Published online Jul 6, 2020. doi: 10.12998/wjcc.v8.i13.2778
Peer-review started: March 15, 2020
First decision: April 22, 2020
Revised: May 2, 2020
Accepted: June 7, 2020
Article in press: June 7, 2020
Published online: July 6, 2020
Processing time: 111 Days and 22.2 Hours
Nab-paclitaxel plus gemcitabine (AG) has resulted in higher tumor response and survival rates for metastatic or advanced pancreatic ductal adenocarcinoma (PDAC) compared with gemcitabine (GEM) alone.
To examine the feasibility and safety of AG adjuvant chemotherapy of resectable PDAC.
We retrospectively analyzed patients with resected PDAC who received AG or GEM as postoperative adjuvant treatment between January 2013 and December 2016 at the Chinese People’s Liberation Army General Hospital, Beijing, China. The patients adopted combined nab-paclitaxel (125 mg/m2) and GEM (1 g/m2) or GEM (1 g/m2) alone treatment, on days 1 and 8 every 3 wk for six cycles, unless intolerable adverse events or disease progression occurred. The disease-free survival, overall survival (OS) and adverse events of the two groups were statistically analyzed.
Compared with GEM, median disease-free survival (12.2 mo vs 15.8 mo, P = 0.039) and OS (20.6 mo vs 28.3 mo, P = 0.028) were significantly improved in the AG group. The 2-year OS rates were 63.3% and 43.3% in the AG and GEM groups, respectively. However, the incidence of sensory neuropathy was increased significantly in the AG than the GEM group (53.3% vs 23.3%, P < 0.001).
In our initial experience, AG significantly improved disease-free survival and OS of patients with resected PDAC. AG may be a potential option for postoperative adjuvant chemotherapy of resectable PDAC.
Core tip: Nab-paclitaxel plus gemcitabine (AG) has resulted in better survival and tumor response rates for advanced or metastatic pancreatic ductal adenocarcinoma (PDAC) compared with gemcitabine alone. However, its role in adjuvant therapy for resected PDAC remains unclear. We retrospectively reviewed our PDAC database for patients with primary resectable PDAC who were treated with AG or gemcitabine alone as postoperative adjuvant chemotherapy between January 2013 and December 2016. Our results suggested that AG significantly improved disease-free survival and overall survival of patients with resected PDAC. AG may be a potential option for postoperative adjuvant chemotherapy of resectable PDAC.
- Citation: Yin ZZ, Zhao ZM, Tang WB, Jiang N, Zhang KD, Song YY, Wang Y, Li CG, Gao YX, Liu R. Adjuvant nab-paclitaxel plus gemcitabine vs gemcitabine alone for resected pancreatic ductal adenocarcinoma: A single center experience in China. World J Clin Cases 2020; 8(13): 2778-2786
- URL: https://www.wjgnet.com/2307-8960/full/v8/i13/2778.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i13.2778
The mortality rate of patients with pancreatic ductal adenocarcinoma (PDAC) is increasing. In China, PDAC is the sixth leading cause of cancer-related death[1]. Complete resection of the involved portion of the pancreas has shown a curative effect. Although improved surgical techniques have led to more patients undergoing radical resection, the 5-year survival rate after radical resection alone is < 10%[2,3]. Therefore, postoperative adjuvant treatments have been evaluated over the past several decades.
The ESPAC-1[4] and CONKO-001[5] trials showed that postoperative adjuvant systemic chemotherapy [fluorouracil or gemcitabine (GEM)] improves survival of patients with resected PDAC. The ESPAC-4 trial[6], published in 2017, demonstrated the superiority of combination of GEM and capecitabine in the adjuvant setting (median survival 26 mo, 5-year survival rate approximately 30%). Surgery followed by adjuvant GEM monotherapy or GEM plus capecitabine is the standard of care for potentially curable pancreatic cancer. Additionally, the JASPAC 01 trial[7] showed that adjuvant chemotherapy with S-1 can be effective for resected pancreatic cancer in Japanese patients. However, recurrence rates remain high despite microscopically and pathologically compete removal of the tumor and adjuvant chemotherapy with about 69%–75% of patients experiencing recrudescence within 2 years[8-10].
Nab-paclitaxel combination with GEM (AG) significantly improves disease-free survival (DFS), overall survival (OS) and tumor response rates of patients with metastatic or local advanced pancreatic adenocarcinoma[11-13]. Therefore, we explored the efficacy and safety of AG compared with GEM alone as adjuvant therapy for patients with resected PDAC.
We retrospectively reviewed our PDAC database for patients with primary resectable PDAC who were treated with AG or GEM alone as postoperative adjuvant chemotherapy between January 2013 and December 2016. The study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (No. S2016-098-02). Patients aged 18–79 years were recruited and had received complete macroscopic resection (R0 or R1)[14] for histologically confirmed PDAC. The other inclusion criteria were full recovery from surgery (3-12 wk after the operation), Eastern Cooperative Oncology performance status of 0 or 1 and adequate hematological, liver and renal function according to measurements[15] performed 7 d before initiation of adjuvant therapy. Patients with incomplete (R2) resection, nonductal adenocarcinoma, TNM stage IV disease[16] or serious complications (Clavien–Dindo grade III or above) were excluded. Additionally, patients were excluded if they had recurrence or metastatic disease on chest radiography and contrast-enhanced abdominal computed tomography or magnetic resonance imaging before initiation of adjuvant therapy. Postoperative carbohydrate antigen 19-9 level was not restricted.
Patients were assigned to receive the AG regimen or GEM alone. GEM was delivered as a 1 g/m2 intravenous infusion, and AG regimen consisted of 125 mg/m2 nab-paclitaxel (Abraxane) followed by 1 g/m2 GEM. Both regimens were administered on days 1 and 8 every 3 wk (one cycle) for 24 wk (six cycles). During each cycle or whenever clinically indicated, patient status was assessed via a comprehensive physical examination, Word Health Organization performance status assessment, whole blood cell count and blood biochemical examination. Follow-up evaluation included contrast-enhanced computed tomography or magnetic resonance imaging of the chest, abdomen and pelvis and measurement of tumor markers. Clinical examinations were repeated at 3-mo intervals for 2 years and subsequently at 6-mo intervals for 3 years.
Chemotherapy-related toxicity was evaluated and graded by the National Cancer Institute Common Terminology Criteria of Adverse Events, version 4.0[17]. The grade 3 or higher was defined as the severe adverse events. Safety evaluations were carried out before each cycle and until the end of follow-up.
Adjuvant treatment was continued until intolerable adverse events or disease progression occurred. Details of dose modifications, adverse events and treatment cessation are reported elsewhere[8,18]. In the AG group, nab-paclitaxel was adjusted at a dose of 60 mg/m2 for the patients of grade 2 neutropenia or grade 1 throm-bocytopenia. Adjuvant chemotherapy was postponed for a maximum of 2 wk for patients of grade 3 or 4 neutropenia, anemia or thrombocytopenia. If the treatment was delayed because of febrile neutropenia, then granulocyte colony-stimulating factor was used in subsequent cycles.
Statistical analyses were performed with IBM SPSS Statistics, version 20.0 (IBM Corp., Armonk, NY, United States). The continuous variables of normal distribution were expressed as mean ± standard deviation and those of abnormal distribution as median with minimum to maximum values. Differences in continuous variables between two groups were compared by t test or Mann–Whitney test, according to the distribution of the data. The frequencies of categorical variables were examined by χ2 test. If the observed number in one or more cells was > 1 but < 5, or the table was more than 2 × 2, then likelihood ratio statistics were calculated. If at least one expected cell count was < 1 in a 2 × 2 table, then Fisher’s exact test was performed. Two-tailed statistical analyses were conducted. A Kaplan–Meier algorithm was used to compare survival. Differences were considered significant at P < 0.05.
From January 2013 to December 2016, 70 patients with PDAC were assigned to receive an adjuvant regimen, of whom, ten were excluded due to serious complications or R2 resection. As a result, 30 patients each were assigned to the AG and GEM groups. Both groups had similar demographic and clinical characteristics at baseline (Table 1).
| Characteristic | AG | GEM | P value |
| Age in yr | 57 (40-72) | 59 (31-67) | |
| Sex | 0.787 | ||
| Male | 19 (63) | 20 (67) | |
| Female | 11 (37) | 10 (33) | |
| Performance status | 0.791 | ||
| 0 | 19 (63.3) | 18 (60) | |
| 1 | 11 (36.7) | 12 (40) | |
| Diabetes | 0.559 | ||
| No | 23 (77) | 21 (70) | |
| Yes | 7 (23) | 9 (30) | |
| Operative procedure | 0.605 | ||
| Pancreatoduodenectomy | 15 (50) | 17 (57) | |
| Distal pancreatectomy | 15 (50) | 13 (43) | |
| Residual tumor status | 0.687 | ||
| R0 | 27 (90) | 26 (87) | |
| R1 | 3 (10) | 4 (13) | |
| Primary tumor status | 0.638 | ||
| T1 | 6 (20.0) | 3 (10.0) | |
| T2 | 17 (56.7) | 20 (66.7) | |
| T3 | 5 (16.6) | 6 (20.0) | |
| T4 | 2 (6.7) | 1 (3.3) | |
| Regional lymph node status | 0.273 | ||
| N0 | 22 (73) | 18 (60) | |
| N1 | 8 (27) | 12 (40) | |
| Pathological stage | 0.405 | ||
| I (IA/IB) | 17 (5/12, 56.7) | 13 (1/12, 43.4) | |
| II (IIA/IIB) | 11 (4/7, 36.6) | 16 (5/11, 53.3) | |
| III | 2 (6.7) | 1 (3.3) | |
| Tumor grade | 0.105 | ||
| Well differentiated | 3 (10) | 2 (6.7) | |
| Moderately differentiated | 23 (76.7) | 17 (56.7) | |
| Poorly differentiated | 4 (13.3) | 11 (36.6) | |
| Pre-adjuvant CA19-9, median | 48.2 | 24.6 | 0.795 |
| ≤ 37 U/mL | 13 (43.4) | 14 (46.7) | |
| > 37 U/mL | 17 (56.6) | 16 (53.3) |
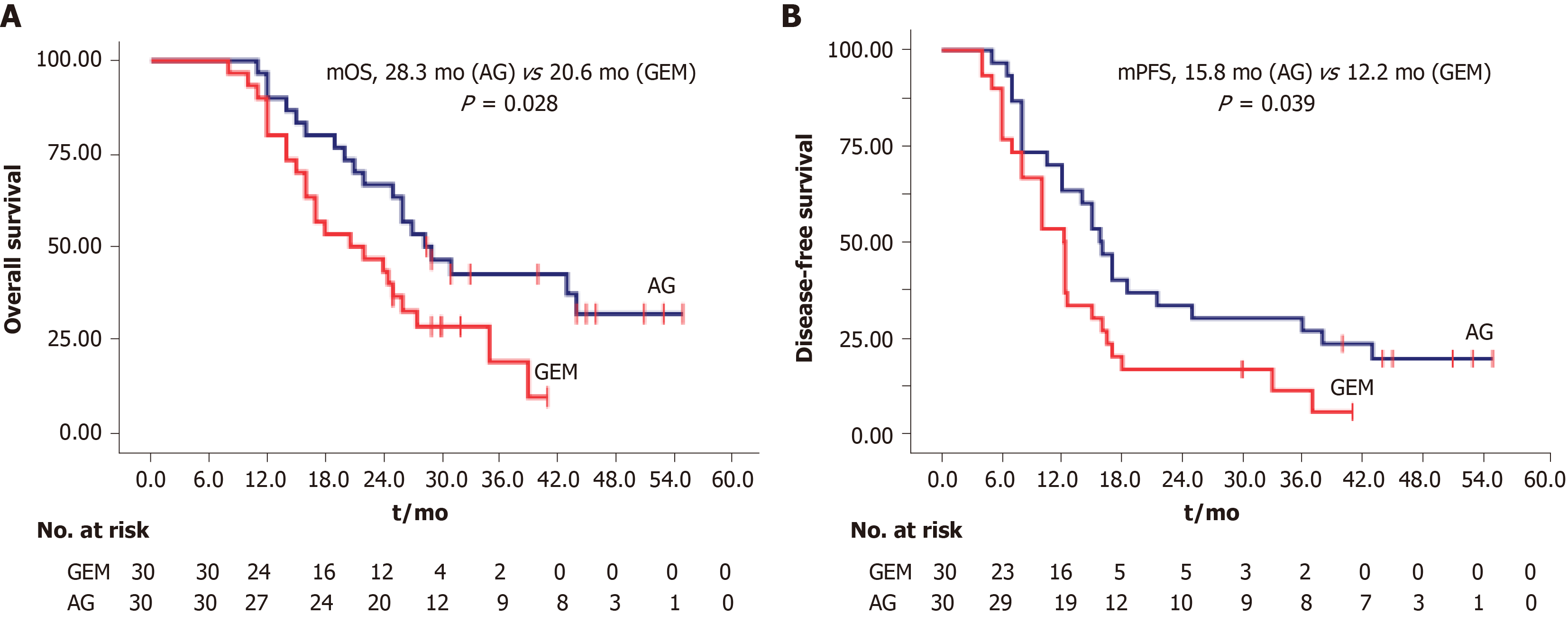
At the last follow-up on January 20, 2019, the median follow-up duration was 22 mo in the GEM group and 27 mo in the AG group. Patients alive and without disease progression at the time of the final analysis were censored at the date of last follow-up. The median OS was 20.6 mo [95% confidence interval (CI): 11.2-29.9 mo] in the GEM group and 28.3 mo (95%CI: 21.9–34.6 mo) in the AG group (P = 0.028). The median DFS was 12.2 mo (95%CI: 9.6-14.8 mo) in the GEM group and 15.8 mo (95%CI: 13.1-18.5 mo) in the AG group (P = 0.039) (Figure 1). Recurrence was observed in 27 of 30 (90%) patients in the GEM group and in 24 of 30 (80%) patients in the AG group. Seven patients (23.3%) in the GEM group and eleven (36.7%) in the AG group were alive at the end of the analysis. The 2-year OS rates were 63.3% and 43.3% in the AG and GEM groups, respectively.
There were 15 patients (50%) in the AG group and 17 patients (56.7%) in the GEM group who received all planned cycles of chemotherapy. The median chemotherapy cycles were 5.5 (range 1-6) in the AG group and six (range 2-6) in the GEM group (P = 0.402). All patients were in the safety set and were analyzed for adverse events.
In the GEM group, 13 (43.3%) patients had 81 adverse events. In the AG group, 18 patients (60%) had 143 adverse events (Table 2). The incidence of decreased white blood cell count, neutropenia, anemia, thrombocytopenia, fatigue, vomiting, diarrhea, fever, febrile neutropenia and infection were similar in both groups. However, the incidence of sensory neuropathy was significantly increased in the AG group than the GEM group (53.3% vs 23.3%, P < 0.001). Additionally, grade 3 or higher neutropenia was more common in the AG group than GEM group (43.3% vs 30%), but the frequency of granulocyte colony-stimulating factor use did not differ significantly between the two groups (41.9% in the AG group vs 24.1% in the GEM group, P = 0.177).
| AG | GEM | P value | P value | |||||
| Grade 1/2 (%) | Grade 3/4 (%) | Grade 5 (%) | Grade 1/2 (%) | Grade 3/4 (%) | Grade 5 (%) | Grade ≥ 3 | ||
| White blood cell count decreased | 8 (26.7) | 9 (30.0) | 0 | 7 (23.3) | 6 (20.0) | 0 | 0.549 | 0.371 |
| Neutropenia | 5 (16.7) | 13 (43.3) | 0 | 4 (13.3) | 9 (30.0) | 0 | 0.435 | 0.284 |
| Anemia | 10 (33.3) | 3 (10.0) | 0 | 6 (20.0) | 3 (10.0) | 0 | 0.489 | 1.000 |
| Thrombocytopenia | 5 (16.7) | 3 (10.0) | 0 | 2 (6.7) | 2 (6.7) | 0 | 0.394 | 0.639 |
| Fatigue | 9 (30.0) | 4 (13.3) | 0 | 6 (20.0) | 2 (6.7) | 0 | 0.382 | 0.385 |
| Vomiting | 10 (33.3) | 3 (10.0) | 1 (3.3) | 8 (26.7) | 0 | 1 (3.3) | 0.168 | 0.148 |
| Diarrhea | 8 (26.7) | 1 (3.3) | 0 | 6 (20.0) | 0 | 0 | 0.392 | 1.000 |
| Fever | 8 (26.7) | 2 (6.7) | 0 | 5 (16.7) | 1 (3.3) | 0 | 0.496 | 0.550 |
| Febrile neutropenia | 2 (6.7) | 4 (13.3) | 1 (3.3) | 3 (10.0) | 1 (3.3) | 0 | 0.296 | 0.073 |
| Infection | 2 (6.7) | 1 (3.3) | 1 (3.3) | 1 (3.3) | 1 (3.3) | 0 | 0.615 | 0.550 |
| Sensory neuropathy | 14 (46.7) | 15 (50.0) | 1 (3.3) | 7 (23.3) | 0 | 0 | < 0.001 | < 0.001 |
In this retrospective study involving patients with resected PDAC, adjuvant chemotherapy with AG significantly increased OS and DFS compared with GEM alone. The safety and toxicity profiles of AG were acceptable and manageable, as in previous trials of advanced metastatic pancreatic adenocarcinoma.
The median OS and DFS in the GEM group were 20.1-26.5 and 11.3-15.3 mo, respectively, which was similar to previous studies[6-8,10]. However, the median OS was 7.7 mo longer in the AG group than in the GEM group (28.3 mo vs 20.6 mo, P = 0.028). The median DFS was 15.8 mo in the AG group and 12.2 mo in the GEM group (P = 0.039). Treatment with AG led to a 20% improvement in the 2-year OS rate (63.3% in the AG group vs 43.3% in the GEM group).
Nab-paclitaxel possesses antitumor activity and acts synergistically with GEM. Nab-paclitaxel also improves the intratumoral concentration of GEM[19]. The efficacy and safety of AG as first-line treatment for borderline resectable and locally advanced and metastatic pancreatic cancer have been reported[11,13,18,20-21]. Although this was a single-center retrospective study, the outstanding OS observed suggests the efficacy of the AG combination, which warrants further investigation for resectable PDAC.
As expected, the rates of serious life-threatening adverse events in the AG and GEM groups were similar and acceptable. Compared with the GEM group, the grade 3 or higher neutropenia was more frequent in the AG group (30% vs 43.3%), but there was no significant difference in the frequency of granulocyte colony-stimulating factor use (P = 0.177), febrile neutropenia (P = 0.073) or infection (P = 0.550). However, the incidence of sensory neuropathy differed significantly between the AG and GEM groups (53.3% vs 23.3%, P < 0.001). If grade 3 or higher sensory neuropathy was diagnosed, then the dose of nab-paclitaxel was subsequently reduced or temporarily discontinued.
Conroy et al[22] reported that adjuvant chemotherapy with a modified FOLFIRINOX (combination of fluorouracil, oxaliplatin, irinotecan and leucovorin) treatment led to significantly longer survival than GEM did among patients with resected PDAC (DFS, 21.6 mo vs 12.8 mo; OS, 54.4 mo vs 35 mo). However, there was a significant increase in the incidence of adverse effects. Therefore, the modified FOLFIRINOX regimen is not frequently applied in China, especially in the adjuvant setting for resected PDAC[23].
Results of the global phase III APACT trial have been reported at ASCO 2019. The APACT trial revealed that there was no statistical benefit for adjuvant chemotherapy with AG by independent central review[24,25]. The median DFS by independent review was 19.4 mo in the AG group and 18.8 mo in the GEM group (P = 0.1824). However, the sensitivity analysis of investigator assessment demonstrated a significant improvement for both DFS (16.6 mo vs 13.7 mo, P = 0.0168) and OS (40.5 mo vs 36.2 mo, P = 0.045) with the use of AG as compared to GEM only. The ongoing Phase III APACT study is investigating survival for adjuvant GEM compared with AG for resected PDAC. We look forward to seeing the results of this study that are expected by 2022.
The present study had several limitations due to its retrospective study design. It was also a single-center, nonrandomized controlled study with potential selection bias. However, our results initially indicated the efficacy and safety of AG for resectable PDAC. In conclusion, AG may be a potential option for postoperative adjuvant chemotherapy of resectable PDAC.
Nab-paclitaxel plus gemcitabine (AG) has resulted in better tumor response and survival rates for metastatic or advanced pancreatic ductal adenocarcinoma (PDAC) compared with gemcitabine (GEM) alone. However, its role in adjuvant therapy for resected PDAC remains unclear.
This study aimed to examine the effects of AG and GEM alone as adjuvant therapy for resected PDAC.
This study examined the safety and efficacy of AG adjuvant therapy for resected PDAC.
We retrospectively reviewed our PDAC database for patients with primary resectable PDAC who were treated with AG or GEM alone as postoperative adjuvant chemotherapy between January 2013 and December 2016.
The median follow-up duration was 22 mo in the GEM group and 27 mo in the AG group. Compared with GEM, median disease-free survival (12.2 mo vs 15.8 mo, P = 0.039) and overall survival (20.6 mo vs 28.3 mo, P = 0.028) were significantly improved in the AG group. The 2-year overall survival rates were 63.3% and 43.3% in the AG and GEM groups, respectively. However, the incidence of sensory neuropathy was significantly higher in the AG than in the GEM group (53.3% vs 23.3%, P < 0.001).
This study suggested that AG significantly improved disease-free survival and overall survival of patients with resected PDAC. AG may be a potential option for postoperative adjuvant chemotherapy of resectable PDAC.
The present study had several limitations due to its retrospective study design. It was also a single-center, nonrandomized controlled study with potential selection bias. However, our results initially indicated the efficacy and safety of AG for resectable PDAC. The ongoing Phase III APACT study is investigating survival for adjuvant GEM compared with AG for resected PDAC. We look forward to seeing the results of this study that are expected by 2022.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aktekin A, Karamouzis MV S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, Li H, Zou X, He J. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 704] [Article Influence: 100.6] [Reference Citation Analysis (2)] |
| 2. | Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1442] [Cited by in RCA: 1343] [Article Influence: 149.2] [Reference Citation Analysis (2)] |
| 3. | Reynolds RB, Folloder J. Clinical Management of Pancreatic Cancer. J Adv Pract Oncol. 2014;5:356-364. [PubMed] |
| 4. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 1908] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 5. | Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1361] [Article Influence: 113.4] [Reference Citation Analysis (0)] |
| 6. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1394] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 7. | Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y; JASPAC 01 Study Group. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 784] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 8. | Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1763] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 9. | Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, Padbury R, Moore MJ, Gallinger S, Mariette C, Wente MN, Izbicki JR, Friess H, Lerch MM, Dervenis C, Oláh A, Butturini G, Doi R, Lind PA, Smith D, Valle JW, Palmer DH, Buckels JA, Thompson J, McKay CJ, Rawcliffe CL, Büchler MW; European Study Group for Pancreatic Cancer. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 10. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 224] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 11. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4882] [Article Influence: 406.8] [Reference Citation Analysis (0)] |
| 12. | Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, Javle MM, Eads JR, Allen P, Ko AH, Engebretson A, Herman JM, Strickler JH, Benson AB, Urba S, Yee NS. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2654-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 13. | Olowokure O, Torregroza-Sanchez MP, Bedoya-Apraez ID. Gemcitabine plus Nab-Paclitaxel with chemoradiation in locally advanced pancreatic cancer (LAPC). J Gastrointest Oncol. 2013;4:E16-E18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Campbell F, Smith RA, Whelan P, Sutton R, Raraty M, Neoptolemos JP, Ghaneh P. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology. 2009;55:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Ielpo B, Duran H, Diaz E, Fabra I, Caruso R, Ferri V, Malavé L, Hidalgo M, Alvarez R, Plaza C, Quijano Y, Vicente E. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42:1394-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 16. | Allen PJ, Kuk D, Castillo CF, Basturk O, Wolfgang CL, Cameron JL, Lillemoe KD, Ferrone CR, Morales-Oyarvide V, He J, Weiss MJ, Hruban RH, Gönen M, Klimstra DS, Mino-Kenudson M. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg. 2017;265:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 17. | Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, Lacouture ME. Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67:1025-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 239] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 18. | Miyasaka Y, Ohtsuka T, Kimura R, Matsuda R, Mori Y, Nakata K, Kakihara D, Fujimori N, Ohno T, Oda Y, Nakamura M. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann Surg Oncol. 2019;26:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, Tuveson DA. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 351] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, Tortora G, Van Laethem JL, Young R, Penenberg DN, Lu B, Romano A, Von Hoff DD. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Xu J, Hua J, Liu J, Liang C, Meng Q, Ni Q, Shi S, Yu X. Nab-paclitaxel plus gemcitabine as first-line treatment for advanced pancreatic cancer: a systematic review and meta-analysis. J Cancer. 2019;10:4420-4429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1942] [Article Influence: 277.4] [Reference Citation Analysis (0)] |
| 23. | Li X, Ma T, Zhang Q, Chen YG, Guo CX, Shen YN, Sun PW, Li GG, Gao SL, Que RS, Lou JY, Yu RS, Yuan Y, Wei QC, Wei SM, Zhang Y, Zheng L, Bai XL, Liang TB. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett. 2017;406:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Okusaka T, Furuse J. Recent advances in chemotherapy for pancreatic cancer: evidence from Japan and recommendations in guidelines. J Gastroenterol. 2020;55:369-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Parmar A, Chaves-Porras J, Saluja R, Perry K, Rahmadian AP, Santos SD, Ko YJ, Berry S, Doherty M, Chan KKW. Adjuvant treatment for resected pancreatic adenocarcinoma: A systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2020;145:102817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |