Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3097
Peer-review started: May 18, 2020
First decision: June 4, 2020
Revised: June 9, 2020
Accepted: June 29, 2020
Article in press: June 29, 2020
Published online: July 26, 2020
Processing time: 67 Days and 8.4 Hours
Follicular dendritic cell (FDC) sarcomas are rare neoplasms that occur predominantly in the lymph nodes. They can also occur extranodally. Extranodal FDC sarcomas most commonly present as solitary masses. Inflammatory pseudotumor (IPT)-like FDC sarcomas, a subcategory of FDC sarcomas, are rarer than other sarcoma subtypes. They are composed of spindle or ovoid neoplastic cells and exhibit an admixture of plasma cells and prominent lymphoplasmacytic infiltration. Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is a rare autoimmune bullous disease that is associated with underlying neoplasms. PNP has a high mortality, and its early diagnosis is usually difficult.
We describe a 27-year-old woman who presented with stomatitis, conjunctivitis, and skin blisters and erosions as her first symptoms of PNP with an intra-abdominal IPT-like FDC sarcoma. The patient underwent surgical tumor resection and received tapering oral corticosteroid treatment. She showed no recurrence at the 1-year follow-up.
IPT-like FDC sarcomas are rare underlying neoplasms that have an uncommon association with PNP. PNP-associated FDC sarcomas predominantly occur in intra-abdominal sites and suggest a poor prognosis. Surgical resection is an essential and effective treatment for PNP and primary and recurrent FDC sarcomas.
Core tip: To date, 32 cases of paraneoplastic pemphigus (PNP)-associated follicular dendritic cell (FDC) sarcomas have been reported in the English literature. Inflammatory pseudotumor-like FDC sarcoma was described as an underlying neoplasm of PNP in only two cases. Here, we report a case that PNP was the patient’s first symptom of an intra-abdominal inflammatory pseudotumor-like FDC sarcoma, and review the related literature.
- Citation: Zhuang JY, Zhang FF, Li QW, Chen YF. Intra-abdominal inflammatory pseudotumor-like follicular dendritic cell sarcoma associated with paraneoplastic pemphigus: A case report and review of the literature. World J Clin Cases 2020; 8(14): 3097-3107
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3097.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3097
Follicular dendritic cell (FDC) sarcoma was first described in 1986 by Monda et al[1] as a nonlymphomatous lymph node malignancy with features suggesting a FDC origin. It is classified into two types: (1) Conventional FDC sarcomas that are histologically characterized by spindle cell proliferation with fascicles, trabecular, or diffuse sheets; and (2) Inflammatory pseudotumor (IPT)-like FDC sarcomas, an entity proposed by Cheuk et al[2] in 2001, which are characterized by dispersed spindle or ovoid tumor cells against a background of abundant lymphocytes and plasma cells. However, in contrast to conventional FDC sarcomas, IPT-like FDC sarcomas predominantly arise in intra-abdominal sites, especially the liver and spleen. Paraneoplastic pemphigus (PNP), which was first described in 1990, is a rare and life-threatening mucocutaneous autoimmune disease that is associated with underlying neoplasms, especially lymphoproliferative disorders[3]. A total of 32 cases of FDC sarcomas associated with the occurrence of PNP have been previously reported (Table 1)[4-30]. Only two case wherein an IPT-like FDC sarcoma is an underlying neoplasm of PNP have been described[20,31]. Here, we report a 27-year-old woman who presented stomatitis, conjunctivitis, and polymorphic cutaneous lesions, which are consistent with the features of PNP, as her first symptoms of intra-abdominal IPT-like FDC sarcoma, and review the related literature. This work intends to serve as a reference for the correct identification of this disease by clinicians. It also aims to broaden clinicians’ understanding of this kind of tumor, PNP, and their rare relationship.
| Ref. | Sex/age | Tumor pathology | Location | Maximum diameter (cm) | Clinical manifestations | Treatments | Follow-up |
| Walters et al[4] | M/48 | FDCS | Anterior mediastinum | NA | Lichenoid skin lesions, BO, MG | Tumor resection, multiple immunosuppressive therapies | Progressive respiratory disease |
| M/88 | FDCS | Retropharynx | 8 | Mucosal lichenoid erosions | Tumor resection | DOD within 1 yr, status unknown | |
| F/59 | FDCS | Axillary lymph node | NA | Lichenoid skin lesions, mucocutaneous blisters, BO | Tumor resection | DOD within 6 mo | |
| M/23 | FDCS associated with CD | Cervical lymph node | NA | Mucosal lichenoid erosions, BO | Partial tumor resection and residual mass was radiated | Tumor recurrence and DOD within 2 yr | |
| Lu et al[5] | F/49 | FDCS | Pancreatic tail | 6 | Stomatitis, MG, pulmonary infection | Tumor resection, antifungal and anti-infection therapies | DOD 12 d after surgery |
| Jonkman et al[6] | F/35 | FDCS | Intra-abdomen | NA | Stomatitis, punctate keratoses with central ulceration on the palms and soles. | Tumor resection and intensive immunosuppression | DOD with respiratory failure |
| Akel et al[7] | M/39 | FDCS | Intra-abdomen | 18 | Lichenoid skin lesions, mucocutaneous blisters, febrile neutropenia | Tumor resection and high-dose steroids | DOD with severe pneumonia and acidosis |
| Wang et al[8] | F/56 | FDCS associated with CD | Retroperitoneum | 10 | Stomatitis, polymorphous skin lesions, BO | Tumor resection, IVIg and steroid therapies | Alive at 4 yr follow-up |
| Wang et al[9] | F/27 | FDCS | Retroperitoneum | 8 | Stomatitis, conjunctivitis, lichenoid skin lesions | Tumor resection | Tumor recurrence 5 yr after surgery |
| Su et al[10] | M/43 | FDCS | Retroperitoneum | 5 | Stomatitis, lichenoid skin lesions | Tumor resection and lymphadenectomy, IVIg and steroid therapies | DOD with multiple organ failure |
| Chow et al[11] | M/62 | FDCS | Anterior mediastinum | 7.5 | Stomatitis, conjunctivitis, mucocutaneous blisters | Right thoracotomy and tumor resection, adjuvant radiotherapy | DOD with respiratory failure |
| Garza-Chapa et al[12] | M/20 | FDCS | Right-side mediastinum | 7 | Stomatitis, conjunctivitis, lichenoid skin lesions | Right thoracotomy and tumor resection, chemotherapy with R-CVP (rituximab, cyclophosphamide, vincristine, prednisone) | Resolution of skin lesions and no evidence of tumor recurrence at 1-yr follow-up |
| Streifel et al[13] | M/72 | FDCS | Right-side mediastinum | NA | Stomatitis, conjunctivitis, and glans penis involvement, lichenoid skin lesions, MG | Thymectomy and partial pericardiectomy, rituximab, IVIg, steroids and mycophenolate mofetil | Improvement at 9-mo follow-up |
| Kim et al[14] | M/68 | FDCS | Small bowel mesentery | 9 | Abdominal palpable mass, stomatitis, conjunctivitis, MG | Tumor resection | Metastatic tumors found in the liver 1 yr after surgery; DOD within 2 yr |
| Seishima et al[15] | F/64 | FDCS | Retroperitoneum and small intestine | 15 | Stomatitis, conjunctivitis, severe skin erosions | Tumor resection and steroid therapy | Tumor recurrence; DOD with fungal infective embolisms in the lungs |
| Liu et al[16] | F/54 | FDCS | Retroperitoneum | 10.8 | Stomatitis, Lichenoid skin lesions | Tumor resection, systemic corticosteroid, and cyclosporine therapies | DOD with respiratory failure |
| Baghmar et al[17] | M/20 | FDCS | Right hemipelvis | 6 | Stomatitis, conjunctivitis, lichenoid skin lesions | Unresectable, chemotherapy with rituximab, cyclophosphamide, adriamycin, vincristine, and prednisolone | DOD with respiratory pseudomonas infections |
| Hwang et al[18] | F/46 | FDCS | Liver | 16 | Abdominal pain, Stomatitis, lichenoid skin lesions | Tumor resection, rituximab and ciclosporin therapies | Tumor recurrence 1 yr after surgery; DOD with pneumonia |
| Lee et al[19] | M/67 | FDCS associated with CD | Small bowel mesentery | NA | Stomatitis, conjunctivitis, MG | Tumor resection, IVIg, prednisolone, and cyclosporine therapies | Metastatic tumors found in the liver 1 yr after surgery |
| Zhao et al[20] | F/28 | IPT-like FDCS | Intra-abdomen | 9 | Stomatitis, blisters and erosions of the underarm, groin and perineum, and labia majora, BO | Tumor resection | Improvement after surgery |
| Sugiura et al[21] | M/28 | FDCS associated with CD | Left retroperitoneum | 7.7 | Stomatitis, polymorphic cutaneous lesions, papillomatous hyperplasia on the tongue | Tumor resection, chemotherapy of COP (cyclophosphamide, vincristine, and prednisolone) | Skin lesions healed completely except for the papillomatous hyperplasia on the tongue, and follow-up is not clear |
| Marzano et al[22] | F/53 | FDCS | Right retroperitoneum | 9 | Stomatitis, conjunctivitis, lichenoid skin lesions, dyspnea | Tumor resection, systemic corticosteroid and IVIg therapies | DOD with respiratory failure within 2 yr |
| Meijs et al[23] | M/60 | FDCS | Mediastinum | 6 | Stomatitis, conjunctivitis, mucocutaneous blisters and erosions, BO | Tumor resection, chemotherapy with rituximab, IVIg, plasmapheresis, corticosteroid, azathioprine and cyclophosphamide | DOD with respiratory failure |
| Lee et al[24] | M/66 | FDCS associated with CD | Right retroperitoneum | 12 | Stomatitis, conjunctivitis, mucocutaneous blisters and erosions, lichenoid skin lesions | Tumor resection and antibiotics therapy | DOD with sepsis 8 d after surgery |
| Choi et al[25] | F/39 | FDCS | NA | NA | Stomatitis, mucocutaneous blisters and erosions | Tumor resection | Alive for 5 yr, and skin lesions healed except for oral persistent mucositis |
| Zhang et al[26] | M/20 | FDCS associated with CD | NA | NA | Stomatitis, conjunctivitis and genital involvement, skin blisters | Tumor resection | Alive for 3 yr without recurrence |
| M/16 | FDCS associated with CD | NA | NA | Stomatitis, conjunctivitis and genital involvement, skin blisters | Tumor resection | DOD with severe infections 2 wk after surgery | |
| Yamada et al[27] | M/68 | FDCS | Retroperitoneum | NA | Stomatitis, conjunctivitis and genital involvement, polymorphic cutaneous lesions | Tumor resection, plasmapheresis, and steroid pulse therapies | DOD with septicemia 2 mo after surgery |
| Ogawa et al[28] | M/28 | FDCS | Retroperitoneum | NA | Stomatitis, skin blisters and erosions | Partial resection, IVIg, chemotherapy with cyclophosphamide, vincristine, and prednisolone therapies | Alive for 3 yr, and skin lesions healed except for oral persistent mucositis |
| Raco et al[29] | F/61 | FDCS associated with CD | Intra-abdomen | 10 | Stomatitis, lichenoid skin lesions, dyspnea | Previous: splenectomy and chemotherapy 3 yr agoRecent: IVIg, rituximab, steroid and antibiotic therapies | Tumor metastasis or recurrence; DOD with respiratory failure |
| Rice et al[30] | F/41 | FDCS | Retroperitoneum | 8 | Stomatitis, conjunctivitis, lichenoid skin lesions dyspnea, | Tumor resection, IVIg, systemic corticosteroid, rituximab, and daclizumab therapies | Progressive respiratory failure |
| Wang et al[31] | F/60 | IPT-like FDCS | Left axillary and cervical lymph nodes | 6.4 | Stomatitis, polymorphic cutaneous lesions, MG, dyspnea | Tumor resection, IVIg, steroid, and rituximab therapies | DOD with multiple organ failure |
| Present case | F/27 | IPT-like FDCS | Intra-abdomen | 9 | Stomatitis, conjunctivitis, skin blisters and erosions, mild dyspnea | Tumor resection, tapering corticosteroid | No evidence of tumor recurrence at 1-yr follow-up |
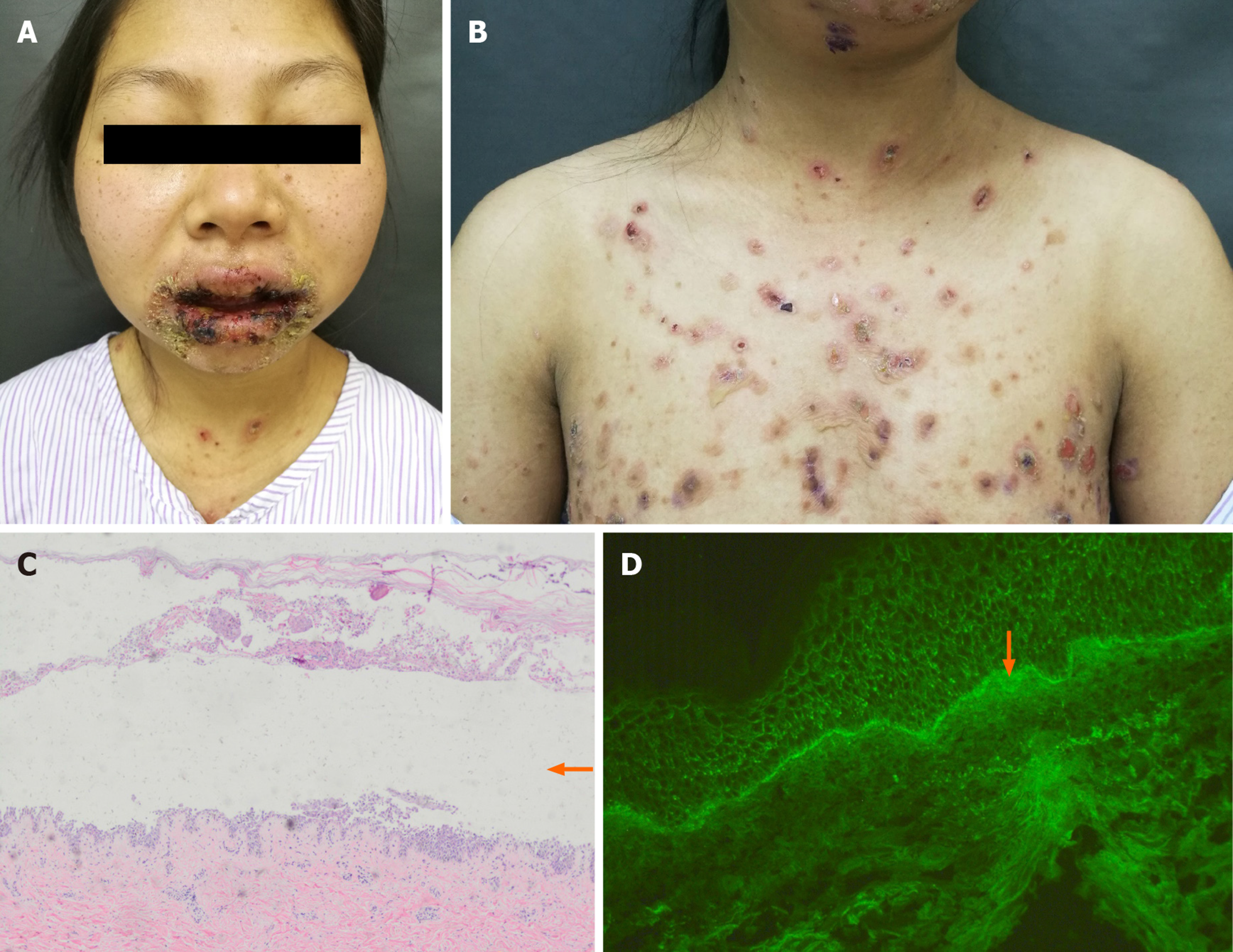
A 27-year-old Chinese woman presented with complaints of painful desquamative stomatitis, conjunctivitis for 2 mo (Figure 1A), and polymorphic skin lesions on the trunk (Figure 1B) for half a month.
Oral blisters and erosions occurred first, and skin lesions, including erythema, vesicles, and erosions, developed on the trunk subsequently. The patient had no abdominal discomfort or other gastrointestinal symptoms.
Oral blisters and erosions involved mucus membranes, the tongue, and lips. The vesicles on the trunk were loose and positive for the Nikolsky sign (a dermatological examination method of acanthocyte loosening that is performed to check whether blisters and bullae are located inside or under the epidermis).
Enzyme-linked immunosorbent assay revealed increased concentrations of circulating serum autoantibodies against desmoglein-1 and desmoglein-3 (two kinds of pemphigus antibodies).
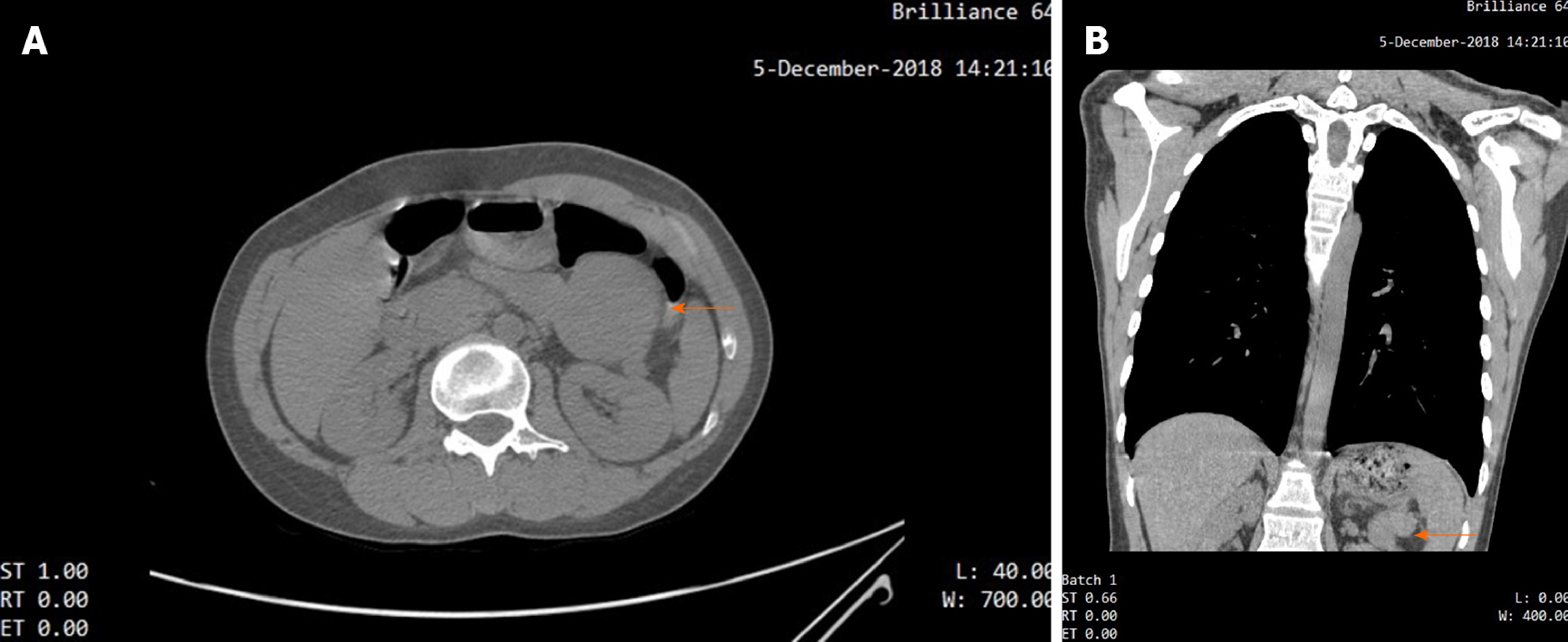
For the patient complained of mild dyspnea, a chest computed tomography (CT) scan was performed, which revealed mild bronchiolitis obliterans on the patient’s lungs. Besides, it happened to scan an iso-dense, well-circumscribed mass in the upper abdominal area (Figure 2A and B).
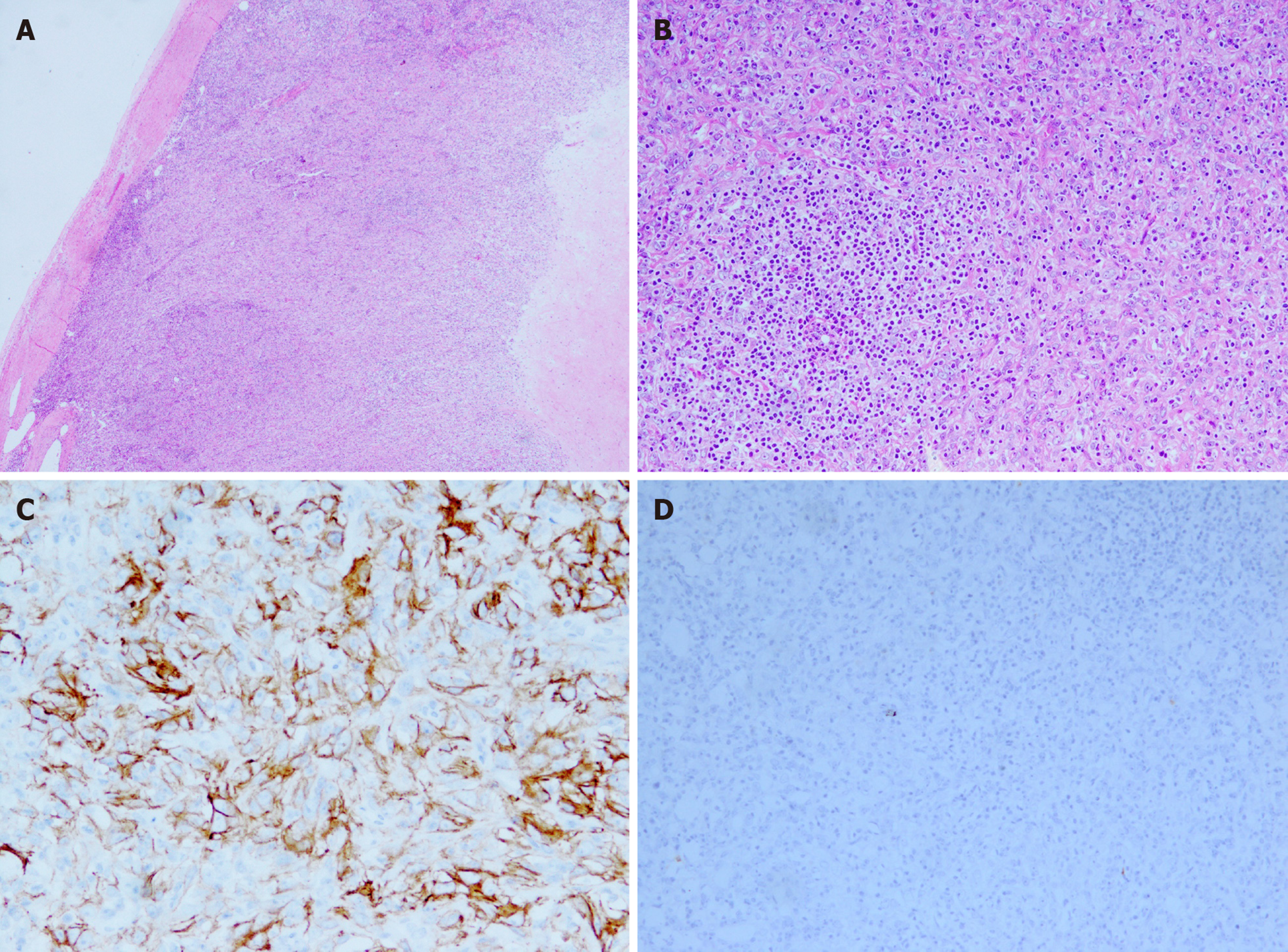
Skin lesion biopsy showed intraepidermal acantholysis and blisters (Figure 1C). Moreover, C3 was detected in the basal stratum through direct immunofluorescence (Figure 1D). The postoperative pathology of the tumor showed that spindle vacuolar tumor cells with mild cellular atypia were distributed against a background of abundant small lymphocytes, especially in pseudofollicles (Figure 3A). Some abnormal nuclear fissions were observed (Figure 3B), and cells resembling Reed–Sternberg cells were captured at times. Immunohistochemical studies revealed that the tumor cells were positive for CD21 (Figure 3C), CD68, and Ki-67 (maximally 20% to 30%) and negative for CK, CD3, HMB-45, CD20, CD30, CD34, CD117 (Figure 3D), and anaplastic lymphoma kinase.
PNP associated with IPT-like FDC sarcoma.
Before the diagnosis of IPT-like FDC sarcoma, the patient received treatments of methylprednisolone combined with cyclophosphamide and showed improvement but shortly relapsed. After the diagnosis of IPT-like FDC sarcoma, the patient underwent surgical resection of the sarcoma. The resected tumor, which had dimensions of 9 cm × 6 cm × 6 cm, was solid and circumscribed. Gradually tapering methylprednisolone treatment was continued as a conservative adjuvant therapy to ensure that the patient’s PNP was controlled.
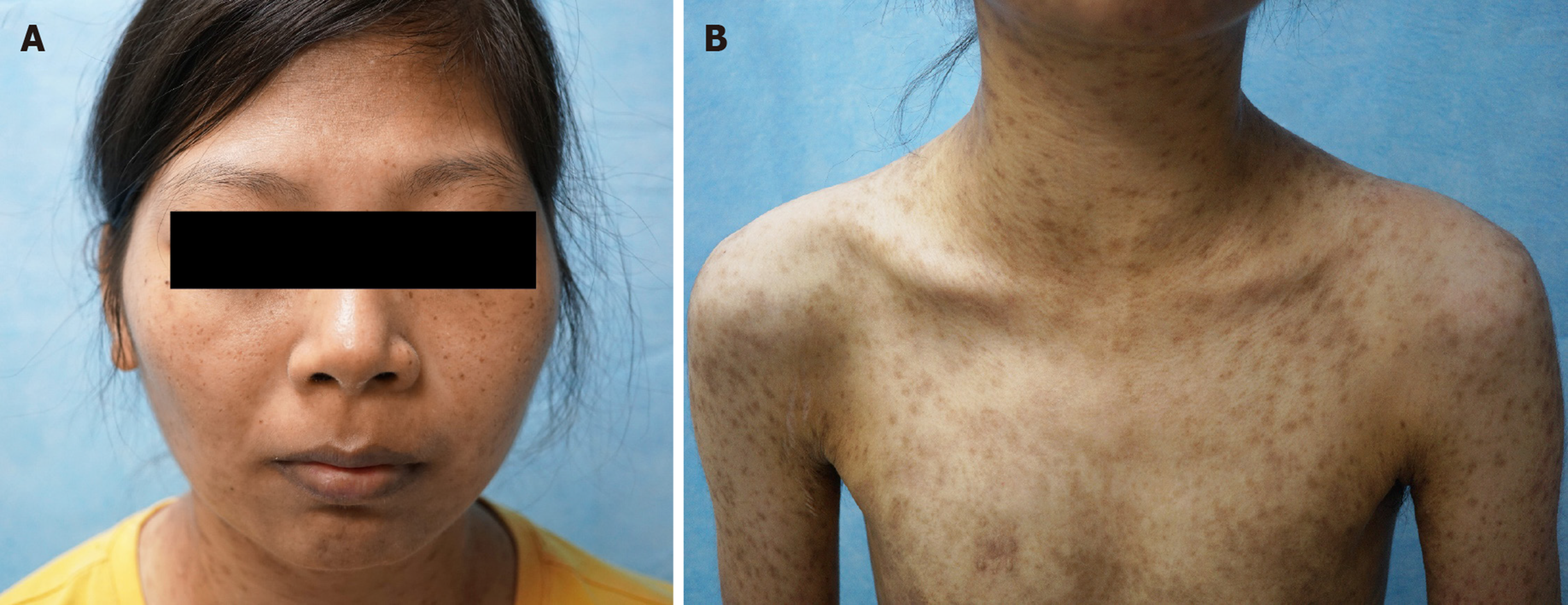
When the patient previously received drug treatments for pemphigus, she improved but her symptoms relapsed shortly. By contrast, after the surgical resection of her sarcoma, the patient was free from pemphigus symptoms. PNP and IPT-like FDC sarcoma showed no recurrence at the 1-year follow-up (Figure 4A and B).
In the case that we described, the existence and disappearance of PNP had a direct relationship with IPT-like FDC sarcoma. This relationship adequately illustrated an extremely unusual association of IPT-like FDC sarcoma with PNP. Sarcoma is an underlying malignancy in approximately 6% of PNP cases; it is involved in leiomyosarcomas, liposarcomas, malignant nerve sheath tumors, poorly differentiated sarcomas, reticulum cell sarcomas, dendritic cell sarcomas, and inflammatory myofibroblastic tumors[32]. The occurrence of PNP with IPT-like FDC sarcoma is rare. To date, 32 cases of PNP-associated FDC sarcomas have been reported in the English literature (Table 1). Given that IPT-like FDC sarcoma was described as an underlying neoplasm of PNP in only two cases[20,31], our presentation is extremely rare. PNP-associated FDC sarcomas tend to show an Asian preference (21/32), especially among Eastern Asians. Previously reported cases involved 18 males and 14 females (male/female ratio of 1.3: 1) with a mean age of 47 years (range, 16-88 years). The locations of FDC sarcomas were the mediastinum (5/29), cervix (3/29), axillary lymph nodes (2/29), and intra-abdominal sites (20/29). Locations were not mentioned in three cases. Nearly 70% cases of PNP-associated FDC sarcomas occurred in intra-abdominal sites, even though FDC sarcomas themselves predominantly occur in the cervical and axillary lymph nodes. This characteristic suggests that intra-abdominal FDC sarcomas may indicate a poor prognosis. Furthermore, in six cases, FDC sarcomas caused PNP were coexisting with myasthenia gravis (MG)[4,5,13,14,19,21]. Paraneoplastic neurologic syndromes are even rarer than other syndromes, occurring in approximately 0.01% of patients with cancer[33]. PNP was the first autoimmune disease that was demonstrated to be associated with FDC sarcoma[24]. Subsequently, Hartert et al[34] first reported in 2010 that FDC sarcoma is associated with MG without PNP. What’s more, Sandri et al[35] once reported that paraneoplastic arthritis is the first symptom of IPT-like FDC sarcoma. FDC sarcoma is one of the underlying risk factors for developing paraneoplastic autoimmune diseases, which show a high mortality rate. Its early and correct identification by clinicians is crucial.
Previous reports have revealed that diseases associated with PNP predominantly underlie B-cell lymphoproliferative diseases, for example, non-Hodgkin lymphomas, chronic lymphocytic leukemia, and Castleman disease, such that 84% of neoplasms are associated with PNP[31]. Interestingly, we found that Castleman disease was involved in eight cases of PNP-associated FDC sarcoma (Table 1)[4,8,19,21,24,26,29]. In China, PNP is frequently found in association with Castleman’s tumors[36]. Some researchers assumed that FDC sarcoma arose from hyaline vascular Castleman disease, possibly through a mechanism involving epidermal growth factor receptors[37]. A recent genetic study has suggested that FDC sarcomas associated with unicentric hyaline-vascular Castleman disease show mutations and copy number changes in known oncogenes, tumor suppressors, and chromatin remodeling genes[38]. In addition, histologically, indolent T-lymphoblastic proliferation is frequently found in FDC sarcomas and shows an association with paraneoplastic autoimmune multiorgan syndrome; this association suggests that neoplastic follicular dendritic cells can recruit or foster the proliferation of immature T cells, which may lead to the occurrence of PNP[4,22]. In the present study, we failed to detect the immunohistochemistry of TdT, which is a necessary index for later studies. However, the patient’s manifestations improved and her serum autoantibody titers gradually decreased after tumor resection. We thus hypothesized that B lymphocytes also have a notable role in this associated tumor. Zhu’s research team used a specific peptide to probe the specific immunoglobulin receptors on tumor B lymphocytes from patients with PNP and confirmed that associated tumors can produce autoantibodies against antigens in the epidermis[39]. The relationships among immature T cells–B cells–PNP in FDC sarcomas and other trigger tumors require further studies.
FDC sarcomas are low-grade tumors. Further studies with long follow-up periods have recently indicated that FDC sarcomas are at least intermediate-grade tumors given their local recurrence and occasional distant metastases[40]. PNP-associated FDC sarcomas suggest a poor prognosis and high mortality rate. The risk factors related to mortality are severe infections, such as sepsis and infectious pneumonia; lung bronchiolitis obliterans; multiple organ dysfunction; and tumor recurrences or metastases (Table 1). The stabilization of vital parameters, the evaluation of any underlying malignancy, the accurate diagnosis of PNP, the removal and medical therapy of the trigger tumor, and the treatment of PNP are six indispensable steps to improve the management of patients with PNP[41]. We reckoned that the early treatment and management of PNP helped prevent serious infection and degeneration in the present case. However, PNP, especially stubborn oral mucosa lesions that are observed in most cases, is considered refractory to conventional medical treatments compared with other types of pemphigus. Unified criteria for the therapy and evidence-based treatment of PNP remain lacking. The early detection and resection of trigger tumors are essential for the treatment of PNP since they may produce autoantibodies to impair the epidermis[39]. The 31 patients that we reviewed above underwent complete (29/31) or partial (2/31) surgical resection. Only one case had a tumor that was found to be unresectable during exploratory laparotomy[17]. Surgical resection seems to be the first choice for the treatment of primary and recurrent FDC sarcomas, whereas the role of adjuvant radiotherapy or chemotherapy has not been well defined[42]. A recent study revealed that the local recurrence and distant metastasis rate of IPT-like FDC sarcomas is approximately 17%[43], whereas that of conventional FDC sarcomas is 40%–50%, suggesting that IPT-like FDC sarcomas are a more indolent variant of FDC sarcomas. Our patient showed no recurrence or metastasis of the sarcoma at the 1-year follow-up. Continued long-term follow is required to obtain improved insight into these two diseases.
IPT-like FDC sarcomas are rare underlying neoplasms that have an uncommon association with PNP. To date, only 32 cases of PNP-associated FDC sarcomas have been reported in the English literature. Intra-abdominal FDC sarcomas may suggest a poor prognosis. The immature T cells and B cells of FCD sarcomas might play roles in PNP development, and the mechanisms of these roles require further studies. Surgical resection is an essential and effective treatment for PNP and primary and recurrent FDC sarcomas. IPT-like FDC sarcomas seem to be more indolent than conventional FDC sarcomas, and long-term follow-up is required.
The authors would like to thank the Pathology Department of Dermatology Hospital, Southern Medical University for their contributions to the acquisition of histopathology images.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shi J, Unger MM S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, Izumi H, Ratrie H, Mutasim D, Ariss-Abdo L. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 685] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 4. | Walters M, Pittelkow MR, Hasserjian RP, Harris NL, Macon WR, Kurtin PJ, Rech KLG. Follicular Dendritic Cell Sarcoma With Indolent T-Lymphoblastic Proliferation Is Associated With Paraneoplastic Autoimmune Multiorgan Syndrome. Am J Surg Pathol. 2018;42:1647-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Lu T, Song B, Pu H, Li X, Chen Q, Yang C. Paraneoplastic pemphigus and myasthenia gravis as the first manifestations of a rare case of pancreatic follicular dendritic cell sarcoma: CT findings and review of literature. BMC Gastroenterol. 2019;19:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Jonkman MF, Pas HH. Image Gallery: Paraneoplastic pemphigus and follicular dendritic cell sarcoma. Br J Dermatol. 2018;178:e146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Akel R, Fakhri G, Salem R, Boulos F, Habib K, Tfayli A. Paraneoplastic Pemphigus as a First Manifestation of an Intra-Abdominal Follicular Dendritic Cell Sarcoma: Rare Case and Review of the Literature. Case Rep Oncol. 2018;11:353-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Wang J, Wang X, Xu J, Song P. Follicular dendritic cell sarcoma aggravated by hyaline-vascular Castleman's disease in association with paraneoplastic pemphigus: study of the tumor and successful treatment. An Bras Dermatol. 2019;94:578-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Wang J, Bu DF, Li T, Zheng R, Zhang BX, Chen XX, Zhu XJ. Autoantibody production from a thymoma and a follicular dendritic cell sarcoma associated with paraneoplastic pemphigus. Br J Dermatol. 2005;153:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Su Z, Liu G, Liu J, Fang T, Zeng Y, Zhang H, Yang S, Wang Y, Zhang J, Wei J, Li Y, Guo Y. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma: report of a case and review of literature. Int J Clin Exp Pathol. 2015;8:11983-11994. [PubMed] |
| 11. | Chow SC, Yeung EC, Ng CS, Wong RH, Fai To K, Wan IY. Mediastinal follicular dendritic cell sarcoma with paraneoplastic pemphigus. Asian Cardiovasc Thorac Ann. 2015;23:732-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Garza-Chapa JI, Ocampo-Garza J, Vázquez-Herrera NE, Miranda-Maldonado IC, Rendón-Ramírez E, González-Chávez JM, García-García SC, Montero-Cantú CA, Ocampo-Candiani J. Paraneoplastic pemphigus associated with primary pulmonar follicular dendritic cell sarcoma showing good response to treatment. J Eur Acad Dermatol Venereol. 2016;30:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Streifel AM, Wessman LL, Schultz BJ, Miller D, Pearson DR. Refractory mucositis associated with underlying follicular dendritic cell sarcoma of the thymus: Paraneoplastic pemphigus versus malignancy-exacerbated pemphigus vulgaris. JAAD Case Rep. 2019;5:933-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kim WY, Kim H, Jeon YK, Kim CW. Follicular dendritic cell sarcoma with immature T-cell proliferation. Hum Pathol. 2010;41:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Seishima M, Oda M, Oyama Z, Yoshimura T, Yamazaki F, Aoki T, Nei M, Hashimoto T. Antibody titers to desmogleins 1 and 3 in a patient with paraneoplastic pemphigus associated with follicular dendritic cell sarcoma. Arch Dermatol. 2004;140:1500-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Liu KL, Shen JL, Yang CS, Chen YJ. Paraneoplastic pemphigus as the first manifestation of follicular dendritic cell sarcoma. J Dtsch Dermatol Ges. 2014;12:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Baghmar S, Kumar S, Gupta SD, Raina V. Follicular dendritic cell sarcoma with paraneoplatic pemphigus: Rare case and a brief review of literature. Indian J Med Paediatr Oncol. 2013;34:317-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Hwang YY, Chan JC, Trendell-Smith NJ, Kwong YL. Recalcitrant paraneoplastic pemphigus associated with follicular dendritic cell sarcoma: response to prolonged rituximab and ciclosporin therapy. Intern Med J. 2014;44:1145-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lee SE, Kim HR, Hashimoto T, Kim SC. Paraneoplastic pemphigus developed shortly after resection of follicular dendritic cell sarcoma. Acta Derm Venereol. 2008;88:410-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Zhao C. A case in which paraneoplastic pemphigus and bronchiolitis obliterans are the main manifestations of inflammatory pseudotumour-like follicular dendritic cell sarcoma. Australas J Dermatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Sugiura K, Koga H, Ishikawa R, Matsumoto T, Matsubara M, Hagiwara R, Muro Y, Hashimoto T, Akiyama M. Paraneoplastic pemphigus with anti-laminin-332 autoantibodies in a patient with follicular dendritic cell sarcoma. JAMA Dermatol. 2013;149:111-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Marzano AV, Vezzoli P, Mariotti F, Boneschi V, Caputo R, Berti E. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma and Castleman disease. Br J Dermatol. 2005;153:214-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Meijs M, Mekkes J, van Noesel C, Nijhuis E, Leeksma O, Jonkman M, Hoekzema R. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma without Castleman's disease; treatment with rituximab. Int J Dermatol. 2008;47:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Lee IJ, Kim SC, Kim HS, Bang D, Yang WI, Jung WH, Chi HS. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma arising from Castleman's tumor. J Am Acad Dermatol. 1999;40:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Choi Y, Nam KH, Lee JB, Lee JY, Ihm CW, Lee SE, Oh SH, Hashimoto T, Kim SC. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol. 2012;39:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Zhang J, Qiao QL, Chen XX, Liu P, Qiu JX, Zhao H, Zhao JX, Liu YC, Wan YL. Improved outcomes after complete resection of underlying tumors for patients with paraneoplastic pemphigus: a single-center experience of 22 cases. J Cancer Res Clin Oncol. 2011;137:229-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Yamada H, Nobeyama Y, Matsuo K, Ishiji T, Takeuchi T, Fukuda S, Hashimoto T, Nakagawa H. A case of paraneoplastic pemphigus associated with triple malignancies in combination with antilaminin-332 mucous membrane pemphigoid. Br J Dermatol. 2012;166:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Ogawa M, Sugiura K, Muro Y, Matsumoto T, Koga H, Hashimoto T, Akiyama M. Long-term survival of a patient with paraneoplastic pemphigus due to follicular dendritic cell sarcoma. J Am Acad Dermatol. 2015;72:AB121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Roca B, Pitarch G, Soler F, Penades M, Resino E, Roca M. Paraneoplastic pemphigus associated with dendritic cell neoplasm and Castleman’ s disease: report of a new case and review of the literature. Cent Eur J Med. 2013;8:153-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Rice BL, Bedocs LA, Sahi H. A 41-year-old woman with severe dyspnea and painful oral mucosal ulcerations. Chest. 2010;137:1236-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Wang L, Deng H, Mao M. Paraneoplastic pemphigus and myasthenia gravis, associated with inflammatory pseudotumor-like follicular dendritic cell sarcoma: response to rituximab. Clin Case Rep. 2016;4:797-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol. 2004;40:553-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 662] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 34. | Hartert M, Ströbel P, Dahm M, Nix W, Marx A, Vahl CF. A follicular dendritic cell sarcoma of the mediastinum with immature T cells and association with myasthenia gravis. Am J Surg Pathol. 2010;34:742-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Levi Sandri GB, Colasanti M, Vennarecci G, Ettorre GM. Paraneoplastic arthritis as first symptom of a liver inflammatory pseudotumor-like follicular dendritic cell sarcoma. Liver Int. 2016;36:1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Wang J, Zhu X, Li R, Tu P, Wang R, Zhang L, Li T, Chen X, Wang A, Yang S, Wu Y, Yang H, Ji S. Paraneoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China. Arch Dermatol. 2005;141:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Sun X, Chang KC, Abruzzo LV, Lai R, Younes A, Jones D. Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman's disease. Hum Pathol. 2003;34:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Nagy A, Bhaduri A, Shahmarvand N, Shahryari J, Zehnder JL, Warnke RA, Mughal T, Ali S, Ohgami RS. Next-generation sequencing of idiopathic multicentric and unicentric Castleman disease and follicular dendritic cell sarcomas. Blood Adv. 2018;2:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Zhu X, Zhang B. Paraneoplastic pemphigus. J Dermatol. 2007;34:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Wu A, Pullarkat S. Follicular Dendritic Cell Sarcoma. Arch Pathol Lab Med. 2016;140:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Frew JW, Murrell DF. Current management strategies in paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome). Dermatol Clin. 2011;29:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Saygin C, Uzunaslan D, Ozguroglu M, Senocak M, Tuzuner N. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol. 2013;88:253-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | Zhang BX, Chen ZH, Liu Y, Zeng YJ, Li YC. Inflammatory pseudotumor-like follicular dendritic cell sarcoma: A brief report of two cases. World J Gastrointest Oncol. 2019;11:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |