Published online Jul 26, 2020. doi: 10.12998/wjcc.v8.i14.3021
Peer-review started: March 28, 2020
First decision: April 24, 2020
Revised: May 30, 2020
Accepted: July 4, 2020
Article in press: July 4, 2020
Published online: July 26, 2020
Processing time: 119 Days and 23.1 Hours
Colorectal cancer is one of the most common cancers globally. In China, its prevalence ranks fourth and fifth among females and males, respectively. Presently, treatment of rectal cancer follows a multidisciplinary comprehensive treatment approach involving surgery, radiotherapy, chemotherapy, and targeted therapy. With deepening theoretical and molecular research on colorectal cancer, randomized controlled trials (RCTs) on colorectal cancer have made significant progress. However, many RCTs have shortfalls.
To investigate the RCTs of global colorectal cancer spanning from 2008 to 2018. To provide suggestions for conducting Chinese RCTs of colorectal cancer.
PubMed and Web of Science databases were searched to obtain RCTs of colorectal cancer carried out between January 1, 2008, and January 1, 2018. The bibliometric method was used for statistical analysis of the publication years, countries/regions, authors, institutions, source journals, quoted times, key words, and authors.
Colorectal cancer RCTs showed an upward trend between 2008 to 2018; the top 10 research institutions in the included literature were from the United States, the United Kingdom, and other countries with a high incidence of colorectal cancer. Most of the related research journals are sponsored by European and American countries. The 15 most cited studies involved international multicenter clinical research, having few participants from Chinese research institutions. Network visualization using key words showed that RCTs on colorectal cancer focus on screening, disease-free survival, drug treatment, surgical methods, clinical trials, quality of life, and prognosis. The result of the coauthorship network analysis showed that Chinese researchers are less involved in international exchanges compared to those from leading publication countries.
High-quality RCTs are increasingly favored by leading international journals. However, there is still a large gap in clinical research between China and leading countries. Researchers should implement standardized and accurate clinical trials, strengthen international multicenter cooperation, and emphasize quality control.
Core tip: Bibliometrics was used to quantitatively analyze 1555 articles from PubMed and Web of Science databases. We compared randomized controlled trials of colorectal cancer from China with those published in other countries. This is the first global analysis of this topic in which we analyzed the year of publication, countries/regions, institutions, journals, citations, key words, and authors. Suggestions on how to conduct clinical research were also given.
- Citation: Wang CY, Zhou SC, Li XW, Li BH, Zhang JJ, Ge Z, Zhang Q, Hu JH. Bibliometric analysis of randomized controlled trials of colorectal cancer over the last decade. World J Clin Cases 2020; 8(14): 3021-3030
- URL: https://www.wjgnet.com/2307-8960/full/v8/i14/3021.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i14.3021
In the past 10 years, advances in basic theoretical research on the pathogenesis and molecular mechanisms of colorectal cancer have promoted clinical research to a great extent. However, the incidence of colorectal cancer has continued to increase annually[1]. In China, colorectal cancer is the fifth most prevalent malignant tumor[2]. The current prevention, screening, diagnosis, and treatment approaches for colorectal cancer are not effective. So far, the international clinical guidelines such as the National Comprehensive Cancer Network and the European Society for Medical Oncology have not included clinical studies from China. Currently, there is increasing research interest among Chinese clinicians to conduct randomized controlled trials (RCTs), but findings from such studies have not provided clear research directions. The systematic methodological knowledge is still lacking, especially for important aspects. Several factors, such as rigorous ethical review, scientific design, research personnel, and financial and time costs, make it difficult to obtain high-quality research results. Therefore, to evaluate the research status of colorectal cancer in China and abroad, this paper used bibliometric analysis to analyze previous RCTs to provide reference data for the design, cooperation, and implementation of colorectal cancer RCTs in China.
Inclusion criteria: Colorectal cancer RCT study, time range from January 1, 2008 to January 1, 2018. Exclusion criteria: Repetition, excerpt, conference papers, monographs, retraction, errata, etc.
The computer retrieved articles from PubMed and Web of Science databases from January 1, 2008, to January 1, 2018. The search terms were colorectal, rectal, rectum, colonic, colon, neoplasm, cancer, tumor, adenocarcinoma, randomized controlled trial, etc.
The publications were independently screened and extracted by two investigators. The extracted information included the publication year, country/region, authors, institutions, source journal, quoted time, and key words. Disagreements were resolved through consultation with a third researcher.
Descriptive statistical analysis was done using Microsoft Excel 2013 software; descriptive analysis included organizational distribution, journal distribution, and citation of published literature. Visual analysis was done using VOSviewer 1.6.4 software and mapped key word co-occurrences and coauthors relationship network diagram.
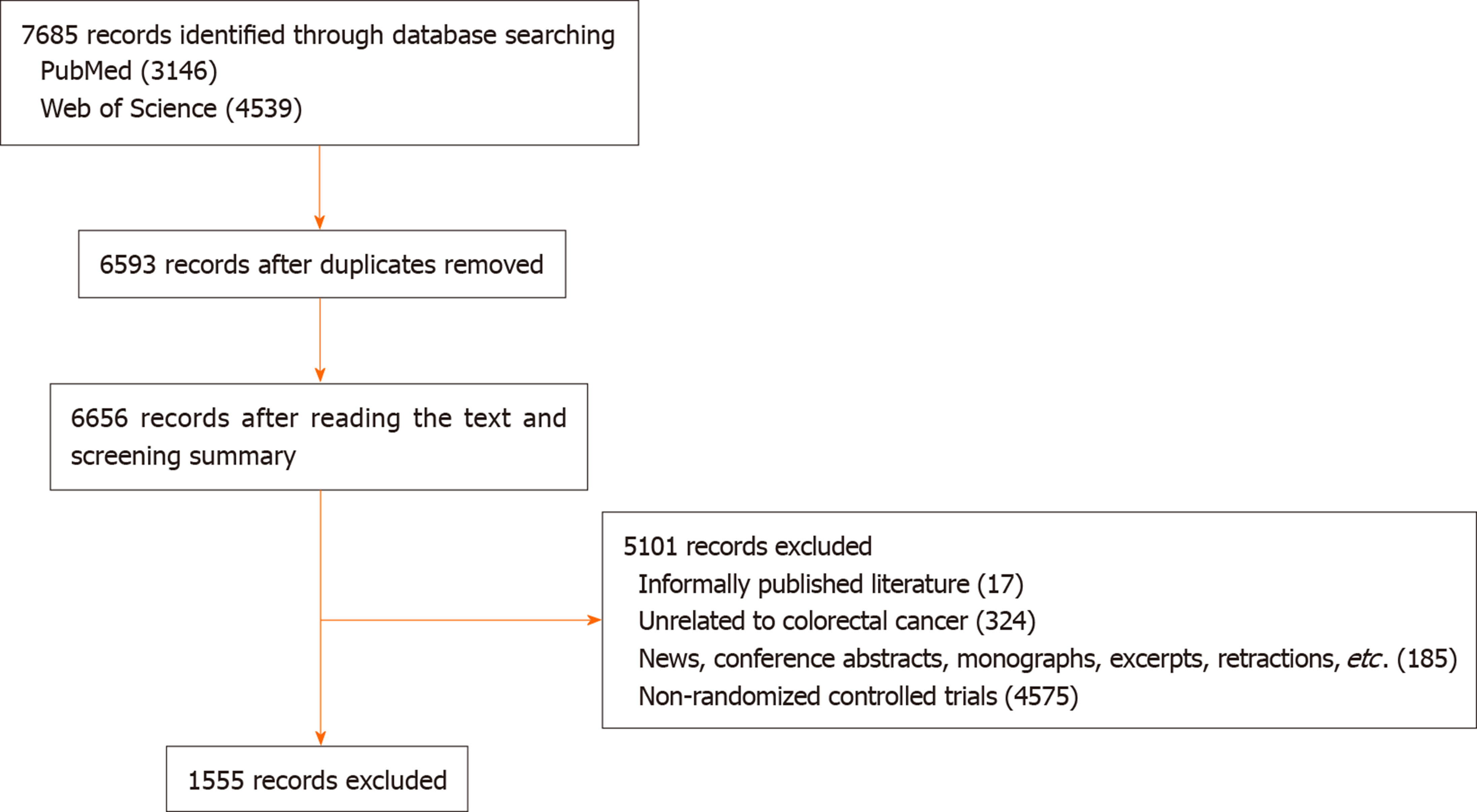
A total of 3146 articles were identified in PubMed and 4539 articles in the Web of Science, and 1029 duplicate articles among these were excluded. We read basic information such as texts and abstracts and excluded 17 informal publications and 324 articles unrelated to colorectal cancer. The types comprised of 185 articles including news, conference abstracts, monographs, excerpts, retractions, and errata, and 4575 non-RCTs. This led to the inclusion of 1555 articles. The literature screening process and results are shown in Figure 1.
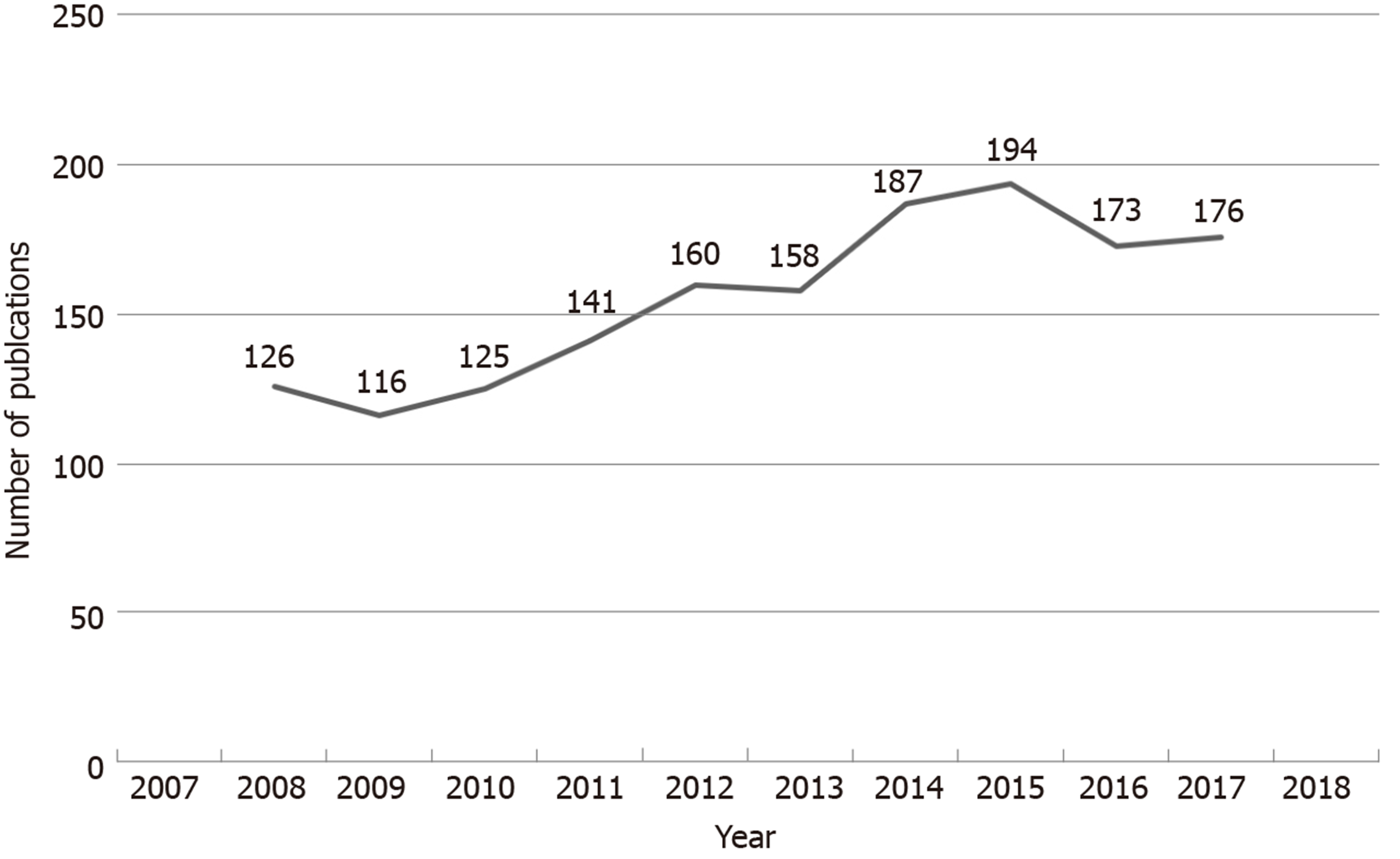
There was a gradual increase in the number of publications on colorectal cancer RCT studies between 2008 and 2018 (Figure 2).
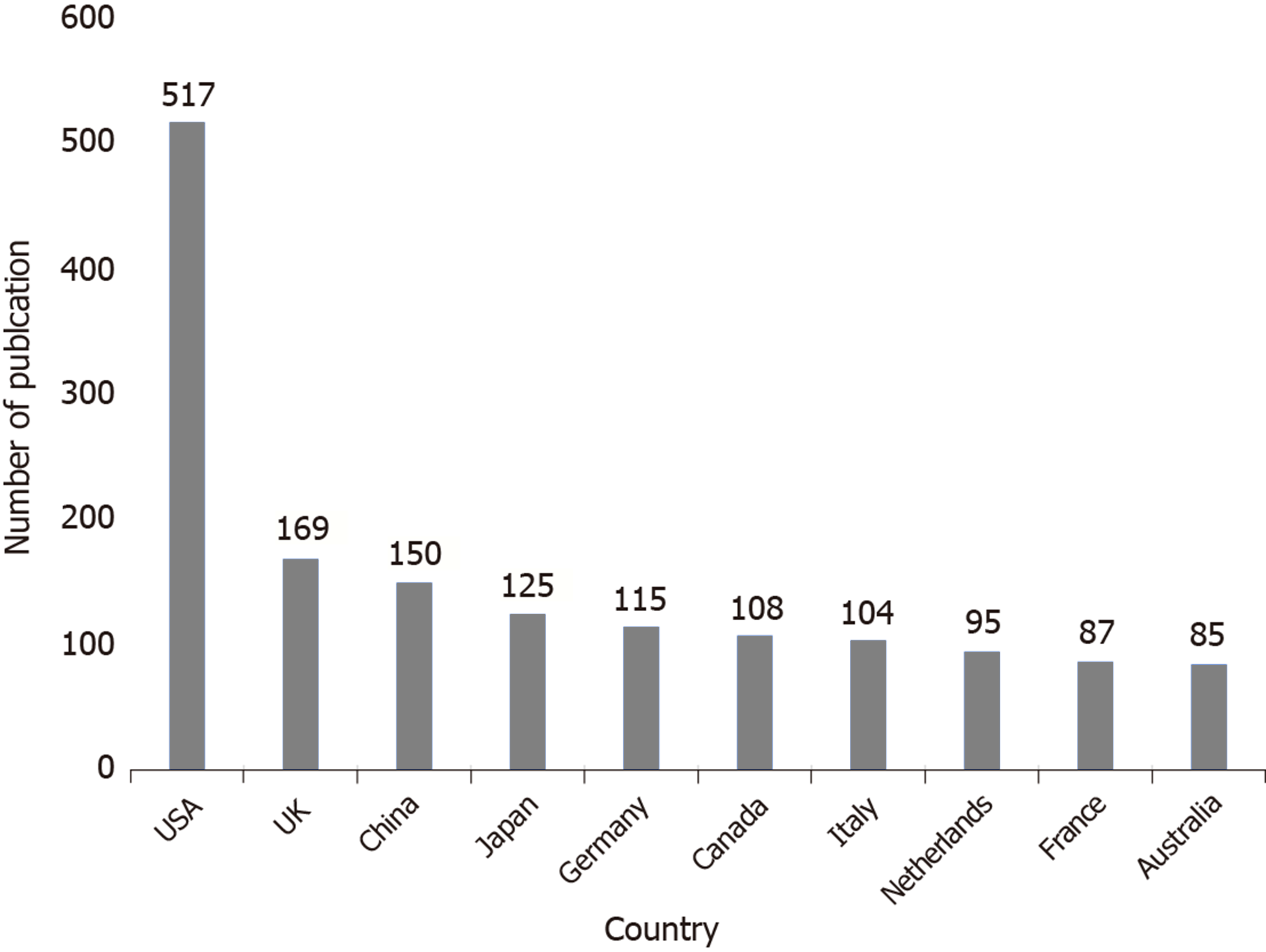
The top 10 countries that published relevant literature and their numbers are shown in Figure 3. The top 10 research institutions that published relevant literature are listed in Table 1.
| Institution | Country | Number |
| Harvard University | United States | 52 |
| University of California | United States | 51 |
| University of Texas | United States | 41 |
| University of London | United Kingdom | 40 |
| University of Toronto | Canada | 33 |
| Boston Healthcare System | United States | 30 |
| NIH National Institute of Health | United States | 29 |
| University College London | United Kingdom | 25 |
| National Cancer Institute NCI | United States | 24 |
| Mayo Clinic | United States | 24 |
The journals were sorted according to the number of RCTs. Most of the journals were concentrated on the fields of cancer, immunology, gastrointestinal surgery, etc. The journals that contained more RCT literature usually has a higher impact factor; high-quality journals were more interested in high-level clinical research such as RCT. The top 20 journals according to the publication volume are shown in Table 2. The three most relevant publications are from the top comprehensive journal of clinical medicine: Lancet. Others are from authoritative journals in various clinical fields. Higher-level clinical research from authoritative journals had higher recognition. The top 15 literature by citation are shown in Table 3[3-12].
| Journal | Number | IF 2017 | Country |
| Journal of Clinical Oncology | 84 | 26.3 | United States |
| Annals of Oncology | 69 | 13.93 | United Kingdom |
| BMC Cancer | 55 | 3.29 | United Kingdom |
| The Lancet Oncology | 49 | 36.42 | United States |
| Trials | 42 | 2.07 | United Kingdom |
| European Journal of Cancer | 38 | 7.19 | United Kingdom |
| British Journal of Surgery | 36 | 5.43 | United Kingdom |
| International Journal of Colorectal Disease | 32 | 2.53 | Germany |
| British Journal of Cancer | 32 | 5.92 | United Kingdom |
| Annals of Surgery | 32 | 9.2 | United States |
| Diseases of the Colon and Rectum | 28 | 3.62 | United States |
| Colorectal Disease | 28 | 2.78 | United Kingdom |
| Cancer | 26 | 6.54 | United States |
| Chinese Journal of Gastrointestinal Surgery | 23 | China | |
| Surgical Endoscopy | 23 | 3.12 | United States |
| Clin Colorectal Cancer | 21 | 3.86 | United States |
| Lancet | 17 | 53.25 | United Kingdom |
| Cancer Epidemiology | 17 | 2.89 | United Kingdom |
| Gut | 15 | 17.02 | United Kingdom |
| BMJ Open | 15 | 2.41 | United Kingdom |
| Ref. | Title | Journal | Cited | Year |
| Nordlinger et al[3] | Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomized controlled trial | Lancet | 1104 | 2008 |
| Atkin et al[4] | Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial | Lancet | 961 | 2010 |
| Grothey et al[5] | Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial | Lancet | 935 | 2013 |
| Tol et al[6] | Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer | New England Journal of Medicine | 904 | 2009 |
| Baxter et al[7] | Association of colonoscopy and death from colorectal cancer | Annals of Internal Medicine | 835 | 2009 |
| Hecht et al[8] | A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer | Journal of Clinical Oncology | 596 | 2009 |
| Maughan et al[9] | Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial | Lancet | 574 | 2011 |
| Peeters et al[10] | Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer | Journal of Clinical Oncology | 549 | 2010 |
| Folprecht et al[11] | Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial | Lancet Oncology | 523 | 2010 |
| Verwaal et al[12] | 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer | Annals of Surgical Oncology | 448 | 2008 |
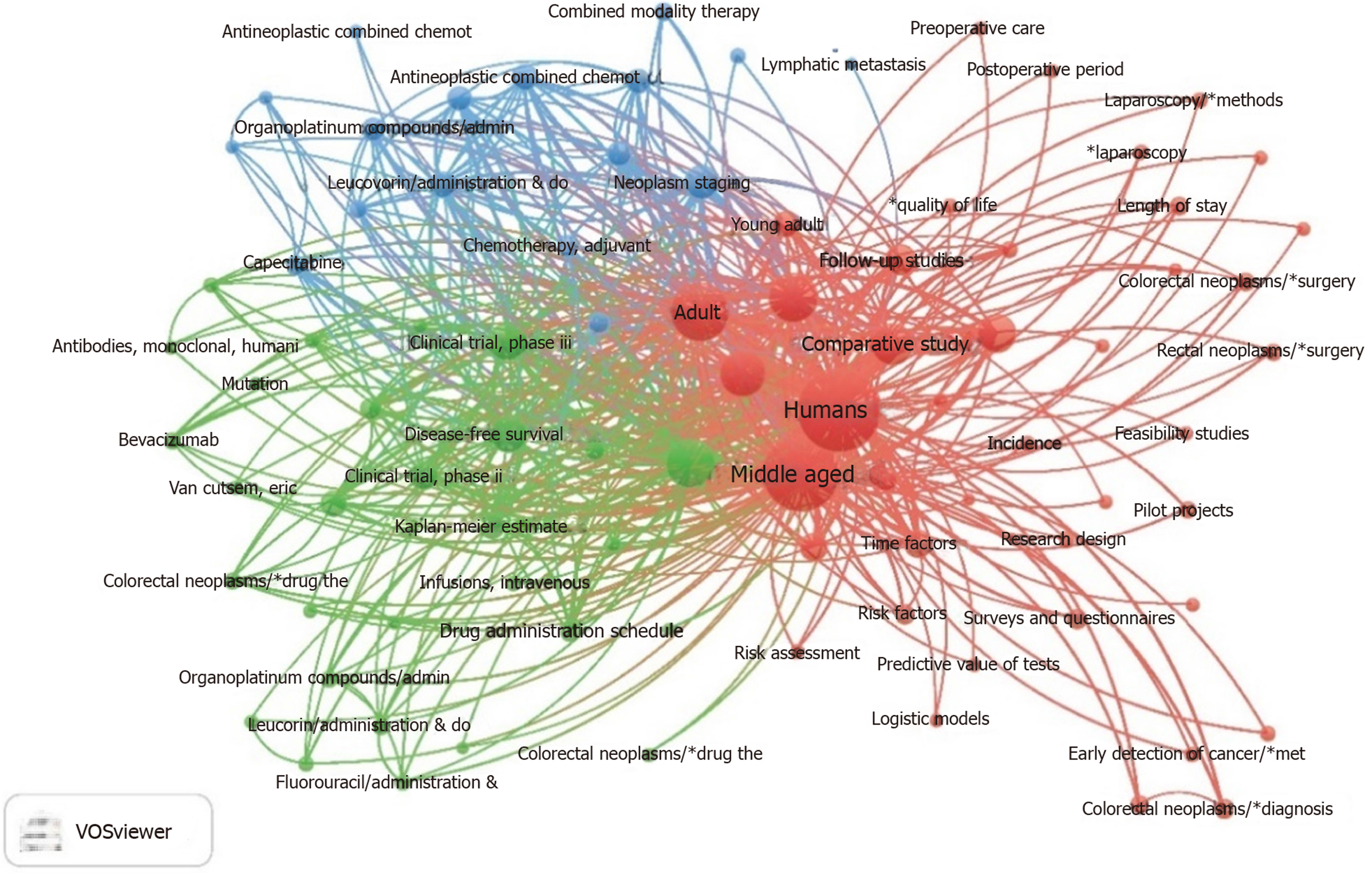
VOSviewer software was used to draw key word co-occurrence map. It can be observed that age, surgery, drug therapy, targeted therapy, neoadjuvant, pathology, comparative study, treatment outcome, disease-free survival, quality of life, and other key words appeared frequently (Figure 4).
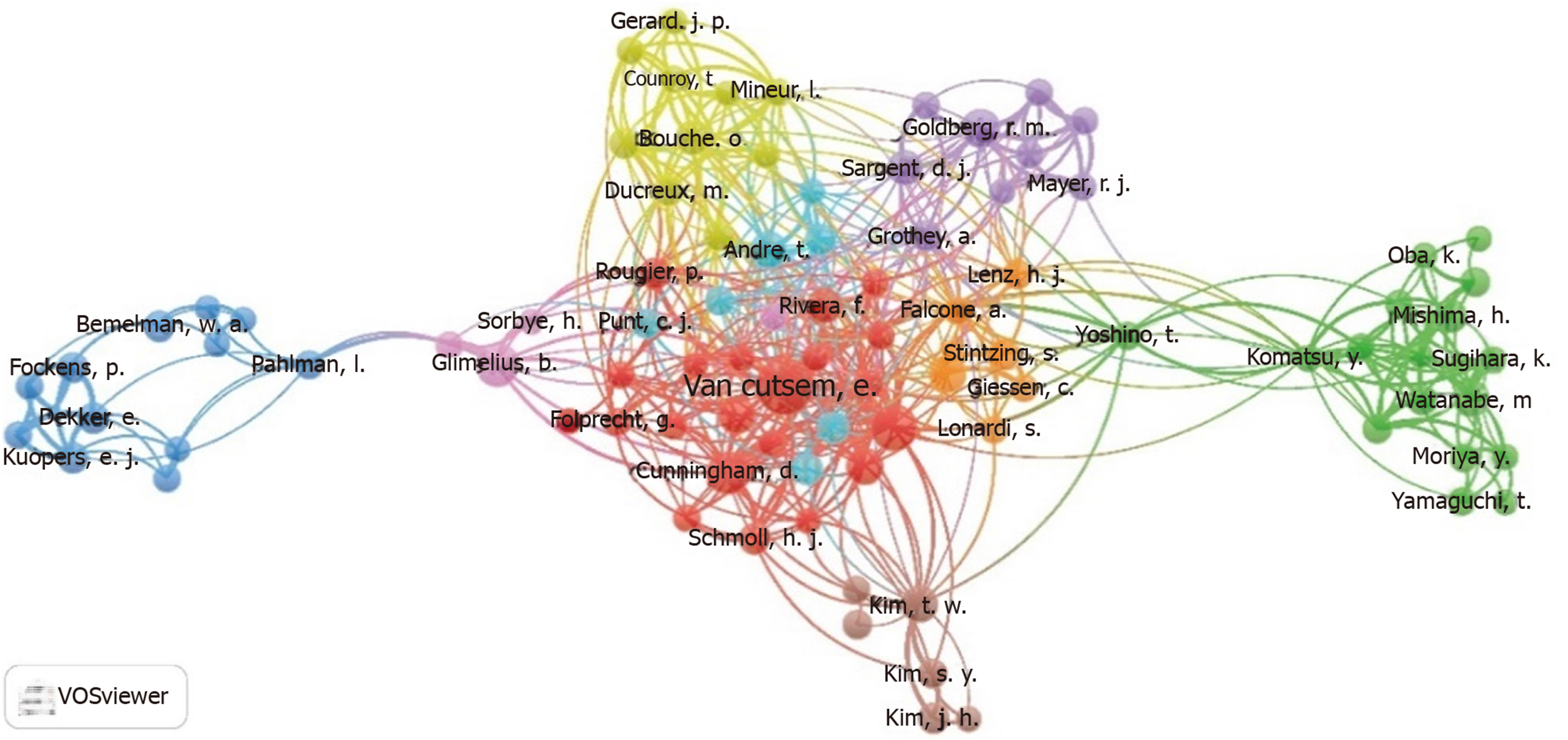
The diagram was drawn according to the coauthor relationship (Figure 5). The colors in the figure indicate the authors’ research areas. Each node represents a researcher and the node size represents the number of documents. The connection between the nodes represents a cohesive relationship between the studies. A thicker line indicates a larger number of coauthors between the two studies.
Early diagnosis of colorectal cancer has significantly improved with better diagnosis and treatment. Innovation and development of minimally invasive technology and the reform concept of diagnosis and treatment has lowered local recurrence after surgery to 5%. Medical workers in the field of colorectal cancer in China have achieved remarkable results in the past decade[13]. However, due to the large population in China, there are nearly 400000 new patients and 200000 deaths every year; the situation is still grim[14]. China’s clinical research started late, and there was lack of experience and attention to the important research aspects. There is still a big gap between China and other countries such as Japan, South Korea, Europe, and the United States[15,16].
Current clinical RCT research hotspots include the debate between laparoscopic and open surgery, the advantages and disadvantages of the “Wait and See” strategy during pathological complete remission after neoadjuvant chemoradiotherapy, 3 or 6 mo for adjuvant chemotherapy, the feasibility of the transanal total mesorectal excision, and the best chemotherapy sequence of neoadjuvant chemotherapy for advanced rectal cancer[17-20]. These research hotspots are based abroad with Chinese researchers and institutions seldom participating. However, domestic clinicians are gradually and actively carrying out evidence-based clinical research.
Chinese clinicians have a wealth of medical information, and multicenter RCTs can utilize it to get high-quality clinical data. For example, the “Radical Extent of lymphadenectomy of Laparoscopic Right Colectomy for colon cancer” study on the scope of laparoscopic right colon cancer lymphatic dissection led by Peking Union Concord Hospital was carried out in strict accordance with the implementation standards. The study conducted multiple rounds of argumentation and consideration for the surgical risks and benefits of complete mesocolic excision. Another study, “The randomized Neoadjuvant FOLFOX6 Chemotherapy with or without Radiation in Rectal cancer”, led by the Sixth Affiliated Hospital of Sun Yat-sen University selected patients with stage II-III rectal cancer and discussed the necessity of receiving oxaliplatin after receiving neoadjuvant therapy. It demonstrated that FOLFOX plus radiotherapy had the best effect, but pure FOLFOX could achieve similar downregulation effects with fewer complications. Although long-term survival is still being followed, the preliminary results have already inspired researchers at home and abroad[21].
Through statistical literature analysis, the top 10 research institutions by publications were found to be in the United States, the United Kingdom, and Canada with China not on the list. Sun Yat-sen University is ranked 20th in colorectal cancer clinical research in China. Lancet and its subdistribution produced 7 of the 15 most cited papers. The articles focused on colonoscopy for colorectal cancer, surgical treatment of metastatic colorectal cancer, surgical procedures, open and laparoscopic surgery, first-line chemotherapy, second-line chemotherapy, targeted therapy, and the safety and efficacy of panitumumab, bevacizumab, and cetuximab. Key co-occurrence map words included age, surgery, drug therapy, targeted therapy, neoadjuvant, pathology, comparative study, treatment outcome, disease-free survival, quality of life, etc. Presently, research hotspots focus on colorectal cancer screening, diagnosis and treatment, surgical methods, choice of drug treatment, and evaluation of treatment outcomes and quality of life.
A co-occurrence map of coauthors showed that most researchers active in international multicenter research were from Europe, the United States, Japan, and South Korea. These coauthorships are relatively close and extensive, and researchers from Asian countries such as Japan and South Korea are less coauthored with European and United States scholars.
In the clinical research on colorectal cancer, authors such as Van Custsem, Cunningham, Rivera, Kim, and Morriya produced the largest number of publications, and there were close relationships among them. They are therefore the key leaders in this field. The Chinese rank first in the number of published literature related to colorectal cancer, but third in RCT studies with fewer multicenter RCTs. The reason may be that the cost of RCT is high, the research team is immature, the research design ideas and research endpoints are unreasonable, and data collection is irregular[22]. Key links in the implementation process are also complex, and the research team needs to address a series of questions such as medical ethics review and good clinical practice. The development of multicenter high-quality RCT research requires a sufficient amount of funding and government agency support. Researchers need to unite, forge ahead, persevere sincerely, and promote exchanges between research institutions that play an important role. Therefore, domestic researchers should pay attention to the various key aspects of RCT research, truth-seeking, pragmatism, and standardize all aspects of clinical RCT research to maintain high accuracy and credibility.
This study has some limitations. Only PubMed and Web of Science databases were searched. There were documents in other languages included in the database, and there may be publication bias. The quality of the literature was not evaluated by the methodological quality evaluation tool.
In summary, the results of bibliometric analysis of colorectal cancer-related RCTs in the past decade, in China and abroad, showed that high-quality RCTs were increasingly favored by top international journals. Although China’s clinical research has achieved positive initial results, there are still large gaps when compared to Europe and the United States. Domestic researchers should implement standardized and accurate clinical trials, strengthen multicenter cooperation at home and abroad, and implement high quality colorectal cancer-related RCT clinical research to promote the field in China.
In the past decade, clinical research on colorectal cancer has made significant progress with deepening theoretical and molecular research on its pathogenesis. However, many randomized controlled trials (RCTs) have shortfalls, such as lacking systematic methodological knowledge, insufficient sample size, etc.
Clinical colorectal cancer research in China has progressed, but the quality of RCTs is still low. Therefore, we compared the RCTs in China with those of other countries to identify deficiencies and improve Chinese research.
We used bibliometric analysis to evaluate the research status of colorectal cancer RCTs in China and abroad and provide references for the design, cooperation, and implementation of colorectal cancer RCTs in China.
We retrieved the RCTs studies related to colorectal cancer published between 2008 and 2018 in PubMed and the Web of Science. The literature was independently screened and extracted by two investigators. The bibliometric methods were used for statistical analysis of the publication years, countries/regions, authors, institutions, source journal, quoted times, key words, and authors. We used Microsoft Excel 2013 and VOSviewer 1.6.4 software to analyze the data.
Colorectal cancer RCTs have shown an upward trend from 2008 to 2018. Most of the top 10 research institutions were from the United States and the United Kingdom, and most of the related research journals were sponsored by European and American countries. The 15 most cited studies were comprised of international multicenter clinical research, with few participants from Chinese institutions. Network visualization using key words showed that RCTs on colorectal cancer focused on screening, disease-free survival, drug treatment, surgical methods, clinical trials, quality of life, and prognosis. The results of the coauthorship network analysis showed that Chinese researchers are less involved in international exchanges.
High-quality RCTs are increasingly favored by top international journals. There is a large gap between Chinese and international clinical research; researchers should gradually standardize clinical trials, ensure accuracy, strengthen international multicenter cooperation, and emphasize quality control.
There is a large gap between Chinese and international clinical research according to our bibliometric analysis. Chinese researchers should gradually standardize clinical trials, ensure accuracy, strengthen international multicenter cooperation, and emphasize quality control.
The authors thank Professor Jun-Hong Hu for his professional advice.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Vynios D S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Liu JH
| 1. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2912] [Article Influence: 364.0] [Reference Citation Analysis (3)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, Jaeck D, Mirza D, Parks RW, Mauer M, Tanis E, Van Cutsem E, Scheithauer W, Gruenberger T; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 921] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 4. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1138] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 5. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2118] [Article Influence: 176.5] [Reference Citation Analysis (0)] |
| 6. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, Richel DJ, Voest EE, Dijkstra JR, Vink-Börger ME, Antonini NF, Mol L, van Krieken JH, Dalesio O, Punt CJ. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 999] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 7. | Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 920] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 8. | Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R, Shahin S, Amado RG. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 9. | Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL, Kay E, Mitchell JK, Madi A, Jasani B, James MD, Bridgewater J, Kennedy MJ, Claes B, Lambrechts D, Kaplan R, Cheadle JP; MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103-2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 766] [Cited by in RCA: 763] [Article Influence: 54.5] [Reference Citation Analysis (2)] |
| 10. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher J, Weitz J, Konopke R, Stroszczynski C, Liersch T, Ockert D, Herrmann T, Goekkurt E, Parisi F, Köhne CH. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 712] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 12. | Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 765] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 13. | Huang HY, Shi JF, Guo LW, Bai YN, Liao XZ, Liu GX, Mao AY, Ren JS, Sun XJ, Zhu XY, Wang L, Song BB, Du LB, Zhu L, Gong JY, Zhou Q, Liu YQ, Cao R, Mai L, Lan L, Sun XH, Ren Y, Zhou JY, Wang YZ, Qi X, Lou PA, Shi D, Li N, Zhang K, He J, Dai M. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: a hospital-based, multicenter, cross-sectional survey. Chin J Cancer. 2017;36:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Pan R, Zhu M, Yu C, Lv J, Guo Y, Bian Z, Yang L, Chen Y, Hu Z, Chen Z, Li L, Shen H; China Kadoorie Biobank Collaborative Group. Cancer incidence and mortality: A cohort study in China, 2008-2013. Int J Cancer. 2017;141:1315-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Zavoral M, Suchanek S, Majek O, Fric P, Minarikova P, Minarik M, Seifert B, Dusek L. Colorectal cancer screening: 20 years of development and recent progress. World J Gastroenterol. 2014;20:3825-3834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Neuman HB, Park J, Weiser MR. Randomized clinical trials in colon cancer. Surg Oncol Clin N Am. 2010;19:183-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ; COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1214] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 18. | Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, Perez RO, Moran BJ. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Ann Surg. 2018;268:955-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 19. | Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 686] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 20. | Penna M, Hompes R, Arnold S, Wynn G, Austin R, Warusavitarne J, Moran B, Hanna GB, Mortensen NJ, Tekkis PP; TaTME Registry Collaborative. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg. 2017;266:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 326] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 21. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J. Modified FOLFOX6 With or Without Radiation Versus Fluorouracil and Leucovorin With Radiation in Neoadjuvant Treatment of Locally Advanced Rectal Cancer: Initial Results of the Chinese FOWARC Multicenter, Open-Label, Randomized Three-Arm Phase III Trial. J Clin Oncol. 2016;34:3300-3307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 22. | Bothwell LE, Greene JA, Podolsky SH, Jones DS. Assessing the Gold Standard--Lessons from the History of RCTs. N Engl J Med. 2016;374:2175-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 355] [Article Influence: 39.4] [Reference Citation Analysis (0)] |