Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2647
Peer-review started: January 14, 2020
First decision: April 8, 2020
Revised: April 25, 2020
Accepted: May 29, 2020
Article in press: May 29, 2020
Published online: June 26, 2020
Processing time: 162 Days and 2.1 Hours
Multiple endocrine neoplasia type 1 (MEN1) is a rare hereditary disorder caused by mutations of the MEN1 gene. It is characterized by hyperparathyroidism and involves the pancreas, anterior pituitary, duodenum, and adrenal gland. Here, we report a 40-year-old male patient with MEN1 who first manifested as thymic carcinoid, then primary hyperparathyroidism and prolactinoma, and a decade later pancreatic neuroendocrine tumor.
The patient underwent a thymectomy because of the thymic carcinoid 10 years prior and a prolactinoma resection 2 years prior. His sister suffered from prolactinoma. His parents displayed a typical triad of amenorrhea, galactorrhea, and infertility. Computed tomography revealed a strong signal in the upper portion of the left lobes and posterior portion of the right lobes of the thyroid and irregular soft tissue densities of the pancreatic body. Positron emission tomography/computed tomography imaging further showed strong 18F-flurodeoxyglucose uptake in the tail of the pancreatic body and segment IV of the liver. The patient underwent pancreatic body tail resection, pancreatic head mass enucleation, and ultrasound-guided radio-frequency ablation for liver cancer. Pathology results reported neuroendocrine tumor grade 2. Whole exome sequencing revealed a verified pathogenic mutation c.378G>A (p.Trp126*) in the MEN1 gene. The diagnosis of MEN1 was confirmed. At the 1.5-year follow-up, the patient appeared healthy without any sign of reoccurrence.
The present case may add some insight into the diagnosis and treatment of patients with MEN1.
Core tip: Genetic screening is recommended in patients with a family history of multiple endocrine neoplasia type 1 who present with primary hyperparathyroidism and pituitary tumors or gastrointestinal neuroendocrine tumors.
- Citation: Ma CH, Guo HB, Pan XY, Zhang WX. Comprehensive treatment of rare multiple endocrine neoplasia type 1: A case report. World J Clin Cases 2020; 8(12): 2647-2654
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2647.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2647
Multiple endocrine neoplasia type 1 (MEN1), a rare hereditary disorder caused by mutations in the tumor suppressor gene MEN1, refers to two or more simultaneous or sequential occurrences of parathyroid adenomas, anterior pituitary adenomas, or entero-pancreatic neuroendocrine tumors (NETs) in an individual[1]. Hormone-secreting and hormone-nonsecreting tumors may occur among some 30 tissues in MEN1[2]. The most common clinical manifestations of MEN1 include parathyroid (> 90%) tumors, followed by pancreatic endocrine tumors (30%-70%) and pituitary tumors (30%-40%)[3]. MEN1 patients also present with other tumors, including adrenocortical tumor, carcinoids, pheochromocytoma, bronchopulmonary NET, thymic NET, gastric NET, lipomas, angiofibromas, collagenomas, and menin-giomas[1,3]. Herein, we report a 40-year-old male patient with MEN1 who first manifested as thymic carcinoid, then primary hyperparathyroidism and prolactinoma 8 years later, followed by a pancreatic NET grade 2 and insulinomas with intrahepatic metastases a decade later.
A 40-year-old male patient experiencing symptoms of hunger, such as fatigue and excessive sweating, for 2 years was admitted to the neurosurgery department at our hospital in August of 2018. The symptoms were relieved with food.
The patient underwent a thymectomy because of thymic carcinoid 10 years ago (July 2008). However, there were intermittent episodes of hypoglycemia symptoms in the following years. The minimum blood glucose level was 2.0 mmol/L. Hypoglycemia-like symptoms can be relieved with glucose infusions. Computed tomography (CT) and magnetic resonance imaging (MRI) findings of the head and neck showed signs of hyperparathyroidism and prolactinoma 2 years prior; thus, the patient underwent a prolactinoma resection (April 2016). Although the surgery was successful, the symptoms of dizziness and hypoglycemia remained unresolved. Therefore, he was readmitted to our department for further treatment and suspected MEN1.
Family history showed that his sister suffered from prolactinoma and underwent gamma knife radiosurgery. His parents, five siblings (sisters), and two offspring displayed the typical triad of amenorrhea, galactorrhea, and infertility.
None.
Laboratory examination findings are summarized in Table 1. The indexes for thyroid-stimulating hormone, β-collagen-specific sequence, total type I collagen amino-terminal extension peptide, fasting insulin, and insulin release were abnormally elevated.
| Items | Factors | Values at admission | Postoperative values | Reference range |
| Thyroid function | Parathyroid hormone | 7.96 pmol/L | 7.11 pmol/L | 1.6-6.9 |
| Thyroid-stimulating hormone | 7.36 μIU/L | 2.24 μIU/L | 0.27-4.2 | |
| Total T4 | 64.68 nmol/L | 79.12 nmol/L | 78.38-157.4 | |
| Pituitary hormone | Prolactin | 17.13 ng/mL | 25.67 ng/mL | 1.61-18.77 |
| Tumor biomarkers | Alpha-fetoprotein | 3.83 ng/mL | - | 0-25 |
| Carbohydrate antigen 19-9 | 15.17 U/mL | - | < 37 | |
| Carcinoembryonic antigen | 3.51 ng/mL | - | < 5 | |
| Bone metabolism | Β-collagen-specific sequence | 1.21 ng/mL | - | 0.3-0.6 |
| Total type I collagen amino-terminal extension peptide | 204.4 ng/mL | - | 20.25-76.31 | |
| Diabetes | Fasting insulin | 55.29 μU/mL | 9.95 μU/mL | 2.6-24.9 |
| Fasting blood glucose | 1.79 mmol/L | 3.98 mmol/L | 3.9-6.1 | |
| Fasting C-peptide | 5.06 ng/mL | 1.81 ng/mL | 1.6-6.9 | |
| Insulin release index | 1.31 | 0.2 | < 0.3 |
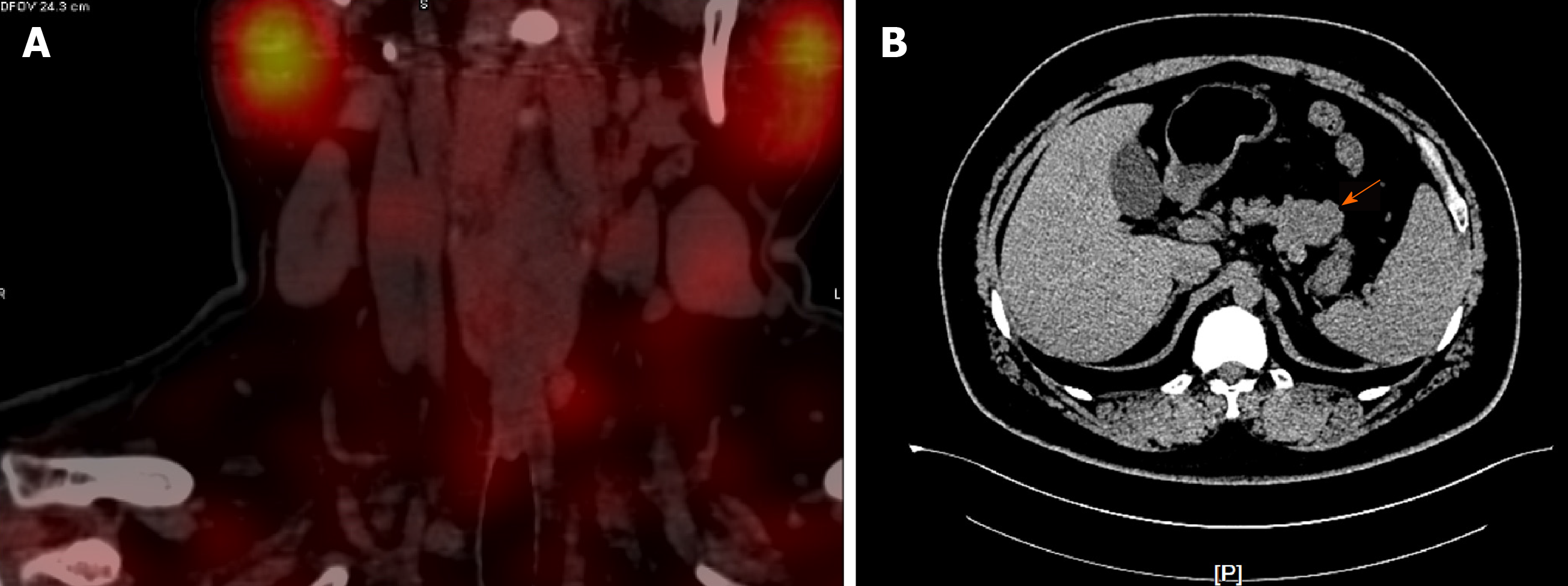
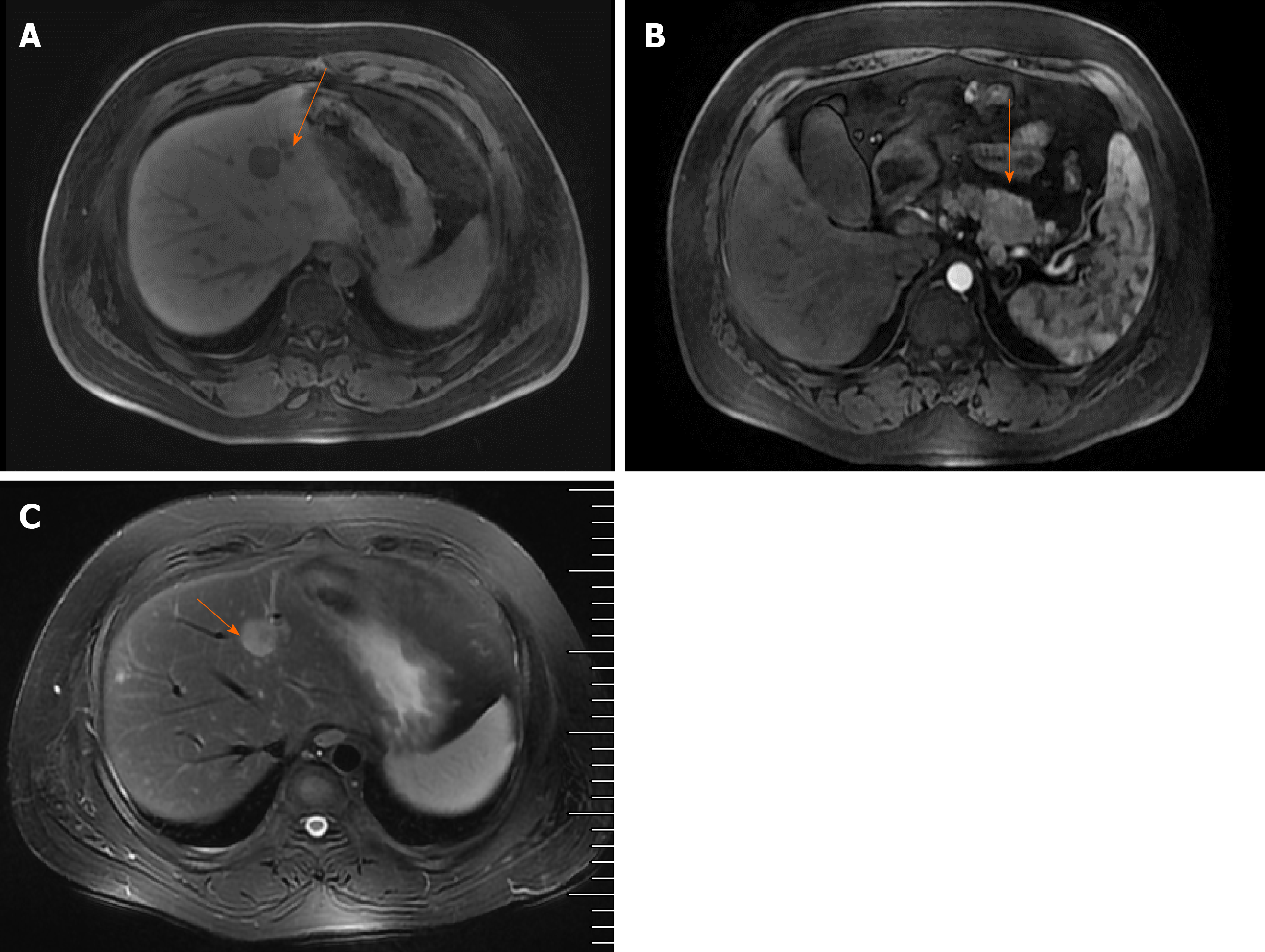
Parathyroid two-phase CT revealed a strong signal in the upper portion of the left lobes and posterior portion of the right lobes of the thyroid, in addition to the parathyroid glands (Figure 1A). Pancreatic perfusion CT imaging showed irregular soft tissue densities of the pancreatic body, which was closely associated with the adjacent stomach wall, with a small reduction in blood volume (BV) and a slight increase in flow extraction product. There was increased blood flow, BV, mean transit time, and flow extraction product in nodules posterior to the pancreatic body; increased blood flow and decreased BV in nodules of the pancreatic head; and multiple circular low or slightly low-density shadows in liver parenchyma (Figure 1B). MRI of the liver and pancreas revealed multiple abnormal signals in liver segment II, segment VIII, and the junction area of liver segments II and IV as well as occupied lesions in the tail of the pancreatic body (Figure 2A). Enhanced MRI revealed occupied lesions in the body and tail of the pancreas (Figure 2B). Enhanced MRI revealed that liver segment IV was occupied, suggesting the possibility of angiomyolipoma. In addition, there were small cysts in segments II and VIII of the liver and occupied lesions in the tail of the pancreatic body (Figure 2C).
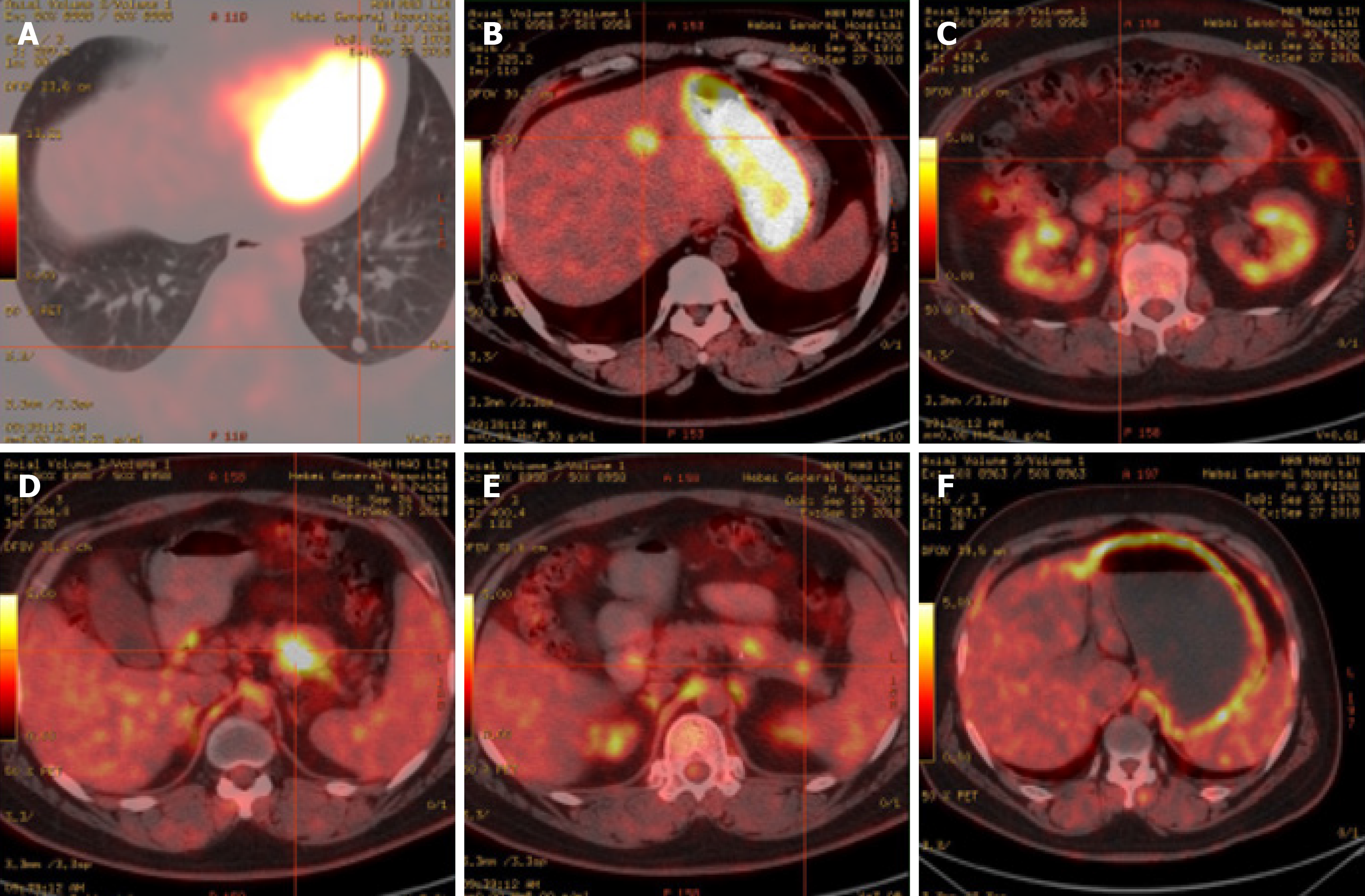
Positron emission tomography (PET)/CT imaging (Figure 3) further showed strong 18F-flurodeoxyglucose uptake in the tail of the pancreatic body and segment IV of the liver, indicating the presence of insulinomas with intrahepatic metastases. There were several circular nodules visible in the right and left lungs, showing partially increased signals in them. After undergoing complete surgical resection of the thymoma, ring-shaped hypermetabolism became visible around the aortic root, which may be a result of fat intake.
A multiple disciplinary team (MDT) consisting of an endocrinologist, oncologist, pathologist, radiologist, thoracic surgeon, and hepatobiliary surgeon was incorporated to manage this patient. MEN1 with thymic carcinoid, thymoma, parathyroid and insulinomas with intrahepatic metastases was initially diagnosed.
Since there were no obvious surgical contraindications, the MDT recommended a radical excision of the lesions to alleviate hypoglycemia symptoms and improve the quality of life for the patient. The patient underwent a resection of the tail of the pancreatic body, enucleation of the pancreatic head mass, and ultrasound-guided radiofrequency ablation for liver cancer. During the operation, a nodule with a diameter of about 0.4 cm was observed in the diaphragmatic surface of the right liver lobe. A mass of about 0.5 cm × 0.5 cm was palpable in the right posterior liver lobe. In addition, a tumor of about 2 cm × 2 cm in the falciform ligament about 4 cm from the hepatic margin, a mass of 1.5 cm × 1.0 cm on the pancreatic head, a mass of 5 cm × 3 cm × 3 cm on the pancreatic body, and a mass of 1.5 cm × 1 cm on the pancreatic tail were found. The intraoperative ultrasonography findings from the liver scan showed the right lobe nodules as cysts, and ultrasound-guided segment IV liver biopsy and radiofrequency ablation were performed.
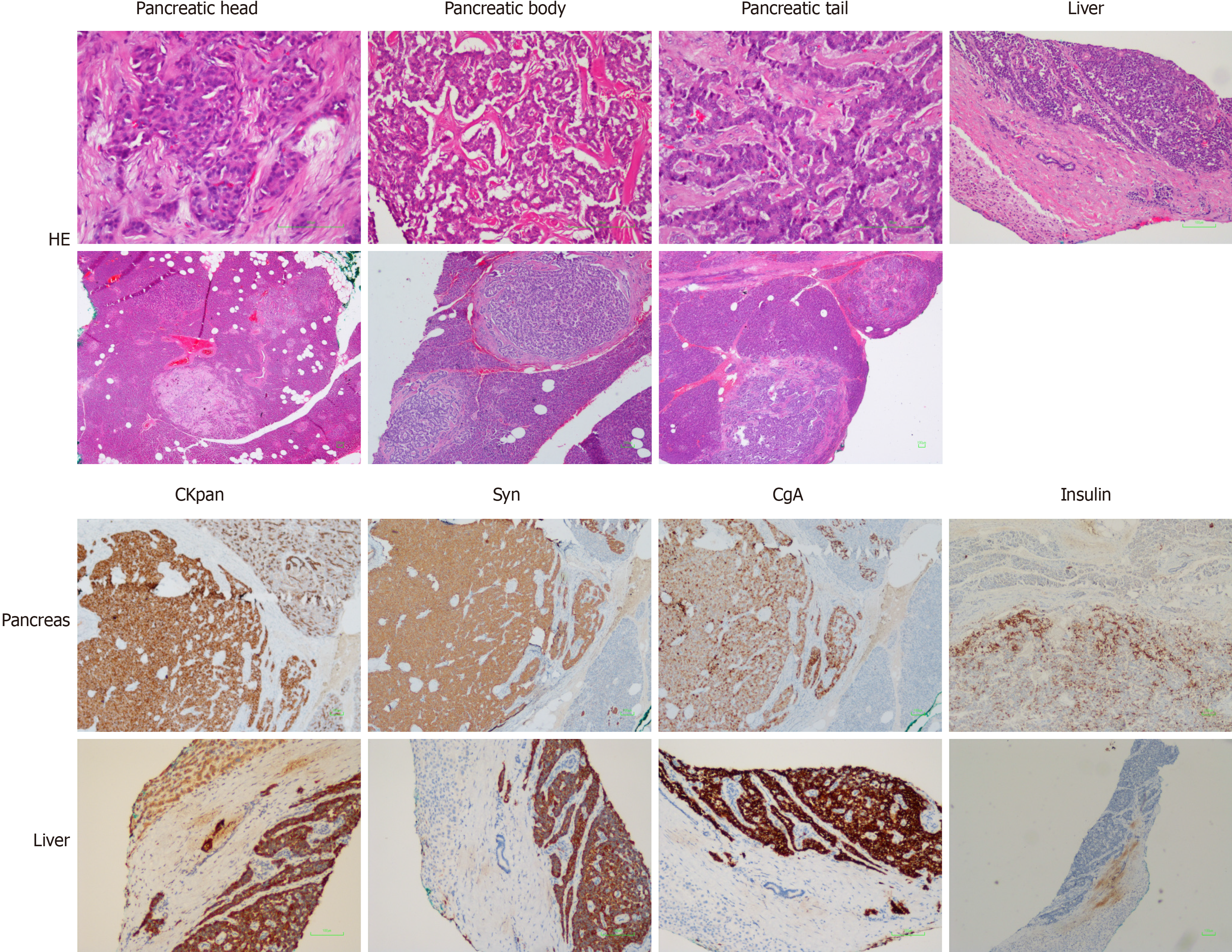
After surgery, all diabetes factors with abnormal serum levels were returned to normal levels (Table 1). The pathological findings are shown in Figure 4. The pancreatic tissues showed CKpan (+), synaptophysin (+), chromogranin A (+), partial CD56 (+), p53 (-), partial PGP9.5 (+), partial SSTR2 (+), CD10 (-), partial vimentin (+), a mitotic count of 6/10 high power fields, and Ki-67 index of about 8%-20%, which confirmed the diagnosis of the pancreas NET grade 2. The liver tissues showed CKpan (+), synaptophysin (+), chromogranin A (+), partial CD56 (+), p53 (-), PGP9.5 (+), a mitotic count of 4/10 high power fields, and Ki-67 index of about 10%-20%, which confirmed the diagnosis of liver NET grade 2. Moreover, there were multiple tiny nodules around the pancreatic tissues, indicating that surgical resection could not completely remove the lesions. NET grade 2 was diagnosed according to the World Health Organization 2010 classifications for gastrointestinal and pancreatic NETs[4]. Furthermore, whole-exome sequencing revealed a verified pathogenic mutation c.378G>A (p.Trp126*) in the MEN1 gene (reference sequence NM_130799.2). Therefore, the final diagnosis of MEN1 was confirmed.
The patient was free of hunger symptoms. During the follow-up visit at 6 mo, single-photon emission CT/CT revealed multiple small nodules in the lungs. After comparing the single-photon emission CT/CT scans with the PET/CT from 4 mo prior, these nodules were suspected of being metastatic lesions. However, the nodules were too small to undergo needle biopsy, so the patient was placed under observation without additional treatment. At the last follow-up in January of 2020, the patient appeared healthy without any signs of disease reoccurrence.
MEN1 is characterized by primary hyperparathyroidism caused by a parathyroid tumor and involves the pancreas, anterior pituitary, duodenum, and adrenal gland. The annual incidence rate of MEN1 is about 1 in 30000[2]. Approximately 25% of patients with thymic carcinoids are also diagnosed with MEN1[5,6], and in patients with MEN1, the incidence of thymic carcinoids is between 2% and 8%, with the disease most commonly occurring in patients 38 to 49 years of age[7,8]. The patient in this study suffered from tumors of multiple organs, including the thymus, parathyroid, pituitary, pancreas, and liver. He first presented with thymic carcinoid, then primary hyperparathyroidism and prolactinoma, and later pancreatic NET with intrahepatic metastases. Moreover, the interval between thymic carcinoid and pancreatic NET was 10 years. Since the diagnosis of the thymic carcinoid, the patient had been alive for more than 11 years. MEN1-insulinoma accounts for about 10% of pancreatic NET cases. The average age of onset is less than 40 years old, oftentimes less than 20 years old, while non-MEN1 insulinoma patients are more than 40 years old[9]. The symptoms of hypoglycemia often appear on an empty stomach or after physical activity, which can be relieved with food. The 72 h starvation test is the most reliable diagnostic method to demonstrate that the hypoglycemia is caused by high insulin[3]. Ultrasound, CT, MRI, and PET/CT are often used for preoperative localization of lesions, and intraoperative ultrasound can improve the success rate of surgery[3]. Endoscopic ultrasound (EUS) can be used to differentiate MEN1-related pancreas NET from sporadic pancreas NET[10,11]. Moreover, preoperative biopsy through EUS/fine needle aspiration is useful for determining the differentiation status of pancreas NET[12-14]. A well-differentiated grade 1/2 tumor is one of the primary surgical indications for pancreas NET management[15]. However, the preoperative diagnosis of pancreas NET is relatively clear based on the patient's medical history, insulin level, Whipple’s triad, CT, MRI, and PET/CT findings. Hence, EUS was not performed, which is a limitation of the present case.
Generally, patients with MEN1 should be managed by a MDT consisting of relevant specialists from endocrinology, radiology, oncology, pathology, and surgery[3]. For this disease, intraoperative monitoring of the insulin release index is useful for determining whether the tumor is successfully removed. Chemotherapy is recommended for patients with metastatic insulinomas and may include streptomycin, 5-fluorouracil, doxorubicin, or hepatic artery embolization[16-18]. The patient in this study suffered from tumors of multiple organs, including the pancreas, parathyroid, and pituitary. The progression of MEN1 was successfully controlled by MDT in the current study.
In conclusion, we presented a case of MEN1 that appeared sequentially as thymic carcinoid, primary hyperparathyroidism and prolactinoma, and pancreatic NET with intrahepatic metastases. Through the consultation of MDT for complete preoperative diagnosis and postoperative treatment and management, the patient was successfully treated in our clinic. If a patient is diagnosed with primary hyperparathyroidism with pituitary tumors or gastrointestinal NET, and the family history shows similar symptoms, MEN1 should be considered, and genetic testing is recommended. We hope that this study will add new insight into the diagnosis and treatment of patients with MEN1.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fusaroli P, Iso Y S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Agarwal SK. The future: genetics advances in MEN1 therapeutic approaches and management strategies. Endocr Relat Cancer. 2017;24:T119-T134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Marx SJ. Recent Topics Around Multiple Endocrine Neoplasia Type 1. J Clin Endocrinol Metab. 2018;103:1296-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 880] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 4. | Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Thakker RV. Multiple endocrine neoplasia type 1 (MEN1). Best Pract Res Clin Endocrinol Metab. 2010;24:355-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Litvak A, Pietanza MC. Bronchial and Thymic Carcinoid Tumors. Hematol Oncol Clin North Am. 2016;30:83-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Singh Ospina N, Thompson GB, C Nichols F, Cassivi SD, Young WF Jr. Thymic and Bronchial Carcinoid Tumors in Multiple Endocrine Neoplasia Type 1: The Mayo Clinic Experience from 1977 to 2013. Horm Cancer. 2015;6:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | de Laat JM, Pieterman CR, van den Broek MF, Twisk JW, Hermus AR, Dekkers OM, de Herder WW, van der Horst-Schrivers AN, Drent ML, Bisschop PH, Havekes B, Vriens MR, Valk GD. Natural course and survival of neuroendocrine tumors of thymus and lung in MEN1 patients. J Clin Endocrinol Metab. 2014;99:3325-3333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Brandi ML, Gagel RF, Angeli A, Bilezikian JP, Beck-Peccoz P, Bordi C, Conte-Devolx B, Falchetti A, Gheri RG, Libroia A, Lips CJ, Lombardi G, Mannelli M, Pacini F, Ponder BA, Raue F, Skogseid B, Tamburrano G, Thakker RV, Thompson NW, Tomassetti P, Tonelli F, Wells SA Jr, Marx SJ. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 906] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 10. | Tamagno G, Scherer V, Caimo A, Bergmann SR, Kann PH. Endoscopic Ultrasound Features of Multiple Endocrine Neoplasia Type 1-Related versus Sporadic Pancreatic Neuroendocrine Tumors: A Single-Center Retrospective Study. Digestion. 2018;98:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Kappelle WF, Valk GD, Leenders M, Moons LM, Bogte A, Siersema PD, Vleggaar FP. Growth rate of small pancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: results from an endoscopic ultrasound based cohort study. Endoscopy. 2017;49:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Fusaroli P, Kypreos D, Alma Petrini CA, Caletti G. Scientific publications in endoscopic ultrasonography: changing trends in the third millennium. J Clin Gastroenterol. 2011;45:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Jenssen C, Hocke M, Fusaroli P, Gilja OH, Buscarini E, Havre RF, Ignee A, Saftoiu A, Vilmann P, Burmester E, Nolsøe CP, Nürnberg D, D'Onofrio M, Lorentzen T, Piscaglia F, Sidhu PS, Dietrich CF. EFSUMB Guidelines on Interventional Ultrasound (INVUS), Part IV - EUS-guided interventions: General Aspects and EUS-guided Sampling (Short Version). Ultraschall Med. 2016;37:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Fusaroli P, Napoleon B, Gincul R, Lefort C, Palazzo L, Palazzo M, Kitano M, Minaga K, Caletti G, Lisotti A. The clinical impact of ultrasound contrast agents in EUS: a systematic review according to the levels of evidence. Gastrointest Endosc. 2016;84:587-596.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA, Minter RM, Lairmore TC, Tseng JF, Zeh HJ, Libutti SK, Singh G, Lee JE, Hope TA, Kim MK, Menda Y, Halfdanarson TR, Chan JA, Pommier RF. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:1-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 16. | Câmara-de-Souza AB, Toyoshima MTK, Giannella ML, Freire DS, Camacho CP, Lourenço DM Jr, Rocha MS, Bacchella T, Jureidini R, Machado MCC, Almeida MQ, Pereira MAA. Insulinoma: A retrospective study analyzing the differences between benign and malignant tumors. Pancreatology. 2018;18:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Pavlidis TE, Psarras K, Symeonidis NG, Pavlidis ET, Sakantamis AK. Current surgical management of pancreatic endocrine tumor liver metastases. Hepatobiliary Pancreat Dis Int. 2011;10:243-247. [PubMed] |
| 18. | Pasaoglu E, Dursun N, Ozyalvacli G, Hacihasanoglu E, Behzatoglu K, Calay O. Comparison of World Health Organization 2000/2004 and World Health Organization 2010 classifications for gastrointestinal and pancreatic neuroendocrine tumors. Ann Diagn Pathol. 2015;19:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |