Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2617
Peer-review started: April 13, 2020
First decision: April 28, 2020
Revised: May 5, 2020
Accepted: May 21, 2020
Article in press: May 21, 2020
Published online: June 26, 2020
Processing time: 71 Days and 18.3 Hours
Thrombotic thrombocytopenic purpura (TTP), a subtype of thrombotic microangiopathy, has a very high fatality rate if there is no timely diagnosis or treatment. Here, we report a case of TTP refractory to high displacement plasma exchange, which was later successfully treated with rituximab.
Here we report a case of refractory TTP in a 63-year-old woman with a low platelet count and decreased ADAMTS13 activity. Her platelet count was 9 × 109/L, hemoglobin level was 81 g/L, and ADAMTS13 was < 5%. She was diagnosed with thrombotic thrombocytopenic purpura. After 8 d of daily plasma exchange (PEX), her platelet levels were still low. However, after 6 d of treatment with rituximab, her platelet count increased and ADAMTS13 activity returned to normal.
PEX can cure most patients, but the relapse rate can be up to 50%-60%. This case suggested that rituximab can improve the curative efficiency of PEX and prevent disease relapse in TTP.
Core tip: Thrombotic thrombocytopenic purpura (TTP) has a very high fatality rate if there is no timely diagnosis or treatment. Here we report a case of refractory TTP in a woman with decreased ADAMTS13 activity. Although the patient was refractory to daily plasma exchange, her platelet count and ADAMTS13 activity returned to normal after 6 d of treatment with rituximab. This case suggested that rituximab can improve the curative efficiency of plasma exchange in TTP.
- Citation: Chen J, Jin JX, Xu XF, Zhang XX, Ye XN, Huang J. Successful treatment of plasma exchange-refractory thrombotic thrombocytopenic purpura with rituximab: A case report. World J Clin Cases 2020; 8(12): 2617-2622
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2617.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2617
Thrombotic thrombocytopenic purpura (TTP), a subtype of thrombotic microangiopathy, is characterized by microangiopathic hemolytic anemia (MAHA) and thrombocytopenia with or without neurological symptoms, renal damage, and fever[1]. Its pathological features include severe diffuse thrombotic microangiopathy, MAHA, platelet consumption, and extensive microthrombus formation. Lesions can occur in multiple organs, such as the kidney and central nervous system. The typical clinical manifestations of TTP are thrombocytopenic purpura, MAHA, central nervous system symptoms, fever, and renal damage. Some patients may suffer from the first three symptoms only, known as “triad syndrome”. The reduced activity of ADAMT13 is the main mechanism responsible for the pathogenesis of TTP[2]. TTP has an acute onset and rapid progression, and misdiagnosis and missed diagnosis often occur. The fatality rate is very high if there is no timely diagnosis or treatment[3]. Herein, we report a case of TTP refractory to plasma exchange, which was later successfully treated with rituximab.
A 63-year-old woman was admitted to our hospital on May 23, 2018 due to weakness for 10 d and unconsciousness for 6 d.
In the 10 d before admission (May 13, 2018), the patient was weak (platelets, 21 × 109/L; hemoglobin, 91 g/L). Her bone marrow test at another hospital showed a moderate number of megakaryocytes with poor function. She was administered with immunosuppressive therapy with dexamethasone. However, scattered ecchymosis in the whole body, irritability, aphasia, and gradual unconsciousness occurred on May 19, 2018.
The patient had no remarkable previous medical history.
The physical examination revealed unconsciousness, lethargy, and scattered ecchymosis in the whole body.
On May 20, 2018, the patient had a platelet count of 9 × 109/L, hemoglobin level of 81 g/L, and ADAMTS13 activity < 5%. She was given immediate treatment with fresh frozen plasma infusion (400 mL) and methylprednisolone 40 mg. However, her coma deepened on May 23, 2018. Upon admission, she had a white blood cell count of 11.5 × 109/L, hemoglobin level of 74 g/L, and platelet count of 19 × 109/L.
A diagnosis of TTP was finally made.
Plasma exchange (PEX) with fresh frozen plasma (2000 mL) was started on May 23, 2018 using a PrismaFlex 113080 system (PA14860; Gambro Lundia AB). On the early morning of the next day, convulsion and delirium developed, and the patient was transferred to the intensive care unit. She underwent tracheal cannulation, PEX, and treatment with methylprednisolone 40 mg. Citrate was used as an anticoagulant during the procedures. After PEX (fresh frozen plasma 2500-3000 mL) was performed once a day for seven times in the intensive care unit, the patient was still unconscious, and her platelet level was still < 20 × 109/L.
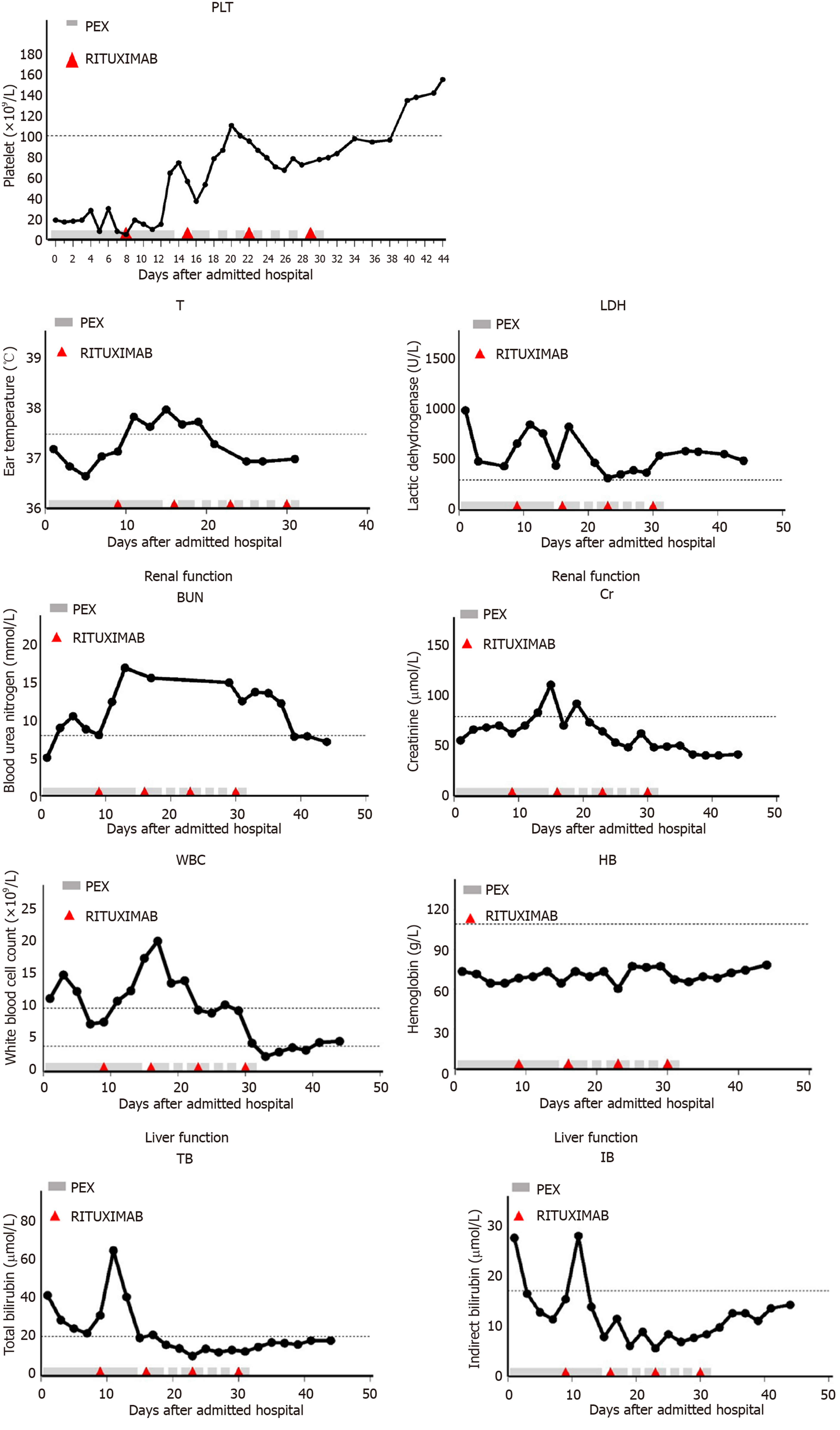
From May 31, 2018, the patient was given therapy with rituximab at 375 mg/m2 per week for 4 wk and methylprednisolone 1 g per day for 3 d on the basis of PEX with fresh frozen plasma (2500 mL). At first, PEX was performed once a day. Then, the frequency of PEX was gradually reduced according to the count of platelets (Figure 1). Methylprednisolone was reduced to 40 mg/d for maintenance therapy after shock treatment. During the period, blood count, liver function, renal function, and ADAMTS13 activity were rechecked.
Platelets did not rise after 8 d of daily PEX. However, the patient had a rise in the platelet count after 6 d of treatment with rituximab and PEX and recovered to normal 13 d later. Platelets were on a rising trend although there were fluctuations. Twenty-seven days later, the platelet count returned to normal (Figure 1), and ADAMTS13 activity was normal. The treatment was well tolerated without infective complications. Currently, the patient is being in clinical remission with a normal platelet count and ADAMTS13 activity.
TTP is a clinical emergency disease with unknown pathogenesis, and the retrospective data revealed that its incidence rate in adults is 3%, mostly at the age of 10-40 years, and it is common in women[4,5]. TTP can be divided into congenital and acquired types. Congenital TTP is often caused by ADAMTS13 gene defect, while acquired TTP is often secondary to infection, drugs, tumors, and pregnancy, mostly due to the ADAMTS13 antibody in the body. In the absence of ADAMTS13, ultralarge multimers of von Willebrand factor cannot be degraded into polymers in normal size, thereby inducing extensive platelet thrombus formation in peripheral blood vessels and capillaries[6]. The characteristic pathological changes of TTP are extensive transparent thrombus formation in capillaries and arterioles, endothelial cell proliferation, and lumen occlusion, affecting various organs in the body.
TTP has rapid progression, and the mortality rate is as high as 90% if not treated in time. Rizzo et al[7] reported that PEX had obtained a great curative effect in TTP since 20 years ago. PEX can rapidly supplement ADAMT13 and remove ADAMTS13 antibody in the body, thus the condition of disease is obviously improved at about 1 wk and can be relieved at 2-4 wk. PEX combined with methylprednisolone at conventional doses as first-line therapy reduces the mortality of TTP to approximately 10%-15%[8]. However, the relapse rate of TTP is 50%-60% after standard treatment[9]. Relapse was defined as a platelet count of less than 50 × 109/L after a minimum of seven procedures of PEX or development of new clinical symptoms[10]. In the present case, after more than 1 wk of PEX, the platelet count was still < 20 × 109/L, suggesting poor efficacy. We then used PEX combined with high-dose methylprednisolone (1 g/d × 3 d) and rituximab (375 mg/m2/wk for 4 wk). As a result, the platelet count and ADAMTS13 activity returned to normal, suggesting that rituximab can improve the curative efficiency of PEX in refractory TTP. The high-dose methylprednisolone pulse therapy can not only inhibit the production of antibodies, but also reduce the damage to platelets, thereby significantly improving the clinical symptoms of the patient. Thumma et al[11] reported that after 12 d of plasmapheresis, there was no improvement in platelet count or ADAMTS13 activity, but after 3 wk of treatment with rituximab at 375 mg/m2, platelet count increased and corticosteroids were tapered.
Controlling the relapse of TTP is a challenge in clinical treatment, and detecting the ADAMTS13 activity during remission can monitor the relapse of disease well[12]. In the present case, the patient has been followed to now without relapse. Rituximab has a significant clinical effect as a salvage therapy for relapse. As a biosynthetic human mouse chimeric monoclonal antibody, rituximab can eliminate B lymphocytes that have an abnormal immune response to autologous antigen. Wieland et al[13] reported that children with refractory relapsing TTP achieved long-term remission after treatment with rituximab. Rituximab not only has good efficacy as a first-line treatment for acquired TTP[14], but can also well prevent relapse. According to foreign reports, rituximab at 375 mg/m2, administered once a week for 4 wk, has a very good curative effect for TTP patients with a low ADAMTS13 activity and positive anti-von Willebrand factor antibody, with a remission rate up to 95%. The reuse of rituximab after relapse can still achieve a high remission rate[15,16]. Hie et al[17] also found that rituximab could reduce the incidence of relapse in adult patients who achieved clinical remission but with severe ADAMTS13 or ADAMT13 < 10% during follow-up. Zwicker et al[18] reported that in 19 TTP patients who received low-dose rituximab combined with PEX, the effective rate was significant and the toxicity was low.
TTP is a clinical emergency disease, and prompt treatment is needed once diagnosed. TTP has rapid progression, and the mortality rate is as high as 90% if not treated in time. PEX can cure most patients, but the relapse rate can be up to 50%-60%. PEX in combination with rituximab can improve the efficacy of PEX in refractory TTP, and prevent disease relapse. Numerous clinical studies have confirmed the low toxicity of rituximab in TTP patients. Therefore, PEX combined with rituximab is an effective and safe treatment for relapse refractory TTP.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mulvihill S, Nault JC S-Editor: Wang JL L-Editor: Wang TQ E-Editor: Zhang YL
| 1. | George JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354:1927-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 373] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059-41063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 581] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 3. | Sadler JE, Moake JL, Miyata T, George JN. Recent advances in thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. 2004;407-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | George JN, Terrell DR, Swisher KK, Vesely SK. Lessons learned from the Oklahoma thrombotic thrombocytopenic purpura-hemolytic uremic syndrome registry. J Clin Apher. 2008;23:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Joly BS, Stepanian A, Leblanc T, Hajage D, Chambost H, Harambat J, Fouyssac F, Guigonis V, Leverger G, Ulinski T, Kwon T, Loirat C, Coppo P, Veyradier A; French Reference Center for Thrombotic Microangiopathies. Child-onset and adolescent-onset acquired thrombotic thrombocytopenic purpura with severe ADAMTS13 deficiency: a cohort study of the French national registry for thrombotic microangiopathy. Lancet Haematol. 2016;3:e537-e546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Gao W, Anderson PJ, Sadler JE. Extensive contacts between ADAMTS13 exosites and von Willebrand factor domain A2 contribute to substrate specificity. Blood. 2008;112:1713-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | Rizzo C, Rizzo S, Scirè E, Di Bona D, Ingrassia C, Franco G, Bono R, Quintini G, Caruso C. Thrombotic thrombocytopenic purpura: a review of the literature in the light of our experience with plasma exchange. Blood Transfus. 2012;10:521-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Sayani FA, Abrams CS. How I treat refractory thrombotic thrombocytopenic purpura. Blood. 2015;125:3860-3867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, Cheung B, Machin SJ; British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 561] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 10. | Allford SL, Hunt BJ, Rose P, Machin SJ; Haemostasis and Thrombosis Task Force, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of the thrombotic microangiopathic haemolytic anaemias. Br J Haematol. 2003;120:556-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 248] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Thumma S, Idrees S, Phuyal P, Manchala V, Mattana J. When the Standard Treatment Fails: Rituximab Therapy for Refractory TTP. Am J Ther. 2019;26:e552-e553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Clinical importance of ADAMTS13 activity during remission in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2016;128:2175-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Wieland I, Kentouche K, Jentzsch M, Lothschütz D, Graf N, Sykora KW. Long-term remission of recurrent thrombotic thrombocytopenic purpura (TTP) after Rituximab in children and young adults. Pediatr Blood Cancer. 2015;62:823-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Chen H, Fu A, Wang J, Wu T, Li Z, Tang J, Shen H, Zhu J, Li J, Zhu Q, Qing L. Rituximab as first-line treatment for acquired thrombotic thrombocytopenic purpura. J Int Med Res. 2017;45:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Galbusera M, Noris M, Remuzzi G. Inherited thrombotic thrombocytopenic purpura. Haematologica. 2009;94:166-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Solak Y, Selcuk NY, Gaipov A, Ucar R, Biyik Z, Acar K. Thrombotic thrombocytopenic purpura secondary to ABO group incompatible blood transfusion in a patient after cardiac surgery. Indian J Crit Care Med. 2013;17:234-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hie M, Gay J, Galicier L, Provôt F, Presne C, Poullin P, Bonmarchand G, Wynckel A, Benhamou Y, Vanhille P, Servais A, Bordessoule D, Coindre JP, Hamidou M, Vernant JP, Veyradier A, Coppo P; French Thrombotic Microangiopathies Reference Centre. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Zwicker JI, Muia J, Dolatshahi L, Westfield LA, Nieters P, Rodrigues A, Hamdan A, Antun AG, Metjian A, Sadler JE; ART Investigators. Adjuvant low-dose rituximab and plasma exchange for acquired TTP. Blood. 2019;134:1106-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |