Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3039
Peer-review started: March 27, 2020
First decision: April 24, 2020
Revised: May 25, 2020
Accepted: July 14, 2020
Article in press: July 14, 2020
Published online: July 26, 2020
Processing time: 119 Days and 5.8 Hours
The endoscopic third ventriculostomy (ETV) is a neuroendoscopical procedure that represents a more suitable alternative to the extracranial shunting. It consists of fenestrating the floor of the third ventricle and thus establishing a free flow of the cerebrospinal fluid from the ventricles to the site of resorption in the subarachnoid space. It offers a more physiological solution and a chance at a shunt-free life for children with hydrocephalus. The main indication for the procedure is obstructive hydrocephalus, however, it can also be useful in patients with other forms of hydrocephalus.
We present a treatment flow of a 9-year-old patient, diagnosed with an obstructive hydrocephalus due to tectal glioma that was successfully treated with an ETV. We review the important factors influencing the success rate such as age, aetiology, shunt history, preoperative planning and visualisation of the basilar artery.
Even though the ETV effectively controls obstructive hydrocephalus in more than 75% of all cases, the overall success rate of the procedure varies and could be approved by the correct preoperative patient selection.
Core tip: The endoscopic third ventriculostomy (ETV) is a suitable alternative to extracranial shunting in obstructive hydrocephalus. It consists of fenestrating the third ventricle floor, thus establishing a free flow of cerebrospinal fluid from the ventricles to the subarachnoid space. It offers a chance for a shunt-free life in children with hydrocephalus. We present a treatment flow of a 9-year-old patient diagnosed with obstructive hydrocephalus due to tectal glioma that was successfully treated with an ETV and review the obstructive hydrocephalus pathology.
- Citation: Munda M, Spazzapan P, Bosnjak R, Velnar T. Endoscopic third ventriculostomy in obstructive hydrocephalus: A case report and analysis of operative technique. World J Clin Cases 2020; 8(14): 3039-3049
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3039.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3039
Hydrocephalus is a disorder of cerebrospinal fluid (CSF) physiology, resulting in an abnormal enlargement of brain ventricles due to excessive accumulation of CSF. The main causes are disturbance of CSF flow, subnormal resorption or, rarely, overproduction[1]. According to hydrocephalus epidemiology meta-analysis, the mean hydrocephalus estimate is approximately 0.8/1000. The prevalence is 0.9/1000 in paediatric population (less than 18 years), 0.1/1000 in adults and 1.7/1000 in the elderly (older than 65 years). Incidence of hydrocephalus diagnosed at birth is 81/100000. The most common cause for congenital hydrocephalus is myelomeningocele and later on, in infants, other cerebral malformations like Dandy-Walker syndrome or aqueductal stenosis. Lower incidence was identified in high-income countries[2].
The total CSF volume in the ventricles and subarachnoid space is age-dependent. The CSF volume of a new-born is approximately 50 mL (15 mL/kg in term neonates). By the age of 5 years, it reaches the adult volume of 150 ml and it is distributed between 125 mL in subarachnoid spaces and 25 mL in the ventricles. Approximately 70% of CSF is produced in the lateral ventricles by the choroid plexus and in the third and fourth ventricles by the tela choroidea. The rest of the CSF secretion derives from the extracellular fluid, cerebral capillaries across the blood-brain barrier, ependymal lining of the ventricles and the spinal dura of the nerve root sleeves. The daily amount of the CSF secreted is between 400 mL to 600 mL (a rate of 0.4 mL per minute), which means that the CSF is renewed three times in every 24 h[3-5].
Hydrocephalus in infants without an obvious extrinsic cause is usually classified as congenital since it is often present at birth. When it occurs as a causal complication of another condition (haemorrhage, infection, tumour) it is called acquired or secondary. Because these conditions as well as some genetic predispositions cause hydrocephalus that is not necessarily evident at birth but develops over time, some authors have decided to distinguish between acquired or extrinsic and developmental or intrinsic forms of hydrocephalus[6].
Regarding the pathophysiological mechanism, we differentiate between obstructive and communicating hydrocephalus. Classically, the obstruction of the CSF flow within the ventricles is classified as obstructive or non-communicating hydrocephalus, whereas the obstruction of the CSF flow or its absorption in the subarachnoid spaces is known as communicating hydrocephalus[1,6]. The obstructive hydrocephalus, as the name implies, is defined as an obstruction of the CSF flow, while the communicating hydrocephalus occurs when full communication between the ventricles and subarachnoid space is present. Main causes include defective absorption, overproduction of the CSF or venous drainage insufficiency. In the acute form of obstructive hydrocephalus, especially in young patients, only minor ventriculomegaly may be present in spite of a significant rise in the intracranial pressure. With long standing CSF pressure on the brain parenchyma, the ventricular system dilates, compressing and thinning the overlying cortex[1,3,4,6]. The primary point of obstruction in a non-communicating hydrocephalus may be proximal (at the level of the third ventricle or the aqueduct) or distal (at the level of the fourth ventricle, fourth ventricular outflow tracts or the foramen magnum). The most common causes for obstruction in the ventricular system are occlusion of cerebral aqueduct, tumours and colloid cysts. In cases of communicating hydrocephalus, the source of obstruction can reside in the basal cisterns or there can be a failure of CSF absorption through the arachnoid granulations. That is most often due to infections or subarachnoid haemorrhage. Some of congenital malformations with more complex mechanisms of hydrocephalus have both obstructive and communicating components. Table 1 shows different causes classified by their underlying mechanism for hydrocephalus formation[1,6].
| Communicating hydrocephalus |
| Impaired absorption |
| Posthaemorrhagic |
| Postinfectious |
| Prematurity-related |
| Congenital CNS malformations |
| Venous congestion/thrombosis |
| Increased secretion: |
| Choroid plexus papilloma/carcinoma |
| Obstructive component with mainly communicating hydrocephalus |
| Tumour |
| Meningitis/encephalitis resulting in secondary obstruction |
| Chiari 2 |
| Dandy Walker |
| Encephalocele |
| Obstructive hydrocephalus |
| Tumour |
| Intracranial cyst |
| Aqueductal occlusion (primary due to stenosis or secondary due to tumour) |
| Atresia of Monro |
| Obstruction of 4th ventricle outlets |
Symptoms of hydrocephalus are nonspecific and may not be related to aetiology. They are caused by raised intracranial pressure. Some infants and children with mild hydrocephalus may be asymptomatic. A classical adult presentation of raised ICP includes headache, vomiting and papilledema, and the same clinical picture can be seen in children. In addition, most common clinical features of progressive hydrocephalus in children are headache, nausea and vomiting, behavioural changes, developmental delays, lethargy or drowsiness and anorexia. Infants with open skull sutures may present with increased occipitofrontal circumference, tense anterior fontanelle, splayed cranial sutures, papilledema and sunsetting (loss of upward gaze)[7].
The best diagnostic technique for covering all of the imaging demands for hydrocephalus is magnetic resonance imaging (MRI). Not only it gives a detailed anatomical information, it may also show the CSF flow dynamics that demonstrates the site of obstruction. It can help us discover the underlying cause for hydrocephalus as it shows most of the intra-axial and extra-axial space occupying lesions. Important for surgery planning is also careful examination of the CSF pathways from the ventricles to the subarachnoid space with its CSF flow. However, the differentiation between asymptomatic ventriculomegaly and hydrocephalus with raised intracranial pressure is not always easy in paediatric population. The most reliable findings that suggest increased intracranial pressure are the enlargement of the recesses of the third ventricle and dilatation of the temporal horn. Other diagnostically helpful indications are periventricular interstitial oedema and effacement of cortical sulci. An important tool for the diagnosis of infant hydrocephalus is transfontanellar ultrasound, which allows a detailed and precise view of the ventricular dimensions and shape[7].
Most cases of paediatric hydrocephalus are progressive, which means that if not treated effectively and in time, neurologic deterioration will progress. The basis of hydrocephalus treatment is to correct the excessive accumulation of the CSF and therefore decreasing the intracranial pressure. The most effective treatment is surgical drainage, which can be done by diverting the excess CSF extracranially (via shunts) or by redirecting the CSF flow to a more physiological site of resorption within the brain system (ventriculostomy). Whereas the extracranial CSF diversion (shunt) has traditionally been the treatment for a communicating hydrocephalus, the endoscopic procedures have become the optimal treatment for purely obstructive type of hydrocephalus[1].
Since the 1990s, the endoscopic third ventriculostomy (ETV) has been the first-line procedure in treating the non-communicating hydrocephalus. The ETV is a surgical procedure, which encompasses the fenestration of the third ventricular floor with a ventriculoscope and it is often assisted with image guidance. The goal of the procedure is to divert the CSF flow to a more physiological site of resorption. That is achieved by establishing a free flow of the CSF from third ventricle into the interpenduncular cistern and onwards to the cortical subarachnoid space, where it is absorbed by the arachnoid villi. The CSF is thus diverted elsewhere in an attempt to bypass an obstruction and to relieve intracranial pressure. Compared to the VP shunt, the ETV represents a more physiological solution for hydrocephalus. It eliminates shunt-related complications and dependency, does not involve the insertion of foreign material and overall has lower complication rates[8-10]. In this report, we present a treatment flow of a 9-year-old boy, who was diagnosed with an obstructive hydrocephalus and has been successfully treated with the ETV.
A 9-year-old boy was admitted to the paediatric department because of an ongoing headache that had been getting worse in the weeks preceding the hospitalisation.
The headache was the main symptom, it was ongoing and had been getting worse in the past weeks. The headache started eight months prior to the visit with a frequency of one per month, then escalated to one per week and has been present every day in the last week. He described it as a dull non-spreading pain above the right eyebrow, with an intensity of 5/10. The episodes lasted five to 15 min and usually disappeared after a short rest. He had no need for analgesics. Several times, he was also woken up during the night by the headaches and vomiting. He denied any history of nausea, flashing of light or scotoma.
The boy had no history of other illness except middle ear inflammation at the age of 9 months and took no medications. He had no known allergies.
At the admission, the physical examination was within normal limits, as was the neurological examination. The dilated fundus examination showed bilateral papilledema, indicating raised intracranial pressure.
Laboratory examinations were within the normal range. The haemostasis was normal, as was the blood count and the biochemistry test. The routine laboratory results were normal.
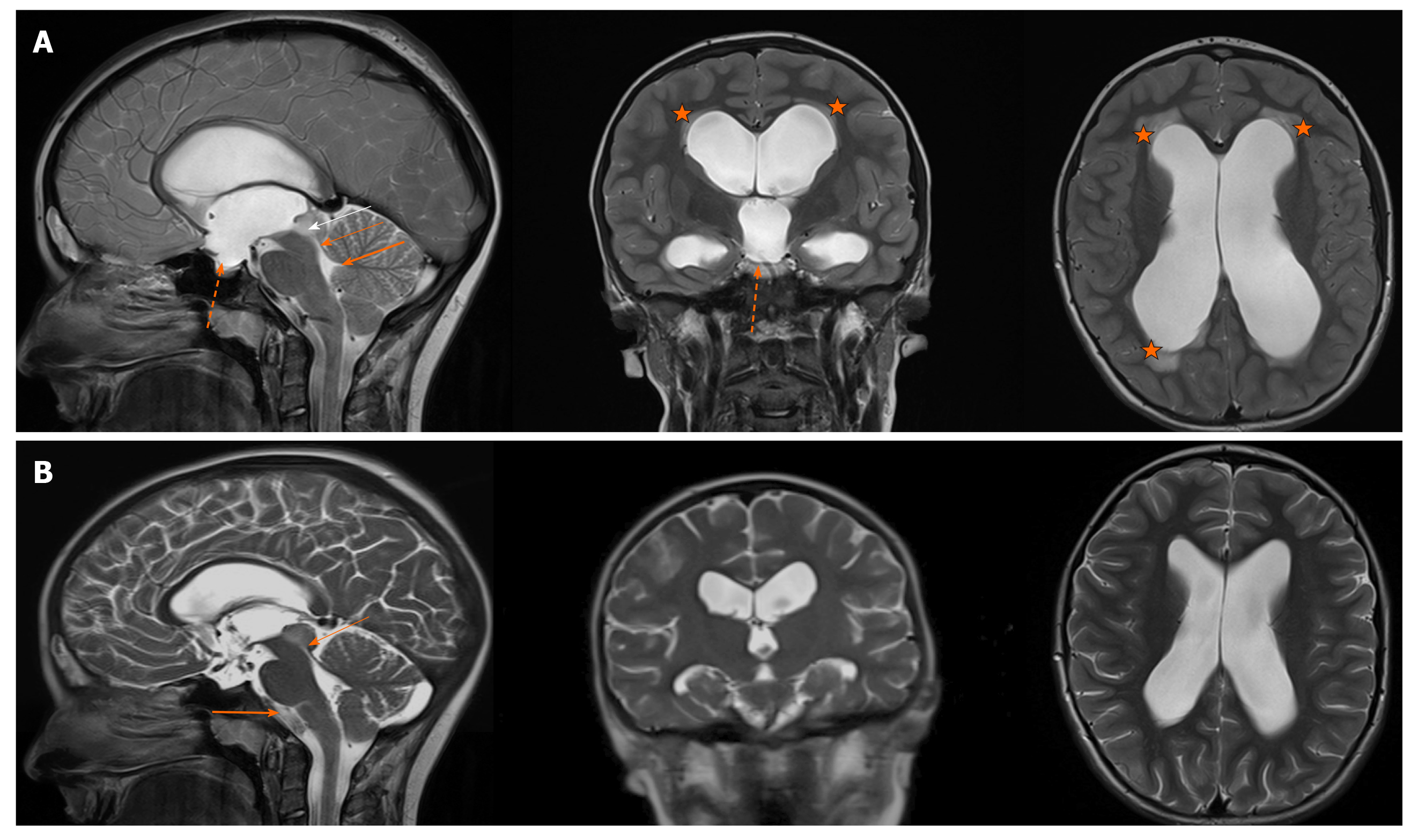
The MRI substantiated the diagnosis by showing hypointensive modality in the right part of the mesencephalic tectum, which was highly suspectful for a tectal low-grade astrocytoma. It was completely obstructing aqueduct of Sylvius, causing extensive supratentorial hydrocephalus with some reliable findings, such as enlarged third ventricle that was bulging into the sella turcica. Additionally, the periventricular hyperintensive area, effacement of cortical sulci, dilatation of lateral ventricles and intracranial hypertension with important narrowing of transverse sinuses were observed (Figure 1A). There were no signs of a hyperdynamic CSF circulation on the MRI at the level of the aqueduct, suggesting a complete flow blockade by the tumour.
The clinical symptoms and the final diagnosis were consistent with the diagnostic results. The main diagnosis of obstructive hydrocephalus has been set.
According to the MRI findings and clinical symptoms of raised intracranial pressure (headache, papilledema), neurosurgical treatment was recommended. Since the diagnosis of the obstruction was unambiguous, it was decided to perform an ETV in order to relieve the symptoms of the obstructive hydrocephalus. The lumbar puncture was not performed in advance as it was contraindicated due to the risk of possible herniation after the CSF removal and consequent neurological deterioration. The CSF sample collection was planned for the surgical stage of treatment.
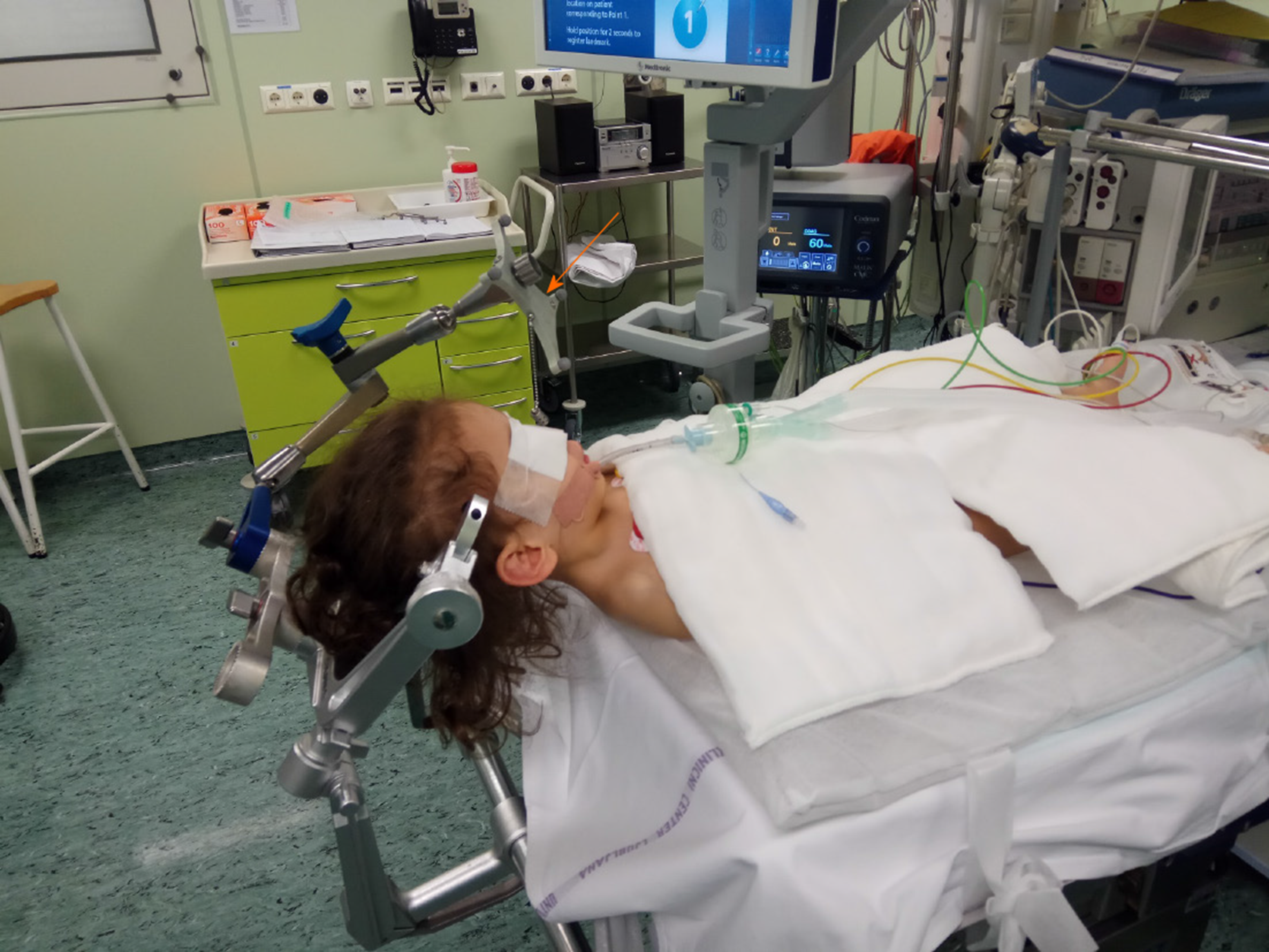
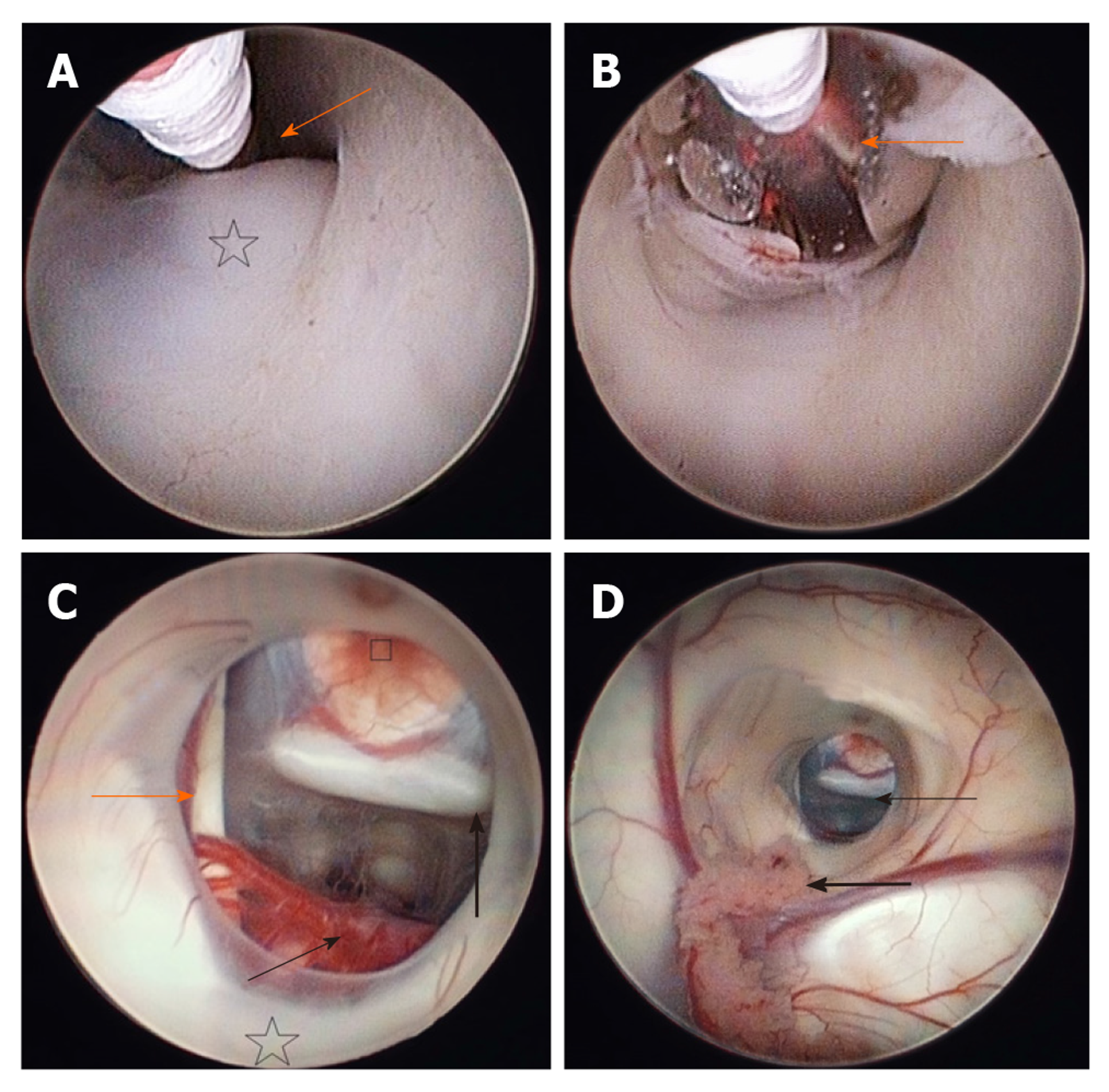
The patient was placed in a supine position under the general anaesthesia (Figure 2). The head was elevated 20 to 30 degrees and the neck was slightly flexed. With this position, we tried to avoid the postoperative pneumocephalus and lower the risk for subdural haematoma. An incision was made and a 1 cm burr hole was drilled about 2 to 3 cm lateral to the midline (at approximately in midpupillary line) just anterior to the coronal suture. This is called Kocher's point and is a common point of entry for lateral ventricle approach. The neuronavigation was used for selecting the optimal position of the burr hole and to choose the safest trajectory to the floor of the third ventricle, thus reducing the risk of any damage to the vital structures, or traction on foramen of Monro. The dura was incised and under the neuronavigational guidance, the rigid endoscope was inserted into the frontal horn of the lateral ventricle through a trans-gyral approach. When reaching the ventricles, the CSF samples were taken for routine microbiological, cytological and biochemical analysis. Once in the ventricles, one must navigate by visual anatomical landmarks on the account of the accuracy limitations of image guidance. The anatomical-topographical knowledge of the ventricular system is essential to perform this procedure successfully[10]. In the lateral ventricle, the choroid plexus and the anterior septal and thalamostriate veins were followed to localise the foramen of Monro, which is in the hydrocephalic ventricles wider than normal and the endoscope can therefore pass through it smoothly and without causing any trauma. The success of the ETV is closely related to the knowledge of third ventricle anatomical landmarks. The floor of the third ventricle has to be inspected and must be thin and translucent enough to permit visualization of the mamillary bodies. Even though not always possible, one should also try to visualize the basilar artery as the tip of the artery needs to be avoided in the fenestration trajectory. Anteriorly to the mammillary bodies, the infundibular recess is located, and between the recess and the bodies, the tuber cinereum lies. This is a triangular transparent area, which is the target for fenestration (Figure 3A).
Here, the fenestration was done in the region of the tuber cinereum, posterior to the infundibular recess, anterior to mammillary bodies and anterior to the tip of the basilar artery. An effective technique for fenestration consists of perforating through the floor of the third ventricle with a blunt probe or a monopolar, especially when the floor is thick. Special caution needs to be paid when coagulating in order not to cause thermal injury to the thalamus. Once the fenestration was made, it was enlarged with a 3 French Fogarty balloon, which we inserted in the fenestration and inflated in order to obtain a diameter of 5 mm to 6 mm (Figure 3B). The proximal end of the balloon should always be visible, as it can accidentally injure the third cranial nerve or nearby vascular structures. In case of some small bleeding during the fenestration, waiting and gentle irrigation with Ringer solution is generally successful. The bleeding results due to the vascularization of the membrane and may be commonly encountered. After the fenestration was enlarged, we explored the prepontine space to eliminate the possibility of a second membrane, the Liliequist's membrane, which would further obstruct the flow (Figure 3C and D). The ventricles were explored for any bleeding and the endoscope was carefully removed. The burr hole was closed with bone chips and the scalp was sutured. The patient was transferred to the intensive care unit. A control computer tomography (CT) scan was made the next day and showed narrowing of the ventricles by 2 mm. The CSF, which was taken during the operation, was normal, with no elevated protein content and no tumour cells present in the cytological analysis.
The rest of the hospitalisation was uneventful and the boy was discharged home after two days. In the following weeks, the patient was again hospitalised two more times on the account of persistent headache episodes, without any nausea or vomiting. His physical and neurological examination were normal. Even though the CT showed smaller ventricles compared to the postoperative scans and no signs of hydrocephalus progression, he was still having multiple headache episodes per day. The dilated fundus examination showed bilateral papilledema, therefore, he was prescribed with acetazolamide and bicarbonate. He was released home and in the following two weeks, the frequency and the intensity of headaches has decreased. The analgesic therapy was not needed any more.
The control MRI after two months showed the same tumour obstruction of aqueduct with no growth dynamics compared to the previous scans. Supraventricular system was less dilated than before the ETV. The absence of transependymal oedema and the normal depth of the brain sulci indicated no signs of hydrocephalus progression. There was a visual CSF flow artefact in the third ventricle, confirming the third ventricle floor opening after the successful ETV. No signs of papilledema or other neurological findings related to the elevated ICP were noted. A control MRI after one year showed even further regression of the ventriculomegaly and no signs of raised ICP (Figure 1B). The tumour was declared inoperable and due to its stable dynamics, the biopsy would have been risky and unnecessary. The radiological findings suggested it was a low-grade astrocytoma and the patient is being followed-up every three month with the MRI. The tumour is stable and no signs of hydrocephalus are present, indicating that the ETV is working normally.
The ETV is one of most commonly used neuroendoscopical procedures[9]. It was primarily designed for use in patients older than two years with the blockage of aqueduct of Sylvius that presented with triventricular hydrocephalus, and those with bulging of the floor of the third ventricle. Over the past two decades, it has become a well-established procedure worldwide, not only for patients with purely obstructive aetiology but also for communicating and even normal pressure hydrocephalus[10,11]. Indications for patients with normal pressure or communicating hydrocephalus have not been defined precisely and the basis of successful outcome is not yet clear. Some authors predict that the ETV might relieve periventricular tissue stress and improve blood flow, which is a hypothetical pathogenetical mechanism in normal pressure hydrocephalus[10]. The main and most common indication for the ETV still remains the noncommunicating hydrocephalus caused by aqueductal stenosis, which may be idiopathic or secondary and congenital or acquired (late onset). Additionally, there are numerous other indications for the ETV, which may be somewhat subjective, offering the patient a chance at a shunt free life[9].
Some authors have stated that indications should be divided into two groups, regarding the necessity of the procedure. Besides aqueductal stenosis, a group of patients with strong indications also includes occlusion of the CSF pathways by posterior fossa or pineal tumours that compress the CSF pathways at the level of aqueduct of Sylvius, Lushka or Magendie foramina or directly compress the fourth ventricle[12]. Other indications include a malfunctioning VP-shunt in patients with obstructive hydrocephalus or malfunctioning shunt in older patients with spina bifida. Some authors believe in lower success rate in patients, who previously had shunts due to obliterated subarachnoid spaces[8]. Weaker indications for the ETV are neonatal hydrocephalus with aqueductal stenosis, numerous congenital abnormalities, communicating hydrocephalus and normal pressure hydrocephalus[12]. There is a lot of debate about some of the indications, such as hydrocephalus in new-borns, hydrocephalus in infants with myelomeningocele, who have deformed ventricles and obliterated CSF pathways, and in patients after shunts with obliterated subarachnoid spaces. All these treatments may result in lower success rates[9].
In our patient, the cause for hydrocephalus lied in the complete compression of the aqueduct of Sylvius due to a mesencephalic infiltrative tumour, which compressed the outflow from the third ventricle causing accumulation of the CSF and enlargement of the supratentorial ventricular system. According to the Monro-Kellie doctrine, stating that volume of all intracranial components is constant, the increase of the CSF due to obstruction resulted in increased intracranial pressure, which caused progressive headache, papilledema and vomiting that brought our patient to the clinic. This was a representative constellation of symptoms of hydrocephalus. Other common findings, though not present in our patient, could be loss of neurocognitive delay, disturbance of the level of consciousness and diplopia[13].
A surgical drainage (either the ETV or a shunt) is generally indicated, when the patient presents with raised ICP symptoms or there is a progression of hydrocephalus or clear obstruction of the CSF flow, visible on neuroimaging. Our patient presented with all three characteristics and we have decided for an ETV because of higher chances for successful outcome with this procedure. Additionally, shunt placement can sometimes be problematic, especially in patients with a high protein content in the CSF, which may be the cause of the shunt malfunction. In our patient, the CSF constituents were within the normal values and devoid of tumour cells. Therefore, a shunt may present a sensible treatment option in case of the ETV failure.
In the analysis made by Kulkarni et al[14] among 618 ETV procedures, the overall success rate after six months was 66.7% and after 2 years was 57.8%. Success rate is strongly related to the cause of occlusion, the age of the patient and clinical and radiological characteristics[12]. Therefore, the outcomes may be improved by correct patient selection. In a purely obstructive aetiology, such as acquired aqueductal stenosis in adult patients, the ETV has a success rate of 80%[12]. However, in patients with components of both noncommunicating and communicating hydrocephalus, the fenestration of the floor of the third ventricle does not always provide long-term success. After reexpansion of the subarachnoid space, the patients are left with insufficient absorption of the CSF. Even though the CSF dynamics are adequate, the hydrocephalus might persist[11]. It is also important to remember that the ICP does not decrease rapidly and may remain elevated for a short postoperative adaptation period, which does indicate failure[9]. Even papilledema normally needs a few days or even weeks to completely resolve and disappear. This scenario might have happened in our patient, who after technically and radiologically successful ETV returned with headaches and papilledema after a few days. He was prescribed with diuretics, which decreased the CSF production and his symptoms disappeared after some time, potentially due to the therapy or adaptation of his ventricular system. Overall, the procedure was successful, which was also confirmed with the flow void through the stoma on the control MRI.
A successful ETV is defined as an improvement of clinical findings with no need for shunt placement[9]. If a patient continues to exhibit persistent headaches, increased ICP or bulging fontanelle without clinical improvement, it is possible that the procedure has failed. The failure may be due to technical problems or most often due to inappropriate indication for the ETV. Sometimes misleading, the postoperative size of the ventricular system is not always a credible predictor for the successful procedure. A blind study of 38 patients made by Buxton et al[15] showed that even though the third ventricular size was characteristically reduced after the ETV, the importance of these findings is of secondary importance compared to the clinical improvement. The predictive value of decrease in the ventricle size, especially during the early stage after the procedure, is unsatisfactory and unreliable[15]. In addition to clinical findings, the most widely used and reliable objective postoperative tests for a successful ETV are the MRI flow studies. They provide detailed information on the subject's CSF flow dynamics[9].
Predicting the ETV success is important in selecting appropriate patients for the procedure (Table 2). In 2011, an ETV success score (ETVSS) was introduced as a six month predictive model for the success of the procedure. Using only preoperative data, the score has been shown to predict the chances of the ETV success with close accuracy[14]. According to the cohort study made by Abhaya et al[16], the overall mean predicted ETVSS among 322 patients was 57.9% and overall actual ETV success rate was 59.2%. In theory, the total of three scores (age, aetiology, shunt history) expressed as a percentage, is the approximate chance of the ETV lasting six months without failure. Score of less than 40% relate to very low chance of success and a score of more than 80% correlate with a better chance of success compared to shunting. Our patient, a 9-year-old boy (40%) with tectal tumour (30%) and no history of shunt (10%) therefore presented with an 80% of successful outcome.
| Age | < 1 mo | 0% |
| 1-6 mo | 10% | |
| 6 mo-1 yr | 30% | |
| 1-10 yr | 40% | |
| > 10 yr | 50% | |
| Etiology | Post-infectious | 0% |
| Myelomeningocele, post IVH, non-tectal brain tumour | 20% | |
| Aqueductal stenosis, tectal tumour, other | 30% | |
| Shunt history | Previous shunt | 0% |
| No previous shunt | 10% |
Current predictive models for the ETV failure are based on the data of a six months follow-up, therefore they are not very representative concerning long term success. Late deterioration of the ETV occurs at a mean of 2.5 years after the ETV. A population-based analysis showed the second round failure at approximately three years after the procedure in children between one to 10 years of age with tumours or aqueductal stenosis[11]. Most common reasons for failure in early period are the presence of a second membrane in the prepontine space, obliterated subarachnoid spaces and an insufficient absorption of CSF. The most frequent cause for late failure is the sealing of the fenestrated floor due to glial fibrosis[9].
In the best ETV candidates with ETVSS higher than 80%, the risk for failure right after surgery is lower than that of shunt insertion. However, in less ideal candidates with ETVSS lower than 70%, the risk of failure is initially higher compared to shunt insertion and only becomes lower after three to six months after surgery[17].
While the ETVSS helps predicting the success of the procedure based on preoperative factors, the identification of other important findings may further help surgeons in decision making and outcome prediction[11]. The main tool for procedure planning is the MRI imaging. Considering the anatomy shown on the preoperative MRI, the surgeon can plan the operative approach and discover certain neurovascular structures or anatomical disorders beneath the floor of the third ventricle, therefore increasing the safety of the procedure and lowering the risk for complications. A longstanding hydrocephalus and raised ICP can lead to different anatomical variations of the floor of the third ventricle, which may increase the risk for operative complications[10].
A study of 25 patients by Rohde and Gilsbach showed that anatomic anomalies of the third ventricle were a common finding with an incidence rate of 36% and that their absence correlated with a higher success rate. In patients without anatomic variations, the ETV was successful in all cases. However, the success rate in patients with anatomic variants dropped to 56%. Therefore, preoperative MRI imaging is an important tool to help neurosurgeons weigh the operative risk for failure[18]. Apart from abnormal anatomy, other negative prognosis indicators might be existing scarred membranes, floppy premammillary membrane, young age, shunt history, infection, intraventricular haemorrhage and myelomeningocele[11]. One of the most important prediction factors is the correct localisation of the basilar artery and its anatomy. Missing an anteriorly placed basilar artery may have catastrophic consequences. Kulkarni et al[14] discovered that among 309 patients success, the rate of those with a clear intraoperative visualization of the basilar artery was 71.7% and among patients where basilar artery was not visible, the success rate was 47.4%.
As noted above, the most serious complication of the procedure is basilar artery injury. It can lead to pseudoaneurysm or even death. Other injuries of the third ventricle floor might appear in the form of damage to the hypothalamus, pons, cerebral peduncle and third cranial nerve. During the ETV, a serious bradycardia and postoperative hyperkalemia might occur due to distortion of the posterior hypothalamus. Other described complications include respiratory arrest, diabetes insipidus, epilepsy, pneumoencephalus, subdural hematoma and psychiatric disorders.
The most common complication is bleeding from the ependymal vessels or choroid plexus[9,10]. According to a systematic review by Bouras and Sgouros, based on 24 case series and reporting outcomes of a total number of 2672 ETV procedures, the overall complication rate was 8.8%. The most common complication was intraoperative haemorrhage (3.9%). The basilar rupture was reported in four cases (0.14%). The overall mortality rate was 0.29% (8 cases)[19].
The ETV effectively controls obstructive hydrocephalus in more than 75% of all cases and should be preferred to extracranial shunts as a primary treatment. The most important factor in the success of the procedure is the correct preoperative selection of patients. Additionally, as the success of the ETV is closely related to the knowledge of the third ventricle anatomical landmarks, special care should be dedicated to the anatomic localisation of the structures and possible peculiarities in this region.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kung WM S-Editor: Gong ZM L-Editor: A E-Editor: Li JH
| 1. | Beni-Adani L, Biani N, Ben-Sirah L, Constantini S. The occurrence of obstructive vs absorptive hydrocephalus in newborns and infants: relevance to treatment choices. Childs Nerv Syst. 2006;22:1543-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Isaacs AM, Riva-Cambrin J, Yavin D, Hockley A, Pringsheim TM, Jette N, Lethebe BC, Lowerison M, Dronyk J, Hamilton MG. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS One. 2018;13:e0204926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 3. | Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 445] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 4. | Filis AK, Aghayev K, Vrionis FD. Cerebrospinal Fluid and Hydrocephalus: Physiology, Diagnosis, and Treatment. Cancer Control. 2017;24:6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Rochette A, Malenfant Rancourt MP, Sola C, Prodhomme O, Saguintaah M, Schaub R, Molinari N, Capdevila X, Dadure C. Cerebrospinal fluid volume in neonates undergoing spinal anaesthesia: a descriptive magnetic resonance imaging study. Br J Anaesth. 2016;117:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Tully HM, Dobyns WB. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet. 2014;57:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 7. | Dinçer A, Özek MM. Radiologic evaluation of pediatric hydrocephalus. Childs Nerv Syst. 2011;27:1543-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Melot A, Curey-Lévêque S, Derrey S, Gérardin E, Borden A, Fréger P, Proust F. Endoscopic 3rd ventriculocisternostomy: procedural complications and long-term dysfunctions? Neurochirurgie. 2013;59:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Cataltepe O. Endoscopic third ventriculostomy: Indications, Surgical Technique and Potential Problems. Turk Neurosurg. 2002;12:65-73. |
| 10. | Hellwig D, Grotenhuis JA, Tirakotai W, Riegel T, Schulte DM, Bauer BL, Bertalanffy H. Endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurg Rev. 2005;28:1-34; discussion 35-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Feng Z, Li Q, Gu J, Shen W. Update on Endoscopic Third Ventriculostomy in Children. Pediatr Neurosurg. 2018;53:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mugamba J, Stagno V. Indication for endoscopic third ventriculostomy. World Neurosurg. 2013;79:S20.e19-S20.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Kahle KT, Kulkarni AV, Limbrick DD, Warf BC. Hydrocephalus in children. Lancet. 2016;387:788-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 14. | Kulkarni AV, Riva-Cambrin J, Holubkov R, Browd SR, Cochrane DD, Drake JM, Limbrick DD, Rozzelle CJ, Simon TD, Tamber MS, Wellons JC, Whitehead WE, Kestle JR; Hydrocephalus Clinical Research Network. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2016;18:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 15. | Buxton N, Turner B, Ramli N, Vloeberghs M. Changes in third ventricular size with neuroendoscopic third ventriculostomy: a blinded study. J Neurol Neurosurg Psychiatry. 2002;72:385-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Kulkarni AV, Riva-Cambrin J, Browd SR. Use of the ETV Success Score to explain the variation in reported endoscopic third ventriculostomy success rates among published case series of childhood hydrocephalus. J Neurosurg Pediatr. 2011;7:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S; Canadian Pediatric Neurosurgery Study Group. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Rohde V, Gilsbach JM. Anomalies and variants of the endoscopic anatomy for third ventriculostomy. Minim Invasive Neurosurg. 2000;43:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Bouras T, Sgouros S. Complications of endoscopic third ventriculostomy: a systematic review. Acta Neurochir Suppl. 2012;113:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |