Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2641
Peer-review started: March 9, 2020
First decision: April 22, 2020
Revised: May 26, 2020
Accepted: May 28, 2020
Article in press: May 28, 2020
Published online: June 26, 2020
Processing time: 106 Days and 14.5 Hours
Pleomorphic rhabdomyosarcoma (RMS) of the spermatic cord is a group of rare neoplasms, and a secondary hydrocele testis occasionally occurs. The misdiagnosis of paratesticular mass may lead to a therapeutic delay.
A 79-year-old man presented to our clinic complaining of a 1-mo history of painless scrotal swelling. Physical examination revealed approximately a 15 cm × 10 cm × 5 cm inguinal mass with limited mobility. Contrast-enhanced magnetic resonance imaging showed a hydrocele testis, several enlarged inguinal lymph nodes, and a heterogeneously enhanced lesion with a relatively well-defined margin in the left inguinal region. Due to the imaging findings, he was diagnosed with pleomorphic RMS and received a wide resection of the mass, an inguinal incision with a high section of the left spermatic cord, and a left radical orchiectomy. He experienced local relapse 1 mo postoperatively and received radiotherapy and anlotinib hydrochloride-based immunotherapy as adjuvant therapy. The patient died 3 mo after the surgery.
The optimal interventions for advanced-stage pleomorphic RMS patients should be investigated by more preclinical studies and clinical trials. Physicians need to be aware of the occurrence of pleomorphic RMS in unusual locations, especially when accompanied by a hydrocele testis.
Core tip: Pleomorphic rhabdomyosarcoma of the spermatic cord is rare and develops commonly in adults, which is still clinically challenging for definitive diagnosis and differentiation from other pathologies, especially when accompanied by a secondary hydrocele testis. The present case report as well as state-of-the-art literature review highlights the importance of comprehensive appreciation of laboratory, radiographic, and pathologic findings for the ultimate differential diagnosis of spermatic cord rhabdomyosarcoma.
- Citation: Chen X, Zou C, Yang C, Gao L, Bi LK, Xie DD, Yu DX. Pleomorphic rhabdomyosarcoma of the spermatic cord and a secondary hydrocele testis: A case report. World J Clin Cases 2020; 8(12): 2641-2646
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2641.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2641
Rhabdomyosarcoma (RMS) is a highly malignant sarcoma originating from immature striated muscle, which mostly occurs in children[1,2]. Paratesticular RMS accounts for about 7% of all the rhabdomyosarcomas[3]. More than 200 cases of paratesticular RMS have been reported, including tumors of the testis, epididymis, and spermatic cord[2-7]. Although the therapeutic outcome of paratesticular RMS has been remarkably improved, it still depends on the tumor grade, distant disease, and tumor cell histology[4,5]. The 5-year overall survival rate of confined paratesticular RMS after combined therapeutic arms including surgery, radiotherapy, and chemotherapy was 94.6%[6].
Pleomorphic RMS of the spermatic cord is one of the rarest histological types of paratesticular RMS and develops commonly in adults. Currently, only case reports with limited patient numbers and expert opinion are available for therapeutic instruction. The diagnosis of pleomorphic RMS of the spermatic cord is based on histological and specific immunohistochemistry features of the tumors. The imagological diagnosis of pleomorphic RMS of the spermatic cord is still challenging if the mass is not confined to paratesticular tissue, and is especially mislead by a concomitant secondary hydrocele.
We herein report a case of pleomorphic RMS of the spermatic cord with a secondary hydrocele testis, treated by radical orchiectomy, high ligation of the spermatic cord, radiotherapy, and immunotherapy. An up-to-date literature review of pleomorphic RMS of the spermatic cord was also performed.
A 79-year-old man presented to our clinic and complained of a 1-mo history of painless scrotal swelling.
A previous computed tomography (CT) scan at a local hospital identified a left 3 cm × 2 cm × 3 cm inguinal mass. The patient did not receive any treatment before he visited our clinic. His sleep and diet were not disturbed in the past 3 mo.
The patient was in good health in the past and only received an appendectomy 40 years ago.
The patient reported no perceptible personal conditions related to the present clinical manifestation. Family history was also not noticeable.
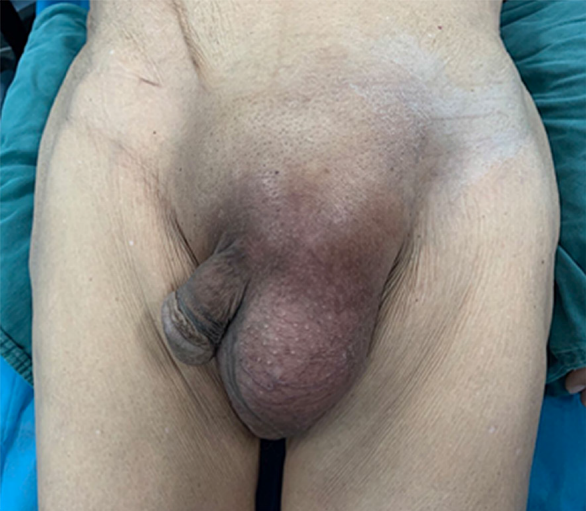
Physical examination revealed approximately a left 15 cm × 10 cm × 5 cm inguinal mass with limited mobility (Figure 1). The scrotum was irreducible, the left testicle was not palpable, and transillumination test was positive.
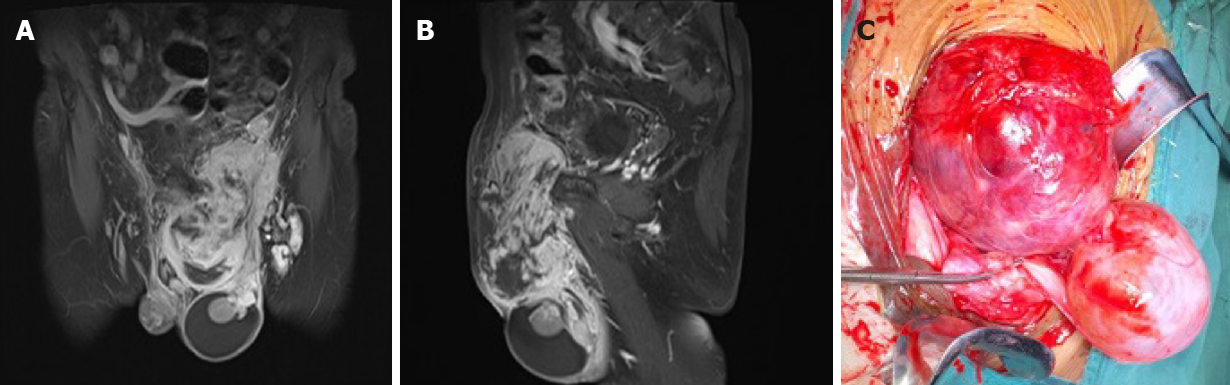
Laboratory tests showed elevated serum β-human chorionic gonadotropin (β-HCG; 240.70 mIU/mL) and squamous cell carcinoma antigen (3.20 ng/mL). Contrast-enhanced magnetic resonance imaging (MRI) showed a hydrocele testis, several enlarged inguinal lymph nodes, and a heterogeneously enhanced lesion (15 cm × 10 cm × 6 cm) with a relatively well-defined margin in the left inguinal region (Figure 2A and B).
The final diagnosis of the presented case was pleomorphic RMS of the spermatic cord and a secondary hydrocele testis.
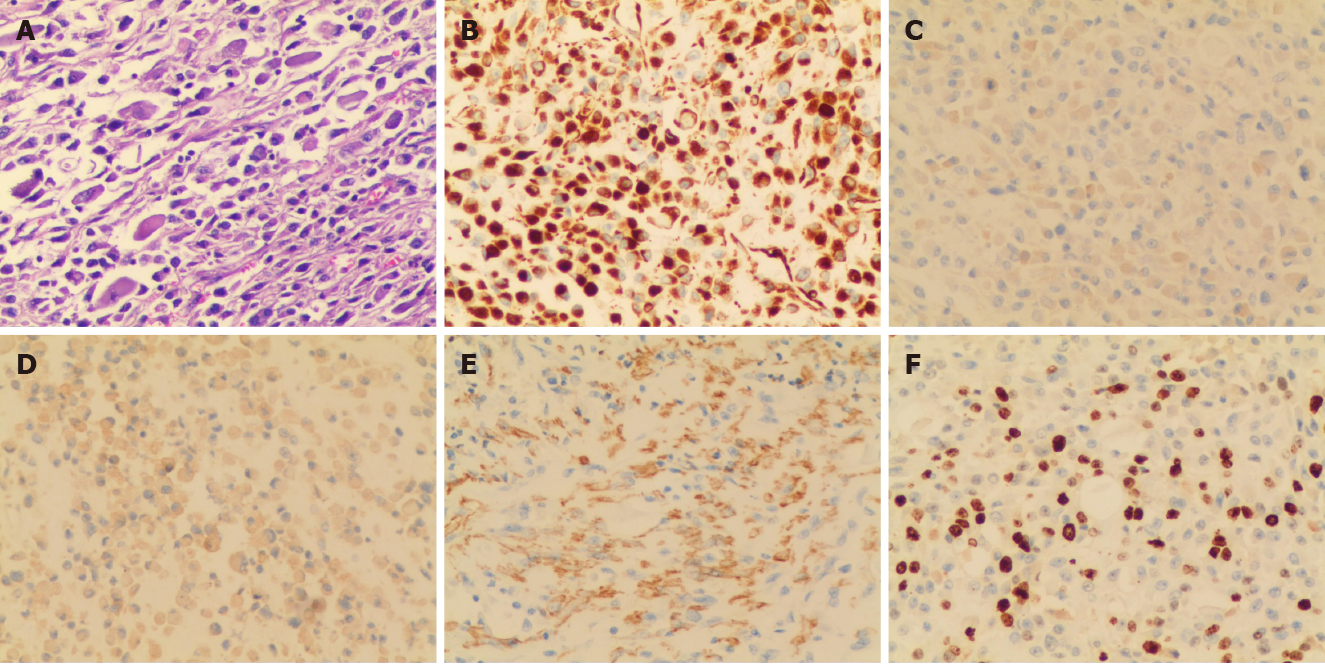
The patient received a wide resection of the mass, an inguinal incision with a high section of the left spermatic cord, and a left radical orchiectomy. Intraoperatively, the encapsulated tumor was found across the spermatic cord and extending into the scrotum, but without invasion to the testis or epididymis (Figure 2C). Pathological analysis identified that the mass was composed of sheets of giant atypical and pleomorphic cells with abundant eosinophilic cytoplasm, which led to a diagnosis of pleomorphic RMS (Figure 3A). Immunohistochemical examination showed a strong positivity for vimentin and Ki-67 (approximately 40%), as well as a weak positivity for desmin, smooth muscle actin (SMA), and myogenin (Figure 3B-F). According to the Intergroup Rhabdomyosarcoma Study Group staging classification, the tumor was in clinical group IIb and clinically staged as T2bN1M0.
The patient experienced local relapse 1 mo postoperatively. With a Karnofsky performance score of 40, the patient received combined radiotherapy and anlotinib hydrochloride-based immunotherapy without additional chemotherapies. Regretfully, the patient died from multiple organ failure 3 mo postoperatively.
Spermatic cord RMS originates from mesenchymal components of the spermatic cord, with a poor prognosis[8]. It was commonly reported in old men[7], but exceptional cases involved male teenagers[3]. Pleomorphic RMS of the spermatic cord is the rarest type of RMS (about 7% of all RMS and about 6% of all paratesticular tumours) with a high metastasis rate and remains a significant challenge for clinical diagnosis and treatment[8,9].
MRI may provide significant information of tumor outline and adjacent bordering. The classic MRI feature of pleomorphic RMS of the spermatic cord is a paratesticular heterogeneously enhanced mass with or without compressing the testis. A paratesticular mass may be diagnosed empirically with hernia[10], or hydrocele[11], which potentially leads to a therapeutic delay. An ultrasound scan may echographically distinguish them. Tumors involving the scrotum might be masqueraded as reproductive system tumors. A secondary hydrocele testis occasionally accompanies the spermatic cord mass, which was only reported in less than ten cases since 2007 (Table 1)[10-17]. To the best of our knowledge, hydrocele testis secondary to pleomorphic RMS of the spermatic cord was not reported previously. Microscopically, the pleomorphic RMS was featured as a pleomorphic architecture of atypical/round rhabdomyoblasts and spindle cells[7]. Its determinant diagnosis is largely based on the positive immunostaining for several highly sensitive and specific markers, such as vimentin, desmin, myoglobin, myosin, and SMA[7,18]. Serum β-HCG generally tends to be normal in RMS patients, however, elevated β-HCG in our case should be highlighted as a potential diagnostic pitfall and raises the differential diagnosis from other germ cell tumors[19].
| Ref. | Yr | Patient’s age (yr) | Side | Histologic diagnosis | Follow-up (mo after surgery) | Outcome |
| Li et al[12] | 2007 | 71 | Right | Lymphangioma | 3 | ANED |
| Lim et al[11] | 2007 | 6 | Right | Sparganosis | NS | NS |
| Domsa et al[13] | 2008 | 75 | - | Mixed liposarcoma | NS | NS |
| Chang et al[14] | 2009 | 38 | Right | Klatskin tumor | 5 | ANED |
| Uehara et al[15] | 2010 | 59 | Left | Liposarcoma | NS | NS |
| Park et al[16] | 2011 | 65 | Left | Mesothelioma | 6 | Died |
| Soda et al[17] | 2012 | 67 | Bilateral | Mesothelioma | 3 | Died |
| Londeree et al[10] | 2014 | 70 | Right | Liposarcoma | 18 | ANED |
Most of therapeutic information on pleomorphic RMS was available from some individual institutions[2-4,7]. A wide resection of tumor with a radical inguinal orchiectomy, followed by adjuvant therapy (e.g., chemotherapy or radiotherapy) was the gold standard therapeutic arm for patients with a localized tumor. This is due to the fact that the tumor commonly recurs locally and invades the pelvic cavity through internal inguinal ring. However, the actual prognosis is dependent on the histology and metastatic stage of the tumors[7]. Lack of pain in the mass contributed sometimes to therapeutic delays. A wide resection of tumors with radical inguinal orchiectomy followed by VAC (vincristine, actinomycin D, and cyclophosphamide) based chemotherapy is the gold standard treatment for localized tumors[20]. The radiotherapy was recommended for controlling tumor local recurrence and remote metastasis[20,21]. The combination of these therapeutic arms may increase the overall survival of RMS towards about 70%-90%[4,22]. A poor prognosis cannot be avoided for advanced tumors. Since chemotherapy was not recommended for cases with a low Karnofsky performance score (< 70), the present patient received only combined radiotherapy and immunotherapy after the surgery[23]. Emerging immunotherapy based on molecular mechanism of RMS is believed to improve the long-term survival of these patients[24]. However, more preclinical studies and clinical trials are warranted to identify the optimal interventions for patients in advanced stages of this disease.
Only scarce reports of paratesticular RMS have been reported, mainly in the form of single neoplasia without a secondary hydrocele testis. Although multi-disciplinary approach including surgery, radiotherapy, and chemotherapy/immunotherapy is performed to maximize survival, an early diagnosis should also be confirmed. Physicians need to be aware of the occurrence of pleomorphic RMS in unusual locations, especially when accompanied by a hydrocele testis.
We thank our colleagues for data processing: Dr. Zhen Hu at Department of Radiology, Dr. Ji-Feng Wu at Department of Pathology, and Dr. Ming-Zhu Gao at Department of Oncology of the Second Hospital of Anhui Medical University, Hefei, China.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tzamaloukas AHH S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Chui CH, Billups CA, Pappo AS, Rao BN, Spunt SL. Predictors of outcome in children and adolescents with rhabdomyosarcoma of the trunk--the St Jude Children's Research Hospital experience. J Pediatr Surg. 2005;40:1691-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Boulma R, Gargouri MM, Sallemi A, Chlif M, Fitouri Z, Kallel Y, Nouira Y. Paratesticular pleomorphic rhabdomyosarcoma: a report of two cases. Case Rep Urol. 2013;2013:807979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Boudahna L, Benbrahim Z, Amaadour L, Mazouz A, Benhayoune K, Tahiri Y, Farih MH, Amarti A, Arifi S, Mellas N. Para testicular rhabdomyosarcoma in adults: three case reports and review of literature. Pan Afr Med J. 2014;19:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Rodríguez D, Barrisford GW, Sanchez A, Preston MA, Kreydin EI, Olumi AF. Primary spermatic cord tumors: disease characteristics, prognostic factors, and treatment outcomes. Urol Oncol. 2014;32:52.e19-52.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Kumar R, Kapoor R, Khosla D, Kumar N, Ghoshal S, Mandal AK, Radotra BD, Sharma SC. Paratesticular rhabdomyosarcoma in young adults: A tertiary care institute experience. Indian J Urol. 2013;29:110-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ferrari A, Bisogno G, Casanova M, Meazza C, Piva L, Cecchetto G, Zanetti I, Pilz T, Mattke A, Treuner J, Carli M. Paratesticular rhabdomyosarcoma: report from the Italian and German Cooperative Group. J Clin Oncol. 2002;20:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Furlong MA, Mentzel T, Fanburg-Smith JC. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol. 2001;14:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 141] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Khoubehi B, Mishra V, Ali M, Motiwala H, Karim O. Adult paratesticular tumours. BJU Int. 2002;90:707-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Stewart LH, Lioe TF, Johnston SR. Thirty-year review of intrascrotal rhabdomyosarcoma. Br J Urol. 1991;68:418-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Londeree W, Kerns T. Liposarcoma of the spermatic cord masquerading as an inguinal hernia. Case Rep Med. 2014;2014:735380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Lim Dh, Kim CS, Kim SI. Sparganosis presenting as spermatic cord hydrocele in six-year-old boy. Urology. 2007;70:1223.e1-1223.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Li DJ, Xu YT, Guo WH, Huang RQ, Gu W, Xu XW, Xie M, Jing YF. [Cystic lymphangioma of the spermatic cord in old man: a case report and review of the literature]. Zhonghua Nan Ke Xue. 2007;13:815-817. [PubMed] |
| 13. | Domşa I, Olinici CD, Crişan D. Spermatic cord mixed liposarcoma. Case report and review of the literature. Rom J Morphol Embryol. 2008;49:105-109. [PubMed] |
| 14. | Chang YH, Chuang CK, Ng KF, Liao SK. Klatskin tumor with spermatic cord metastasis: a case report. Chang Gung Med J. 2009;32:104-107. [PubMed] |
| 15. | Uehara M, Takeda K, Tei H, Shimizu K, Imazu T, Yoshimura K, Kiyohara H. [A case of liposarcoma of spermatic cord with a hydrocele]. Hinyokika Kiyo. 2010;56:127-129. [PubMed] |
| 16. | Park YJ, Kong HJ, Jang HC, Shin HS, Oh HK, Park JS. Malignant mesothelioma of the spermatic cord. Korean J Urol. 2011;52:225-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Soda T, Yamanaka K, Hirai T, Kishikawa H, Nishimura K, Ichikawa Y. [A case of malignant peritoneal mesothelioma presenting with bilateral swelling of spermatic cord]. Hinyokika Kiyo. 2012;58:177-180. [PubMed] |
| 18. | Xi S, Tong W. Pleomorphic rhabdomyosarcoma metastasis to small intestine causing intussusception: A case report. Medicine (Baltimore). 2018;97:e13648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Maryamchik E, Lyapichev KA, Halliday B, Rosenberg AE. Dedifferentiated Liposarcoma With Rhabdomyosarcomatous Differentiation Producing HCG: A Case Report of a Diagnostic Pitfall. Int J Surg Pathol. 2018;26:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Raney RB, Maurer HM, Anderson JR, Andrassy RJ, Donaldson SS, Qualman SJ, Wharam MD, Wiener ES, Crist WM. The Intergroup Rhabdomyosarcoma Study Group (IRSG): Major Lessons from the IRS-I through IRS-IV Studies as Background for the Current IRS-V Treatment Protocols. Sarcoma. 2001;5:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Wolden SL, Anderson JR, Crist WM, Breneman JC, Wharam MD Jr, Wiener ES, Qualman SJ, Donaldson SS. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999;17:3468-3475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | McDowell HP. Update on childhood rhabdomyosarcoma. Arch Dis Child. 2003;88:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | West HJ, Jin JO. JAMA Oncology Patient Page. Performance Status in Patients with Cancer. JAMA Oncol. 2015;1:998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 24. | Crose LE, Linardic CM. Receptor tyrosine kinases as therapeutic targets in rhabdomyosarcoma. Sarcoma. 2011;2011:756982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |