Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3122
Peer-review started: March 14, 2020
First decision: April 22, 2020
Revised: April 26, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: July 26, 2020
Processing time: 132 Days and 9.4 Hours
Hepatosplenic T-cell lymphoma (HSTCL) is a rare subtype of non-Hodgkin’s lymphoma, which has an aggressive clinical course and an extremely poor prognosis. Chidamide is a novel, orally active, benzamide-type histone deacetylase (HDAC) inhibitor that has been used for peripheral T-cell lymphoma (PTCL) treatment. However, to date, there has been no report of the treatment and effect of the HDAC inhibitor chidamide in HSTCL, which is a special subtype of PTCL.
A 45-year-old male patient was admitted with splenomegaly and slight bicytopenia. He was diagnosed with HSTCL via splenectomy. The patient was treated with fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine regiment as inductive therapy. Unfortunately, the disease progressed rapidly during chemotherapy before a suitable allogeneic gene transplant donor was found. The chidamide-combined chemotherapy regimen and single-drug oral maintenance regimen achieved complete remission, duration of response of 9 mo, and overall survival of 15 mo.
The novel agent chidamide can be used in HSTCL to achieve deep remission and improve the duration of response and overall survival.
Core tip: Hepatosplenic T-cell lymphoma is a rare disease that progresses quickly and has a poor prognosis. Patients usually show short-term rapid disease progress with a traditional inductive regimen, leaving no opportunity for hematopoietic stem cell transplantation. We employed the histone deacetylase inhibitor chidamide combined with a dose-adjusted ifosfamide, carboplatin, etoposide regimen, and chidamide single-drug oral maintenance therapy for the management of this patient, which had a satisfactory outcome.
- Citation: Wang XT, Guo W, Sun M, Han W, Du ZH, Wang XX, Du BB, Bai O. Effect of chidamide on treating hepatosplenic T-cell lymphoma: A case report. World J Clin Cases 2020; 8(14): 3122-3129
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3122
Hepatosplenic T-cell lymphoma (HSTCL) is a rare and aggressive subtype of peripheral T-cell lymphoma (PTCL)[1]. This tumor mostly arises from the gamma-delta (γδ) T cells and predominantly affects middle-aged males[2]. Since this is a rare disease, the mechanism has not been well elucidated. Persistent antigen stimulation and immunosuppressive therapy are two possible factors for the development of this disease[3,4]. Unlike other nodal T-cell lymphomas, this disease has no lympha-denopathy, but main manifestations include marked splenomegaly, hepatomegaly, and pancytopenia with bone marrow involvement[5,6]. The diagnosis of HSTCL is usually based on core needle biopsies of the liver or bone marrow; however, patients presenting primarily with splenic enlargement may be diagnosed with splenectomy instead. A prominent sinusoidal infiltrate of medium-sized lymphoid cells with the characteristic immunophenotype (CD2+/CD3+, CD4−/CD8−, CD5−, restricted γ/δ T-cell receptor TCR]) in the liver, spleen, and/or bone marrow is sufficient for the diagnosis of HSTCL with corresponding clinical manifestations[1]. Isochromosome 7q in cytogenetics strongly supports the diagnosis of HSTCL if identified[6]. HSTCL is refractory to chemotherapy with unremitting clinical progression, which leads to an extremely low 5-year overall survival (OS, < 10%)[7] and a quite short median survival (12-14 mo)[8]. The treatment for HSTCLs is still challenging even with high-dose chemotherapy and stem cell transplantation (SCT). Chidamide (CS055/HBI-8000) is an oral histone deacetylase (HDAC) inhibitor that has been prescribed in China for the treatment of relapsed and recurrent PTCL[9]. Chidamide monotherapy showed a good overall response rate (39.06%), and disease control rate (64.45%) in PTCL treatment. Combination with chemotherapy proved longer median progression-free survival (152 d vs 129 d [monotherapy], P = 0.33)[10]. There are still no data on the effect of chidamide on HSTCL.
Here, we report a first of its kind treatment with the HDAC inhibitor chidamide, achieving a satisfactory outcome in an HSTCL patient who showed rapid progress with traditional chemotherapy.
A 45-year-old male patient presented with a 2-mo history of abdominal pain and fatigue, and he was admitted to The First Hospital of Jilin University (Changchun, Jilin, China).
The patient’s symptoms started 2 mo prior when he began a daily fitness routine. There was pain and persistent bloating in the abdomen with fatigue that aggravated slowly until he was referred to the hospital. He had no symptoms of fever or night sweats. Weight loss recorded in the last 5 mo was approximately 5 kg.
The patient had no previous history of immune system disease or immunosuppressive drug use.
The patient had no family history of malignant tumors or blood system diseases.
Physical examination revealed mild epigastric abdominal tenderness and splenomegaly 7 cm below the costal margin without hepatomegaly or peripheral lymphadenopathy.
Laboratory tests showed white blood cell count of 5.05 × 109/L, with a prominent absolute lymphocyte count of 2.47 × 109/L, hemoglobin 9.8 g/dL, and platelet count 109 × 109 /L. The lactate dehydrogenase was 205.1 U/L, erythrocyte sedimentation rate 12 mm/h, and β2-microgloblin 6.72 mg/L. Liver function and renal function tests were normal. The viral markers (hepatitis B, hepatitis C, and human immunode-ficiency viruses), tumor markers (alpha-fetoprotein, carcinoembryonic antigen, and cancer antigen-199), infection profile (Breitbart), and autoimmune profile (antinuclear antibodies and antineutrophil cytoplasmic antibody) were unremarkable.
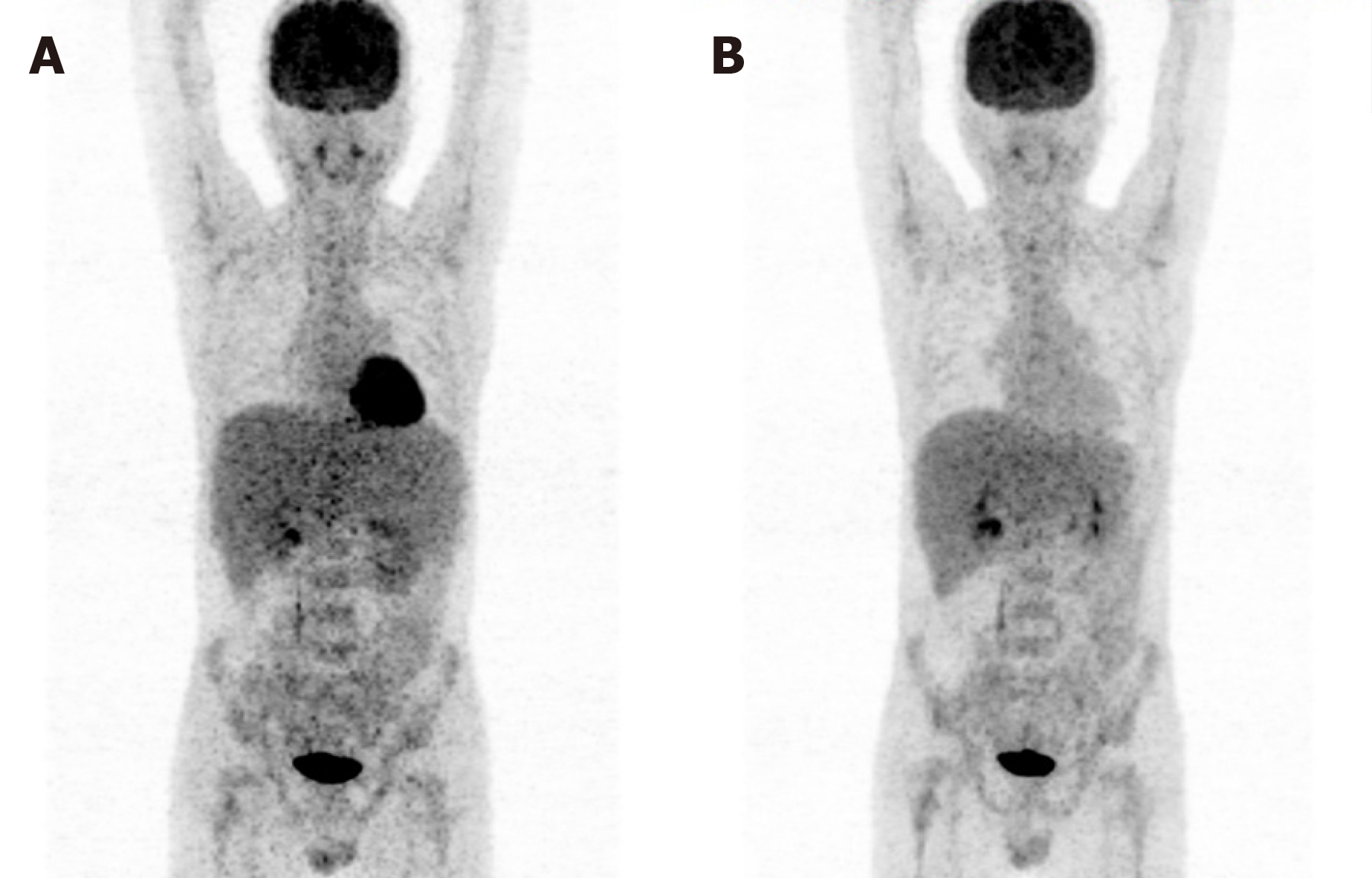
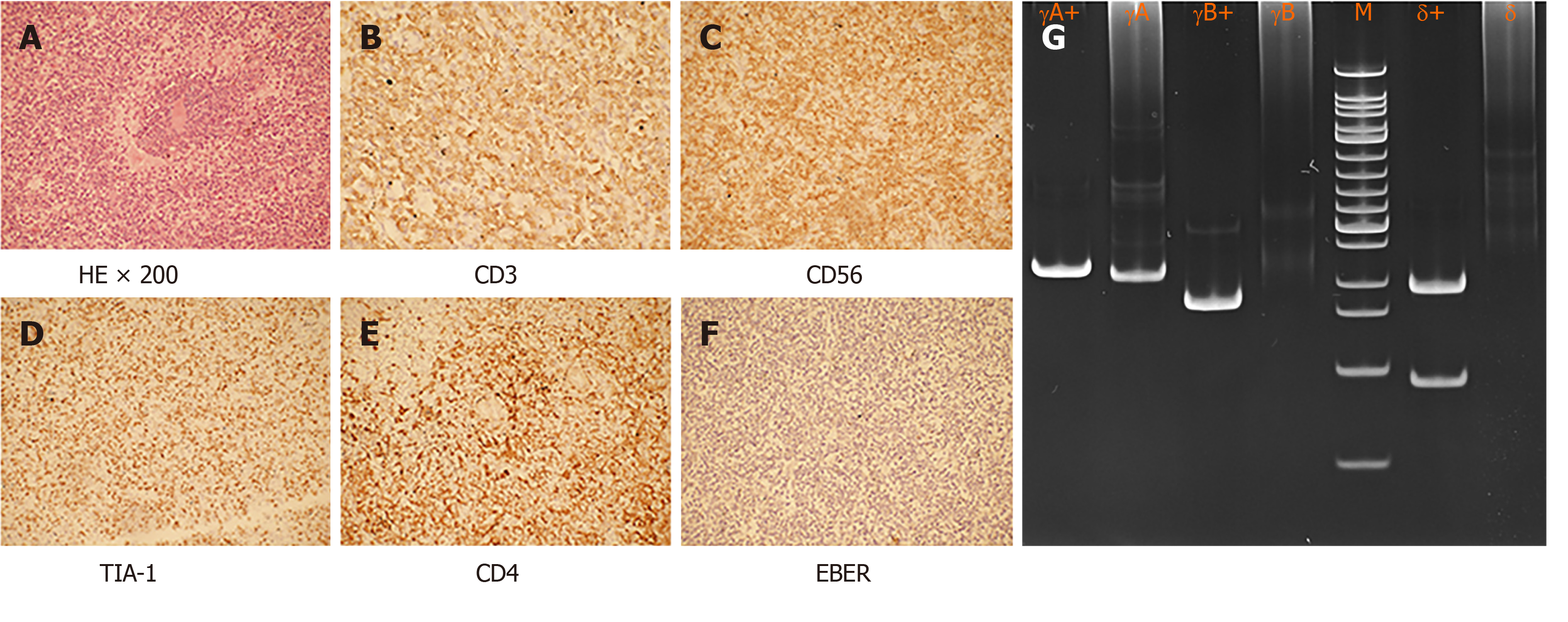
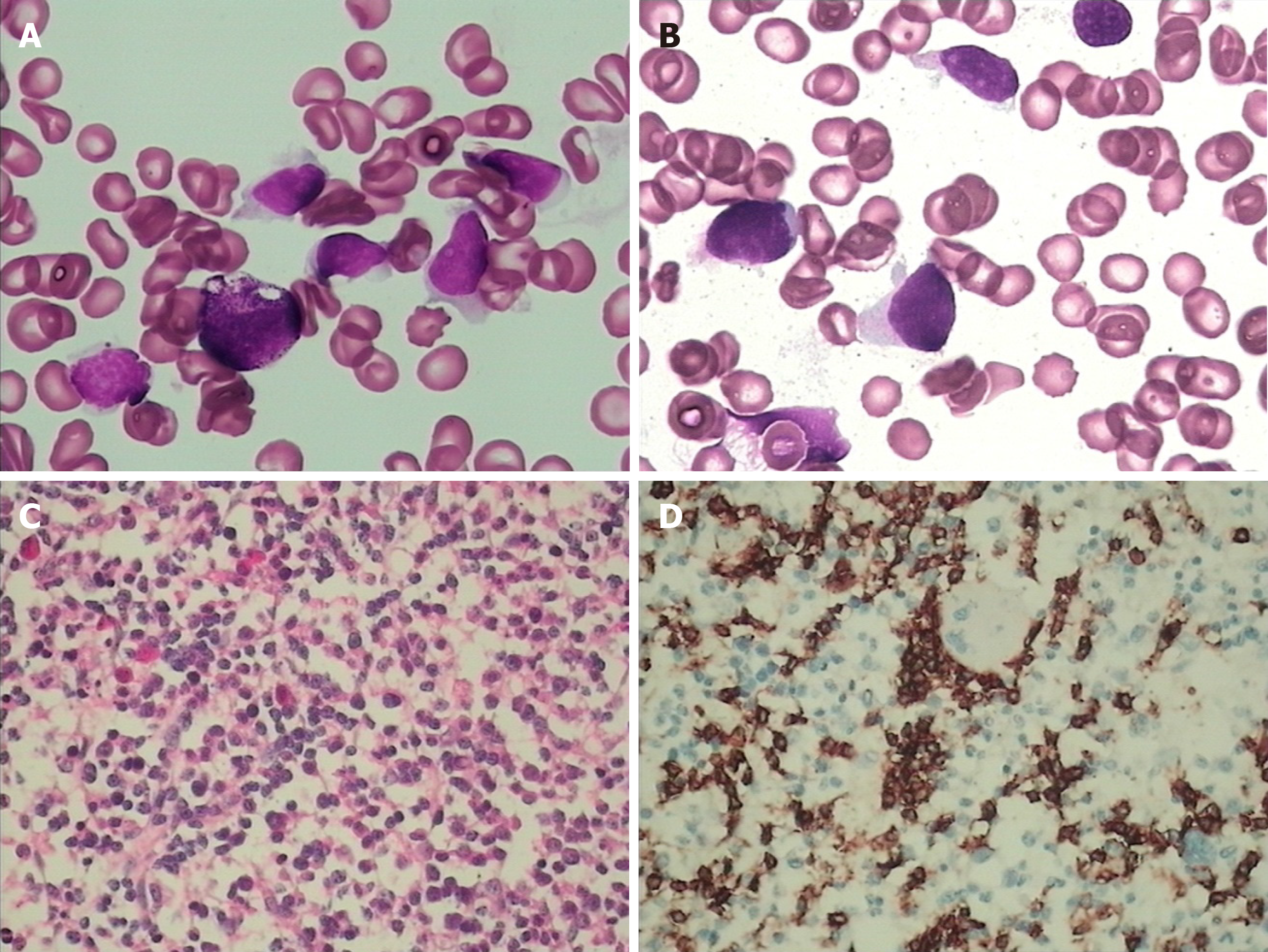
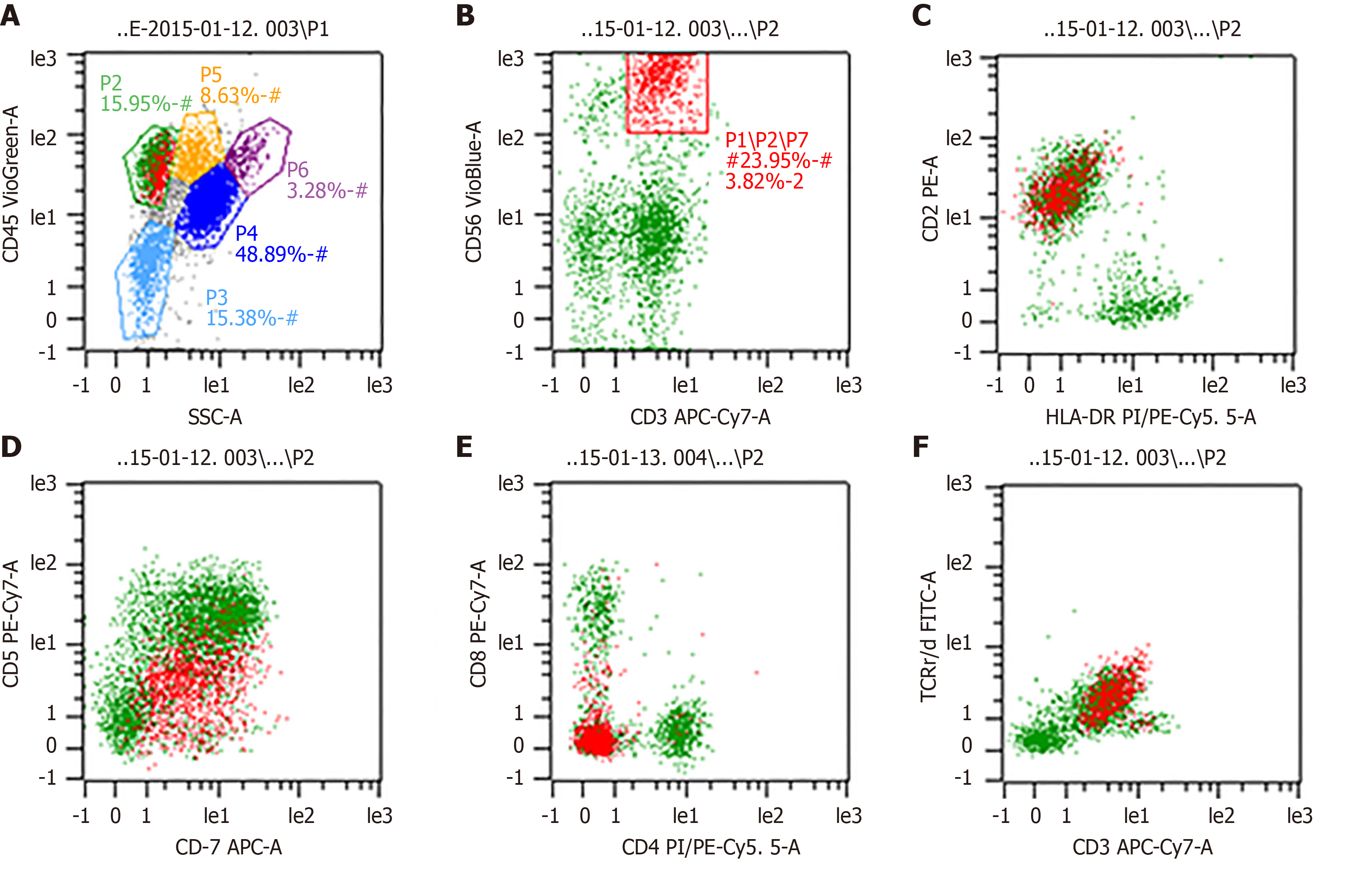
Fluorodeoxyglucose (FDG)-positron emission computed tomography (PET-CT) scan showed significant spleen enlargement with mildly increased FDG uptake (SUV 3.1) and mild liver enlargement (Figure 1A). Histopathology from the splenectomy showed that the tumor tested positive for CD2, surface CD3, CD4, and CD 56 and negative for CD8 and B-cell markers (CD20 and CD79a). The cytotoxic granule protein TIA-1 was expressed, but perforin and granzyme B scatter were positive (Figure 2A-E). In situ hybridization of the Epstein–Barr virus genome showed no abnormality (Figure 2F). Molecular pathology showed positive TCR-γδ rearrangement (Figure 2G), while TCR-αβ was negative. Ki-67 was positive in 70% of atypical cells. Bone marrow and peripheral blood smears revealed the presence of atypical lymphoid cells (Figure 3A and B). Bone marrow biopsy showed a bland infiltration by T lympho-proliferative disease in an intrasinusoidal pattern, supporting the diagnosis (Figure 3C and D). Flow cytometric (FC) analysis of the bone marrow aspirate revealed a population of abnormal cells (23.95%) with higher side scatter expressing CD2, CD3, CD56, and TCR-γδ as compared to normal T-lymphocytes (Figure 4). These cells were negative for CD4, CD8, CD57, CD19, CD10, CD33, CD25, and TDT. The bone marrow cytogenetic study revealed 46, XY, while molecular testing for bone marrow revealed that the clonal immunoglobulin heavy chain was negative, but TCR-γδ was positive.
These findings confirmed the diagnosis of HSTCL (γδ) stage IVB, which involved the spleen and bone marrow.
The process of the therapy is shown in Table 1. Two cycles of inductive chemotherapy and residual disease test by FC showed that the abnormal cells had decreased from before (8.1% vs 23.95%). After the third cycle of chemotherapy, the FC analysis of the bone marrow suggested that aberrant initial cells increased, indicating disease recurrence. The patient was insensitive to chemotherapy, which means that he would not benefit from autogenetic SCT (auto-SCT). The patient agreed to undergo allogeneic SCT (allo-SCT), but no appropriate donor was found. Hence, we chose the novel drug chidamide and administered 20 mg tablets twice a week plus the adjusted-dose regimen of ifosfamide, carboplatin, etoposide (ICE) for salvage therapy. After one cycle, the number of abnormal cells decreased from 21.42% to 9.94%. Additionally, a follow-up PET-CT was performed and showed normal liver size and normalized increased FDG uptake (Figure 1B).
| No. | Regimen | Dose | Abnormalcells, % | Response |
| 1 | Hyper-CVAD | CTX 300 mg/m2 Q12H days 1-3 (mesna rescue); VDS 4 mg day 4, 11; DNR 45 mg/m2 day 1; DEX 40 mg days 1-4, days 11-14 | 8.1 | PR |
| 2 | MA | MTX 1.0 g/m2 day 1; Ara-C 2 g/m2 days 2–3 Q12H; methylprednisolone 30 mg/m2 days 1–3 | ||
| 3 | Hyper-CVAD | CTX 300 mg/m2 Q12H days 1-3 (mesna rescue); VDS 4 mg days 4, 11; DNR 45 mg/m2 day 1; DEX 40 mg days 1-4, days 11-14 | 21.42 | PD |
| 4 | ICE + chidamide | IFO 5 g/m2 day 1, CBP (AUC = 5 mg/mL per min) day 1, VP-16 100 mg/m2 days 3-5, Chidamide: 20mg biw days 1-21 | 9.94 | PR |
| 5 | Chidamide | 30 mg biw days 1-21 | 5.6 | CR |
To ensure a better quality of life, the patient refused to restart chemotherapy and chose to continue with chidamide treatment to prevent disease relapse. Interestingly, after administration of chidamide 30 mg twice a week and monotherapy for 2 mo (3 cycles), the FC analysis showed 0.5% abnormal cells, which indicated complete remission (CR). Apart from the only side effect of grade 2 thrombocytopenia observed, chidamide single drug maintenance was well tolerated.
After 9 mo of chidamide therapy, follow-up bone marrow smear showed substantially increased pathological cells (32%), which indicated lymphoma progression. Though recommend with the rescue chemotherapy and allo-SCT or other targeted drugs (e.g., alemtuzumab), the patient and his family refused to chemotherapy. Unfortunately, the patient died 1 mo after lymphoma progression because of severe pneumonia and respiratory failure which was cause the by leukopenia. Early during this admission, no other abnormalities were found which may imply other occult diseases involved. For this patient, a chidamide combined chemotherapy and single drug maintenance regimen achieved CR, OS of 15 mo, and duration of response of 9 mo.
To the best of our knowledge, this is the first report regarding the management of HSTCL with chidamide, a rare disease that showed recurrence with traditional chemotherapy and DOR of 9 mo.
As a rare disease, there has been no consensus about its treatment. Potent induction chemotherapy and allo-SCT may be the most common treatment strategies for HSTCL[7]. The first-line chemotherapy regimen of chemo-with-anthracycline regimen (CHOP, CVP, or Hyper CVAD/MA) is the top priority in majority of the patients to start with as the induction option. However, poor prognosis with much lower CR response rate (20%-40%)[11] as compared to that of nodal T-cell lymphomas and short time of relapse (8-16 mo) has long been the major concern.
The use of auto-SCT or allo-SCT in HSTCLs has been explored but not well defined in large clinical trials of HSTCL. A recent report from the American Society for Blood and Marrow Transplantation representing consensus opinion has recommended that transplant can be considered in this rare subtype in the case of first remission and relapse-sensitive patients[7]. Due to the limited or questionable benefit shown by auto-SCT in HSTCL, allo-SCT is more promising and well accepted as a front-line consolidation therapy in HSTCL as compared to the commonly adopted auto-SCT for most PTCLs (except ALK-positive anaplastic large cell lymphoma)[12]. A retrospective study performed by the European Bone Marrow Transplant Lymphoma Working Party that included 25 patients with HSTCL and treated with allo-SCT also showed a prominent prolonged median survival of 36 mo[13].
However, due to the low remission rate, only a small proportion of patients underwent SCT after initial remission. The T-Cell Project, which initiated the first prospective worldwide study of patients with aggressive T-cell lymphomas, showed that only 33% of 24 HSTCL patients underwent transplant (auto-SCT, 1; allo-SCT, 4) as consolidation therapy. Additionally, three patients underwent SCT in the salvage setting. Therefore, these patients with HSTCL still had a shortened median OS (13 mo) and a shortened median progression-free survival (11 mo)[11].
Therefore, there is an urgent need to incorporate novel agents in the therapy for HSTCL. New targeted therapies such as pralatrexate, duvelisib, and romidepsin are emerging as potential treatments for PTCL[14]. However, due to its rarity, no large registry study would consider HSTCL as the inclusion criterion. Therefore, no attempt of a combination and maintenance treatment with HDAC inhibitor or other novel drugs for this rare subtype has been tested so far.
The regimen used in our case is described here. First, chidamide is an HDAC inhibitor that modulates chromatin remodeling by interfering with the binding between histone and DNA that increases the level of acetylation, suppresses T lymphoma cell growth, and promotes apoptosis[15]. SETD2 methyltransferase mutations occur in HSTCL and hypermethylation of CpGs around transcription start sites was associated with a lack of protein expression in HSTCL, which showed that the epigenetic drug chidamide would show effective results in HSTCL[16]. Second, chidamide and therapeutic chemotherapy have a synergistic effect of inducing apoptosis with DNA damage accumulation and repair defects. The mechanism is similar to the synergistic effect of low-dose decitabine added to the treatment of acute lymphocyte leukemia[17]. In PTCL, the HDAC inhibitor in combination with the ICE regimen has been shown to have a satisfying effect on relapsed/refractory PTCL, with a 75% (5/7) objective response rate and 100% CR (5/5). The median continuous response time can last for 7.2 mo[18]. To reduce the toxic side effects of co-medication, we reduced the dose of ICE regimen to 2/3 as well as chidamide to 20 mg twice a week, which was well tolerated. Third, HSTCL has already been shown to have a close relationship with immune dysfunction. It was reported that 10% of HSTCL cases occurred in patients with inflammatory bowel disease, who received tumor necrosis factor-alpha inhibitors and/or thiopurines[6,19]. Chidamide enhances the natural killer cells and antigen-specific cytotoxic T-cell-mediated tumor killer effect by inducing the expression of MHC class I-related proteins and NKG2D ligand on tumor cells[15]. Furthermore, chidamide is an orally administered convenient maintenance therapy that can improve the quality of life of the patients.
This case report is the first to demonstrate the effectiveness of chidamide combined with chemotherapy and single-drug maintenance therapy in a patient with HSTCL, who did not show improvement with traditional treatment. This report can be informative for the treatment of rare and poorly studied diseases.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: Chinese Anti-Cancer Association Professional Committee of Lymphoma.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rungsakulkij N S-Editor: Wang JL L-Editor: Filipodia E-Editor: Xing YX
| 1. | Yabe M, Miranda RN, Medeiros LJ. Hepatosplenic T-cell Lymphoma: a review of clinicopathologic features, pathogenesis, and prognostic factors. Hum Pathol. 2018;74:5-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Falchook GS, Vega F, Dang NH, Samaniego F, Rodriguez MA, Champlin RE, Hosing C, Verstovsek S, Pro B. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20:1080-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Ochenrider MG, Patterson DJ, Aboulafia DM. Hepatosplenic T-cell lymphoma in a young man with Crohn's disease: case report and literature review. Clin Lymphoma Myeloma Leuk. 2010;10:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Montgomery M, van Santen MM, Biemond BJ, Diamond RH, Pals ST. Hepatosplenic T-Cell Lymphoma: A Population-Based Study Assessing Incidence and Association With Immune-Mediated Disease. Gastroenterol Hepatol (N Y). 2015;11:160-163. [PubMed] |
| 5. | Jacobs MF, Anderson B, Opipari VP, Mody R. Hepatosplenic αβ T-Cell Lymphoma as Second Malignancy in Young Adult Patient With Previously Undiagnosed Ataxia-Telangiectasia. J Pediatr Hematol Oncol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Carvão J, Magno Pereira V, Jacinto F, Sousa Andrade C, Jasmins L. Hepatosplenic T-Cell Lymphoma: A Rare Complication of Monotherapy with Thiopurines in Crohn's Disease. GE Port J Gastroenterol. 2019;26:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, Reimer P, Gisselbrecht C, d'Amore F, Jantunen E, Ishida T, Bazarbachi A, Foss F, Advani R, Fenske TS, Lazarus HM, Friedberg JW, Aljurf M, Sokol L, Tobinai K, Tse E, Burns LJ, Chavez JC, Reddy NM, Suzuki R, Ahmed S, Nademanee A, Mohty M, Gopal AK, Fanale MA, Pro B, Moskowitz AJ, Sureda A, Perales MA, Carpenter PA, Savani BN. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017;23:1826-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 8. | Youssef RRH, Elmahdy A, Kohla S, Ibrahim F, Sabry AS, Alobaidly A, Saloum N. Role of Imaging in Diagnosis of a Rare Hepatosplenic Gamma Delta T-Cell Lymphoma during Pregnancy: A Case Report and Review of Literature. Case Rep Oncol. 2019;12:935-943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Xu Y, Zhang P, Liu Y. Chidamide tablets: HDAC inhibition to treat lymphoma. Drugs Today (Barc). 2017;53:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Shi Y, Jia B, Xu W, Li W, Liu T, Liu P, Zhao W, Zhang H, Sun X, Yang H, Zhang X, Jin J, Jin Z, Li Z, Qiu L, Dong M, Huang X, Luo Y, Wang X, Wang X, Wu J, Xu J, Yi P, Zhou J, He H, Liu L, Shen J, Tang X, Wang J, Yang J, Zeng Q, Zhang Z, Cai Z, Chen X, Ding K, Hou M, Huang H, Li X, Liang R, Liu Q, Song Y, Su H, Gao Y, Liu L, Luo J, Su L, Sun Z, Tan H, Wang H, Wang J, Wang S, Zhang H, Zhang X, Zhou D, Bai O, Wu G, Zhang L, Zhang Y. Chidamide in relapsed or refractory peripheral T cell lymphoma: a multicenter real-world study in China. J Hematol Oncol. 2017;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 11. | Foss FM, Horwitz SM, Civallero M, Bellei M, Marcheselli L, Kim WS, Cabrera ME, Dlouhy I, Nagler A, Advani RH, Pesce EA, Ko YH, Montoto S, Chiattone C, Moskowitz A, Spina M, Cesaretti M, Biasoli I, Federico M. Incidence and outcomes of rare T cell lymphomas from the T Cell Project: hepatosplenic, enteropathy associated and peripheral gamma delta T cell lymphomas. Am J Hematol. 2020;95:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Okuni M, Yakushijin K, Uehara K, Ichikawa H, Suto H, Hashimoto A, Tanaka Y, Shinzato I, Sakai R, Mizutani Y, Nagao S, Kurata K, Kakiuchi S, Miyata Y, Inui Y, Saito Y, Kawamoto S, Yamamoto K, Ito M, Matsuoka H, Minami H. Successful Bridging Chemotherapy with Gemcitabine, Carboplatin, and Dexamethasone before Unrelated Stem Cell Transplantation for Hepatosplenic T-cell Lymphoma. Intern Med. 2019;58:707-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Tanase A, Schmitz N, Stein H, Boumendil A, Finel H, Castagna L, Blaise D, Milpied N, Sucak G, Sureda A, Thomson K, Vandenberghe E, Vitek A, Dreger P, Lymphoma Working Party of the EBMT. Allogeneic and autologous stem cell transplantation for hepatosplenic T-cell lymphoma: a retrospective study of the EBMT Lymphoma Working Party. Leukemia. 2015;29:686-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Ito Y, Makita S, Tobinai K. Development of new agents for peripheral T-cell lymphoma. Expert Opin Biol Ther. 2019;19:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | McKinney M, Moffitt AB, Gaulard P, Travert M, De Leval L, Nicolae A, Raffeld M, Jaffe ES, Pittaluga S, Xi L, Heavican T, Iqbal J, Belhadj K, Delfau-Larue MH, Fataccioli V, Czader MB, Lossos IS, Chapman-Fredricks JR, Richards KL, Fedoriw Y, Ondrejka SL, Hsi ED, Low L, Weisenburger D, Chan WC, Mehta-Shah N, Horwitz S, Bernal-Mizrachi L, Flowers CR, Beaven AW, Parihar M, Baseggio L, Parrens M, Moreau A, Sujobert P, Pilichowska M, Evens AM, Chadburn A, Au-Yeung RK, Srivastava G, Choi WW, Goodlad JR, Aurer I, Basic-Kinda S, Gascoyne RD, Davis NS, Li G, Zhang J, Rajagopalan D, Reddy A, Love C, Levy S, Zhuang Y, Datta J, Dunson DB, Davé SS. The Genetic Basis of Hepatosplenic T-cell Lymphoma. Cancer Discov. 2017;7:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 17. | Shi P, Zhang L, Chen K, Jiang Z, Deng M, Zha J, Guo X, Li P, Xu B. Low-dose decitabine enhances chidamide-induced apoptosis in adult acute lymphoblast leukemia, especially for p16-deleted patients through DNA damage. Pharmacogenomics. 2017;18:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Strati P, Chihara D, Oki Y, Fayad LE, Fowler N, Nastoupil L, Romaguera JE, Samaniego F, Garg N, Feng L, Wesson ET, Ruben CE, Stafford MD, Nieto Y, Khouri IF, Hosing C, Horowitz SB, Kamble RT, Fanale MA. A phase I study of romidepsin and ifosfamide, carboplatin, etoposide for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Haematologica. 2018;103:e416-e418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-α inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol. 2011;23:1150-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |