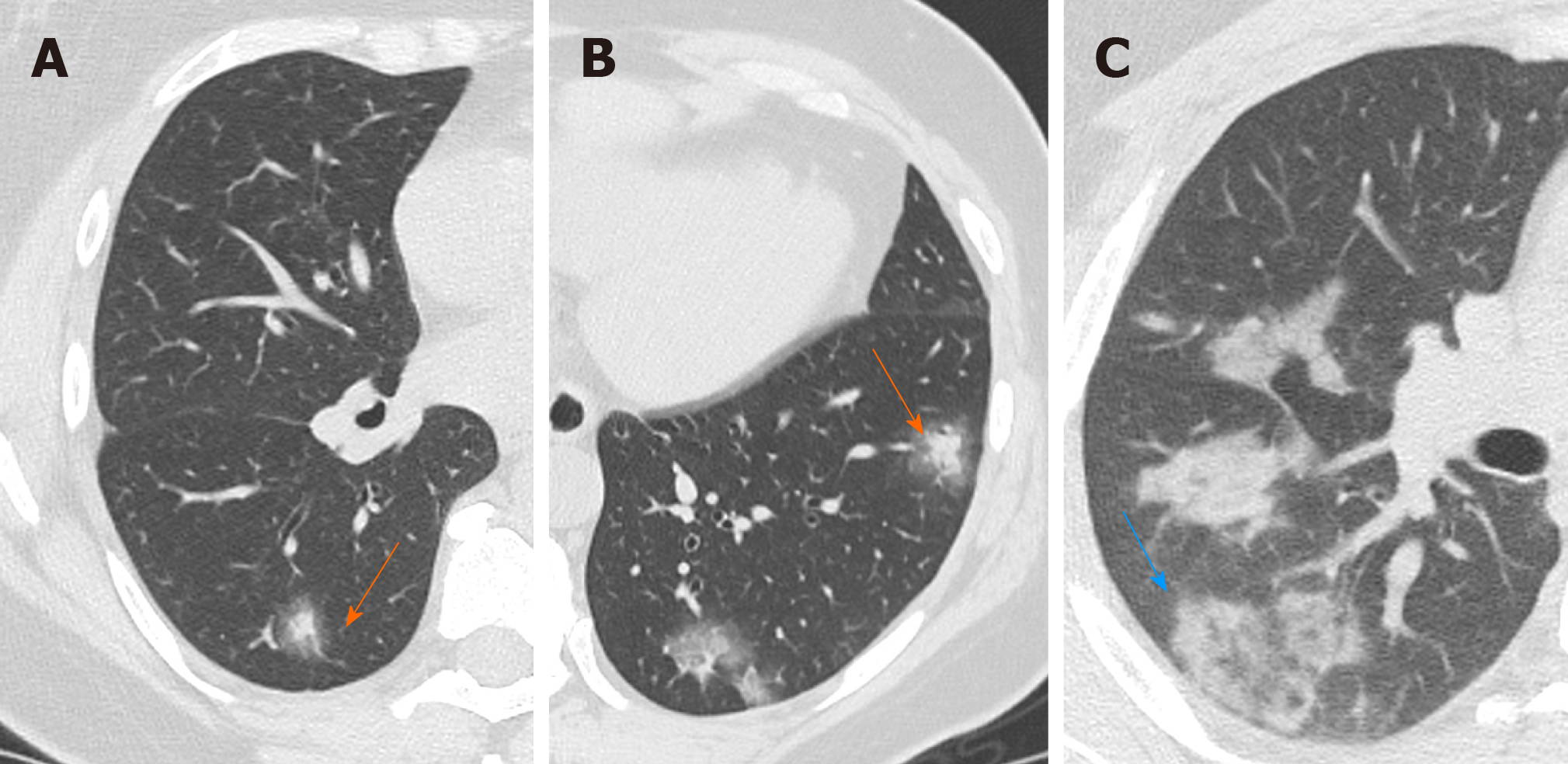
Halo sign is a non-specific sign defined as a circular zone of ground glass attenuation surrounding a pulmonary nodule or mass (Figure 4A and B), classically used to describe hemorrhagic nodules, in pathologies as angioinvasive fungal infections, hypervascular metastases and Wegener granulomatosis vasculitis; Nevertheless, halo sign could be also detected in interstitial pneumonia, such as viral infections and cryptogenic organizing pneumonia[27,28].
Recently, the halo sign was described also in COVID-19 patients as reported by Li et al[29] describing pulmonary nodules with a halo sign in 9 patients (17.6%), while Chen et al[21] observed it in 7 patients (11.3%), considering the halo sign as a feature of the early stage of the disease[21].
Considering the halo sign’s frequency in COVID-19 patients, some differences in literature are emerging, but it can be considered as an uncommon CT finding.
Reversed halo sign (or atoll sign) is a focal rounded ground glass opacity surrounded by a more or less complete ring-like consolidation (Figure 4C). It was initially reported to be specific for cryptogenic organizing pneumonia, but it was subsequently described in a variety of pulmonary diseases, in particular infective pneumonia, tuberculosis and other pathologies such as sarcoidosis and pulmonary infarction[30,31].
Recently, this sign was reported in few cases of COVID-19, which probably may represent a disease progression to initial consolidation around GGO or absorbed lesion leaving a decreased intensity in the center[32]. Bernheim et al[18] investigated CT findings changes based on time lapse incurred between initial CT scan and symptom onset, reporting the absence of reverse halo sign in the early group of patients (0-2 d), and present in 4% of patient in the late group (6-12 d). Reverse halo sign still remained an atypical CT feature of COVID-19.
Consolidation appeared as a homogeneous increase in pulmonary parenchymal attenuation with an obscuration of the margins of vessels and airway walls (Figure 5). It appears in pathological situations where an exudate or other product of disease replaces alveolar air, making the lung solid (as in infective pneumonia)[11]. Histologically, consolidation is due to intra-alveolar fibroblastic granulation tissues, also known as Masson bodies[12].
Jin et al[33] reported that large light consolidative opacities with air bronchograms in the rapid progression stage of the disease (3–7 d of symptomatic presentation), while during the consolidation stage (second week of symptomatic presentation), reduction in density and size of the consolidative opacities may be observed. Lei et al[9] defined consolidation as a common sign of COVID-19 associated to GGO reported in 96% of patients, while Li et al[29] reported the presence of consolidation in 63.9% of population.
Interestingly, during follow-up studies, CT showed increased consolidation suggestive for progression of the disease[9]; However, the appearance of lung consolidation on ground glass area is not related to a worsening of clinical and laboratory data.
Air bronchogram is a pattern of air-filled bronchi on a background of opaque air-less or consolidated lung (Figure 6). The sign implies patency of proximal airways and evacuation of alveolar air by means of absorption (atelectasis) or replacement (pneumonia) or a combination of these processes. Air bronchogram is usually a sign of alveolar involvement[34].
Chen et al[21] described the presence of air bronchogram in 56.2% of COVID-19 cases, mostly in the middle-aged patients who present a more severe lobar involvement. Similarly, Zhou et al[35] in their meta-analysis described the air bronchogram sign as one of the most common signs in COVID-19 patients (44.7%), often in patients with bilateral and multilobe involvement. Lyu et al[36] showed that air bronchogram was a common sign (63%) and increased in severe and critical patients compared to the ordinary cases. Again, according to these authors, air bronchogram could be related to a central representation of disease from COVID-19.
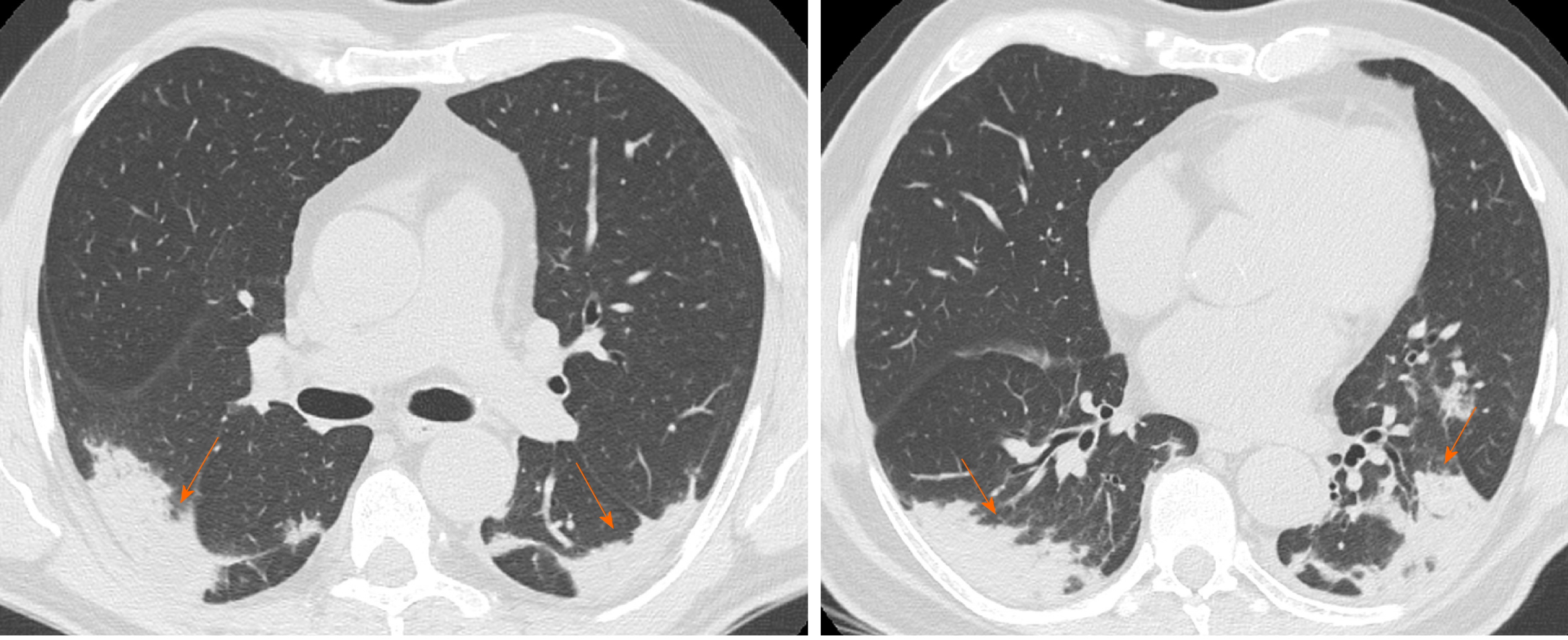
Interlobular septal thickening is usually found on chest radiographs as thin linear opacities at right angles in contact with the lateral pleural surfaces near lung bases (Kerley B lines); this sign commonly occurred in lymphangitic cancer spread or pulmonary edema. Kerley A lines are predominantly situated in the upper lobes, described as fine linear opacities radially oriented toward the hila[37]. In recent years, the anatomically descriptive terms septal lines and septal thickening have gained favor over Kerley lines[38] (Figure 7).
The SARS-CoV-2 usually attacks the components of the septa, thus it may reflect the interlobular septal thickening visible in the CT images. On thin-section CT scans, septal thickening may be smooth or nodular, which may help refine the differential diagnosis[39]. Interlobular septal thickening is also often associated with GGOs in more advanced disease frameworks, in which there is a widespread involvement of all the lobes.
In the study described by Zhu et al[16] the CT sign of interlobular septal thickening was appreciable in 61.6% of COVID-19 cases. Most refer to advanced cases of disease, where, as the inflammatory response progresses, the GGOs gradually tend to consolidate and the signs of interlobular thickening become more evident. Chen et al[21] also showed that interlobular septal thickening was one of the most frequent CT signs from COVID-19, especially in patients with bilateral lung involvement (about 50%).
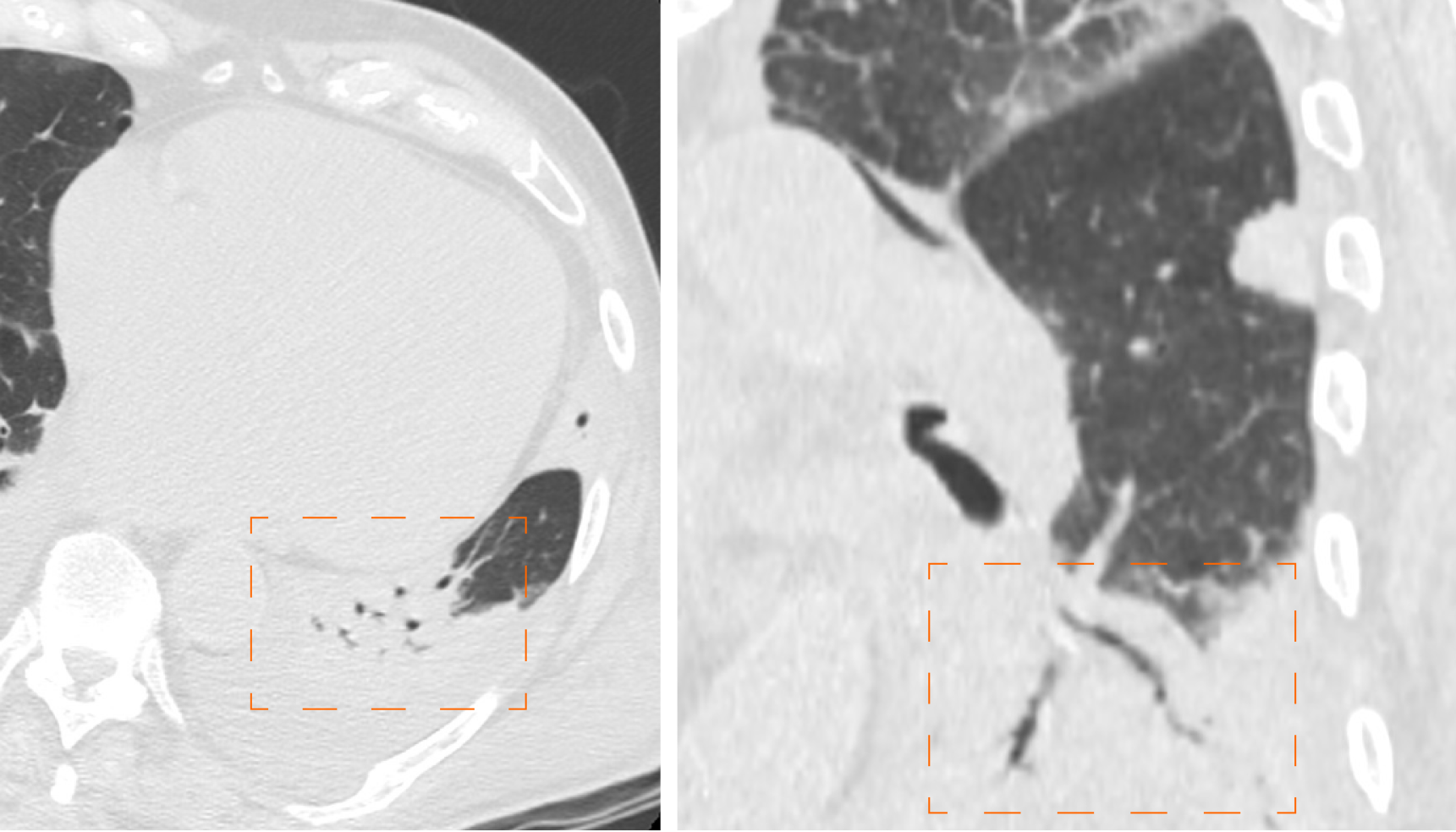
Bronchiectasis is a progressive and irreversible dilatation of the airways, with or without thickening of the corresponding bronchial walls; dilation frequently involves the more distal subsegmental bronchial branches, up to the bronchioles[40] (Figure 8). The causes of bronchiectasis are manifold: Inflammatory processes, specific disease (i.e. cystic fibrosis) and idiopathic forms.
The study performed by Salehi et al[13] showed that bronchiectasis are not a frequent radiological sign in early stage of COVID-19 but they resulted visible in the advanced stages of pathology, whereas Zhao et al[41] reported that traction bronchiectasis is an identifiable finding in almost half of the cases (52.5%). Therefore bronchiectasis, especially traction ones, could be considered as a radiological sign of progression of SARS-CoV-2 lung infection found in the most advanced stages of pathology.
Cavitation or cavity is a circumscribed area of the lung filled with gas visible on CT as a low-density area within a consolidation, mass or nodule; In addition the cavitation is not synonymous of lung abscess and occurs when a necrotic part of a lung injury is drained or expelled through the bronchial tree. It often represents the evolution of a lung consolidation, which regresses leaving only a thin wall[42].
In COVID-19 cavitation is visible especially in the later stages of lung involvement, as described by Salehi et al[13] showing a transition over time from area of pure ground glass to area of ground glass associated with signs of cavitation, indicating the latter progress of the inflammatory involvement. Therefore, cavitation is one of the least frequent CT findings in COVID-19.
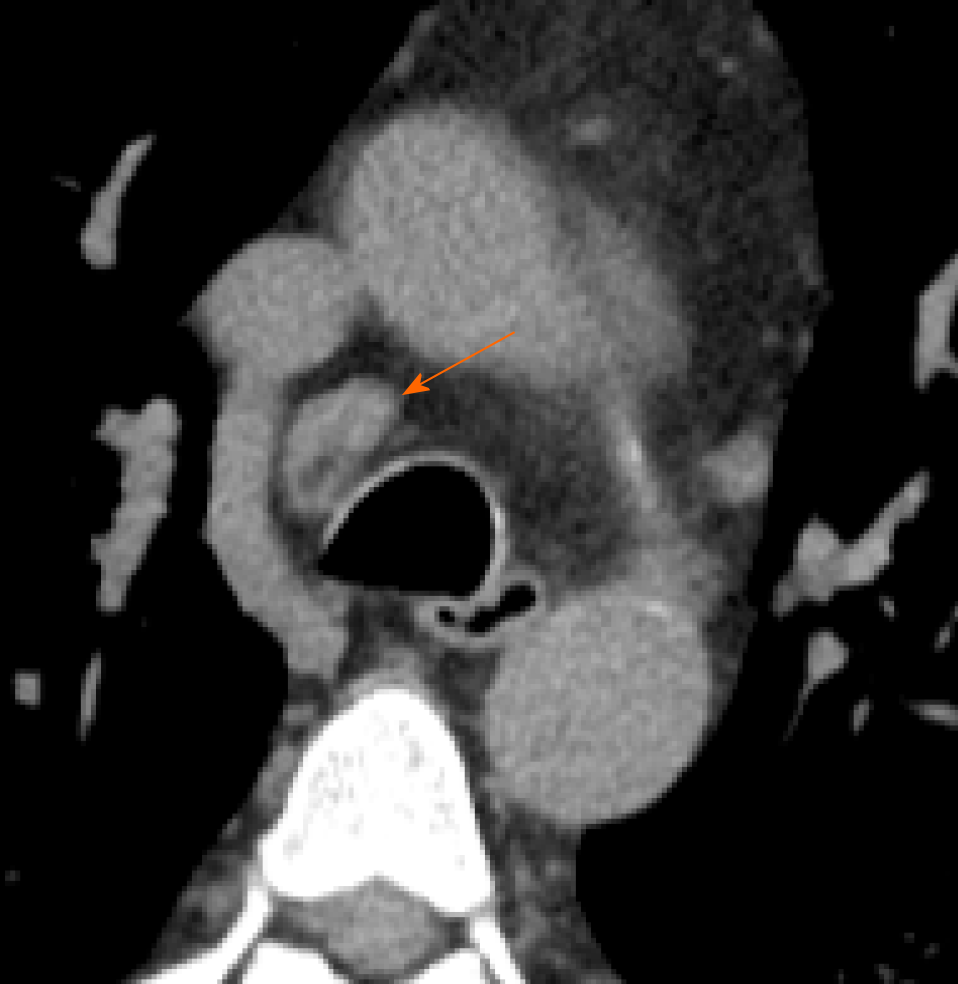
The term lymphadenopathy refers to an enlargement of the lymph nodes. On CT scans, the normal nodes size ranges between sub-CT resolution and 12 mm for mediastinal and hilar stations; The upper limit of normal lymph node can be arbitrary consider of 10 mm in short axis diameter for mediastinal nodes and 3 mm for most hilar nodes (Figure 9). However, measuring method does not allow to differentiate healthy from pathologic lymph nodes[11]. In addition, during inflammation, necrosis or neoplasia, the inflammatory cells drain into lymph nodes leading them to enlarge, without the possibility discriminate the specific etiology, by visual assessment on imaging[43].
This feature is rarely present in the early stage of COVID-19 on chest CT, but it may be observed during disease progression[13]. Li et al[29] observed that severe and critical patients showed higher incidence of lymph nodes enlargement, pericardial effusion and pleural effusion than ordinary patients.
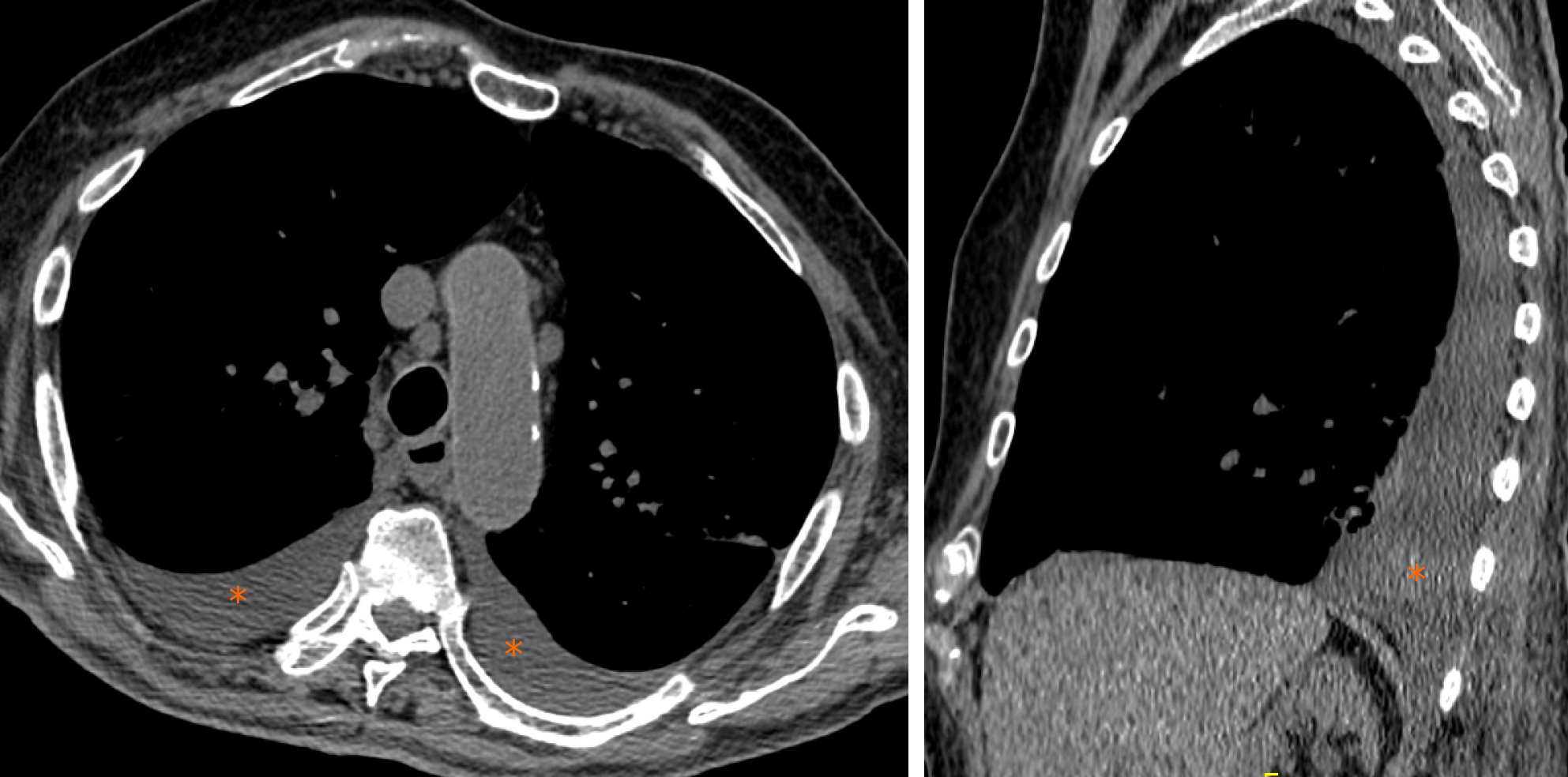
Pleural effusion is defined as the accumulation of fluid in the pleural space usually when the fluid formation exceeds the rate of fluid removal[44]. This may happen with an elevated net hydrostatic pressure gradient (transudation) or an increased permeability of the pleural vessels (exudation). Pleural effusion is a quite rare manifestation in SARS-Cov2 pneumonia (Figure 10).
Compared with early stage of disease, advanced stage is associated with a significantly increased frequency of pleural changes as subpleural line, subpleural transparent line, and pleural effusion as commented by Zhou et al[35]. Further, this manifestation is quite rare and documented in the 7% of COVID-19 patients in the later stage disease by Wang et al[15].
Pericardial effusion occurs when excess fluid collects in the pericardial space (a normal pericardial sac contains about 30 to 50 mL of fluid). Pericardial effusion causes include a wide variety of etiologies as follows: Inflammatory, infectious (both viral and bacterial), tumoral, iatrogenic and post-traumatic.
Salehi et al[13] and Li et al[29] showed in their studies that pericardial effusion is one of the rarest CT features in COVID-19 patients, but it represents an expression of disease progression; In particular, Li et al[29] reported pericardial effusion in all critical patients (4.8% of total population).
The most frequent chest CT findings are GGO, consolidations, vascular enlargement, crazy paving, interlobular septal thickening and air bronchogram. Chest CT has a central role in the initial diagnosis and management of COVID-19 patients. Radiologists need to recognize high suspicious findings of COVID-19 on chest CT, both typical and atypical ones.
The authors would like to acknowledge the entire Radiological Medical, Technical and Nursing Staff for skillful technical assistance.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma GL S-Editor: Zhang L L-Editor: A E-Editor: Xing YX
| 1. | Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1827] [Cited by in RCA: 1696] [Article Influence: 339.2] [Reference Citation Analysis (0)] |
| 2. | Calisher C, Carroll D, Colwell R, Corley RB, Daszak P, Drosten C, Enjuanes L, Farrar J, Field H, Golding J, Gorbalenya A, Haagmans B, Hughes JM, Karesh WB, Keusch GT, Lam SK, Lubroth J, Mackenzie JS, Madoff L, Mazet J, Palese P, Perlman S, Poon L, Roizman B, Saif L, Subbarao K, Turner M. Statement in support of the scientists, public health professionals, and medical professionals of China combatting COVID-19. Lancet. 2020;395:e42-e43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health? Lancet. 2020;395:542-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 581] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 4. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 5. | Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5481] [Cited by in RCA: 4918] [Article Influence: 983.6] [Reference Citation Analysis (1)] |
| 6. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;200642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3285] [Article Influence: 657.0] [Reference Citation Analysis (0)] |
| 7. | Caruso D, Zerunian M, Polici M, Pucciarelli F, Polidori T, Rucci C, Guido G, Bracci B, de Dominicis C, Laghi A. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020;201237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 8. | Cheng Z, Lu Y, Cao Q, Qin L, Pan Z, Yan F, Yang W. Clinical Features and Chest CT Manifestations of Coronavirus Disease 2019 (COVID-19) in a Single-Center Study in Shanghai, China. AJR Am J Roentgenol. 2020;215:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 9. | Lei J, Li J, Li X, Qi X. CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 10. | Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, Park CM, Kim YH. Chest Radiographic and CT Findings of the 2019 Novel Coronavirus Disease (COVID-19): Analysis of Nine Patients Treated in Korea. Korean J Radiol. 2020;21:494-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 374] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 11. | Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2674] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 12. | Zare Mehrjardi M, Kahkouee S, Pourabdollah M. Radio-pathological correlation of organizing pneumonia (OP): a pictorial review. Br J Radiol. 2017;90:20160723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am J Roentgenol. 2020;215:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 860] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 14. | Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi LB, Wang DC, Mei J, Jiang XL, Zeng QH, Egglin TK, Hu PF, Agarwal S, Xie F, Li S, Healey T, Atalay MK, Liao WH. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020;200823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 726] [Article Influence: 145.2] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, Shi H, Zhou M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology. 2020;200843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 493] [Cited by in RCA: 591] [Article Influence: 118.2] [Reference Citation Analysis (0)] |
| 16. | Zhu T, Wang Y, Zhou S, Zhang N, Xia L. A Comparative Study of Chest Computed Tomography Features in Young and Older Adults With Corona Virus Disease (COVID-19). J Thorac Imaging. 2020;35:W97-W101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 17. | De Wever W, Meersschaert J, Coolen J, Verbeken E, Verschakelen JA. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging. 2011;2:117-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1728] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |
| 19. | Verma R, Abdoh M. Crazy paving pattern. Clin Case Rep. 2017;5:533-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, Xie RM, Chen BD. Imaging Features of Coronavirus disease 2019 (COVID-19): Evaluation on Thin-Section CT. Acad Radiol. 2020;27:609-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 21. | Chen Z, Fan H, Cai J, Li Y, Wu B, Hou Y, Xu S, Zhou F, Liu Y, Xuan W, Hu H, Sun J. High-resolution computed tomography manifestations of COVID-19 infections in patients of different ages. Eur J Radiol. 2020;126:108972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | Albarello F, Pianura E, Di Stefano F, Cristofaro M, Petrone A, Marchioni L, Palazzolo C, Schininà V, Nicastri E, Petrosillo N, Campioni P, Eskild P, Zumla A, Ippolito G; COVID 19 INMI Study Group. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: An uncommon radiological presentation. Int J Infect Dis. 2020;93:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 23. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4603] [Article Influence: 209.2] [Reference Citation Analysis (0)] |
| 24. | Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 896] [Cited by in RCA: 792] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 25. | Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170:1136-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1249] [Article Influence: 249.8] [Reference Citation Analysis (2)] |
| 27. | Alves GR, Marchiori E, Irion K, Nin CS, Watte G, Pasqualotto AC, Severo LC, Hochhegger B. The halo sign: HRCT findings in 85 patients. J Bras Pneumol. 2016;42:435-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Ray A, Mittal A, Vyas S. CT Halo sign: A systematic review. Eur J Radiol. 2020;124:108843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, Li C. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020;55:327-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 797] [Article Influence: 159.4] [Reference Citation Analysis (0)] |
| 30. | Torrealba JR, Fisher S, Kanne JP, Butt YM, Glazer C, Kershaw C, Burguete D, Gokaslan T, Batra K. Pathology-radiology correlation of common and uncommon computed tomographic patterns of organizing pneumonia. Hum Pathol. 2018;71:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Zhan X, Zhang L, Wang Z, Jin M, Liu M, Tong Z. Reversed Halo Sign: Presents in Different Pulmonary Diseases. PLoS One. 2015;10:e0128153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;200490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1106] [Cited by in RCA: 952] [Article Influence: 190.4] [Reference Citation Analysis (1)] |
| 33. | Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, Fang C, Huang D, Huang LQ, Huang Q, Han Y, Hu B, Hu F, Li BH, Li YR, Liang K, Lin LK, Luo LS, Ma J, Ma LL, Peng ZY, Pan YB, Pan ZY, Ren XQ, Sun HM, Wang Y, Wang YY, Weng H, Wei CJ, Wu DF, Xia J, Xiong Y, Xu HB, Yao XM, Yuan YF, Ye TS, Zhang XC, Zhang YW, Zhang YG, Zhang HM, Zhao Y, Zhao MJ, Zi H, Zeng XT, Wang YY, Wang XH; for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 881] [Cited by in RCA: 1137] [Article Influence: 227.4] [Reference Citation Analysis (0)] |
| 34. | Collins J. CT signs and patterns of lung disease. Radiol Clin North Am. 2001;39:1115-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Zhou S, Wang Y, Zhu T, Xia L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 451] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 36. | Lyu P, Liu X, Zhang R, Shi L, Gao J. The Performance of Chest CT in Evaluating the Clinical Severity of COVID-19 Pneumonia: Identifying Critical Cases Based on CT Characteristics. Invest Radiol. 2020;55:412-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 37. | Heitzman ER, Ziter FM, Markarian B, McClennan BL, Sherry HT. Kerley's interlobular septal lines: roentgen pathologic correlation. Am J Roentgenol Radium Ther Nucl Med. 1967;100:578-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Loebelenz LI, Ebner L, Obmann VC, Huber AT, Christe A. Kerley B lines in the lung apex - a distinct CT sign for pulmonary congestion. Swiss Med Wkly. 2019;149:w20119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Oikonomou A, Prassopoulos P. Mimics in chest disease: interstitial opacities. Insights Imaging. 2013;4:9-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Marini T, Hobbs SK, Chaturvedi A, Kaproth-Joslin K. Beyond bronchitis: a review of the congenital and acquired abnormalities of the bronchus. Insights Imaging. 2017;8:141-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020;214:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 696] [Article Influence: 139.2] [Reference Citation Analysis (0)] |
| 42. | Parkar AP, Kandiah P. Differential Diagnosis of Cavitary Lung Lesions. J Belg Soc Radiol. 2016;100:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Elmore SA. Histopathology of the lymph nodes. Toxicol Pathol. 2006;34:425-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: a structured approach to care. Br Med Bull. 2004;72:31-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |