Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2610
Peer-review started: January 20, 2020
First decision: April 1, 2020
Revised: April 24, 2020
Accepted: May 23, 2020
Article in press: May 23, 2020
Published online: June 26, 2020
Processing time: 155 Days and 18.9 Hours
There are many disputes about the definition, diagnosis, therapy, and prognosis of collision tumors.
We describe a rare patient with a collision tumor consisting of neuroendocrine carcinoma (NEC) and squamous cell carcinoma (SCC) in the nasal cavity and paranasal sinus. She received operation, concurrent chemoradiotherapy, and then two cycles of palliative chemotherapy. Follow-up at 12 mo after diagnosis showed that this patient experienced a complete response with no signs of recurrence or metastasis. A literature review of previous 26 cases diagnosed with collision tumor of NEC and SCC in the head and neck was also undertaken.
It is challenging to manage collision tumors because there are two morphologically and etiologically distinct tumors. Well-designed multimodality therapy including surgery and chemoradiotherapy might lead to a long survival in these patients.
Core tip: It is rare to see collision carcinoma composed of squamous carcinoma and neuroendocrine carcinoma (NEC) in the head and neck region. In this paper, we present a case of squamous cell carcinoma and NEC colliding in the nasal cavity and paranasal sinus. Besides, 26 cases of collision carcinoma in the head and neck were also reviewed to further comprehend the multimodality therapy of collision carcinoma.
- Citation: Wu SH, Zhang BZ, Han L. Collision tumor of squamous cell carcinoma and neuroendocrine carcinoma in the head and neck: A case report. World J Clin Cases 2020; 8(12): 2610-2616
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2610.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2610
Collision tumor of squamous cell carcinoma (SCC) and neuroendocrine carcinoma (NEC) in the head and neck appears rare[1]. This type of tumor in the nasal cavity and paranasal sinus is an even less likely occurrence[2]. A multidisciplinary approach including surgery, chemotherapy, and radiotherapy should be carefully designed once the diagnosis is confirmed. The real challenge is how to treat two synchronous malignancies. Here, we present a case of SCC and NEC colliding in the nasal cavity and paranasal sinus to promote the understanding of collision carcinoma.
A 47-year-old female patient presented with frequent intermittent bleeding in the right nasal cavity for 6 mo.
The symptom of nosebleed was exacerbated with the passage of time. The patient had no fever, headache, chest tightness, or shortness of breath.
The patient used to be in good health. No history of major past illnesses was found.
No relevant personal or family history was found.
No obvious abnormalities were found in physical examination.
No obvious abnormalities were found in laboratory examinations.
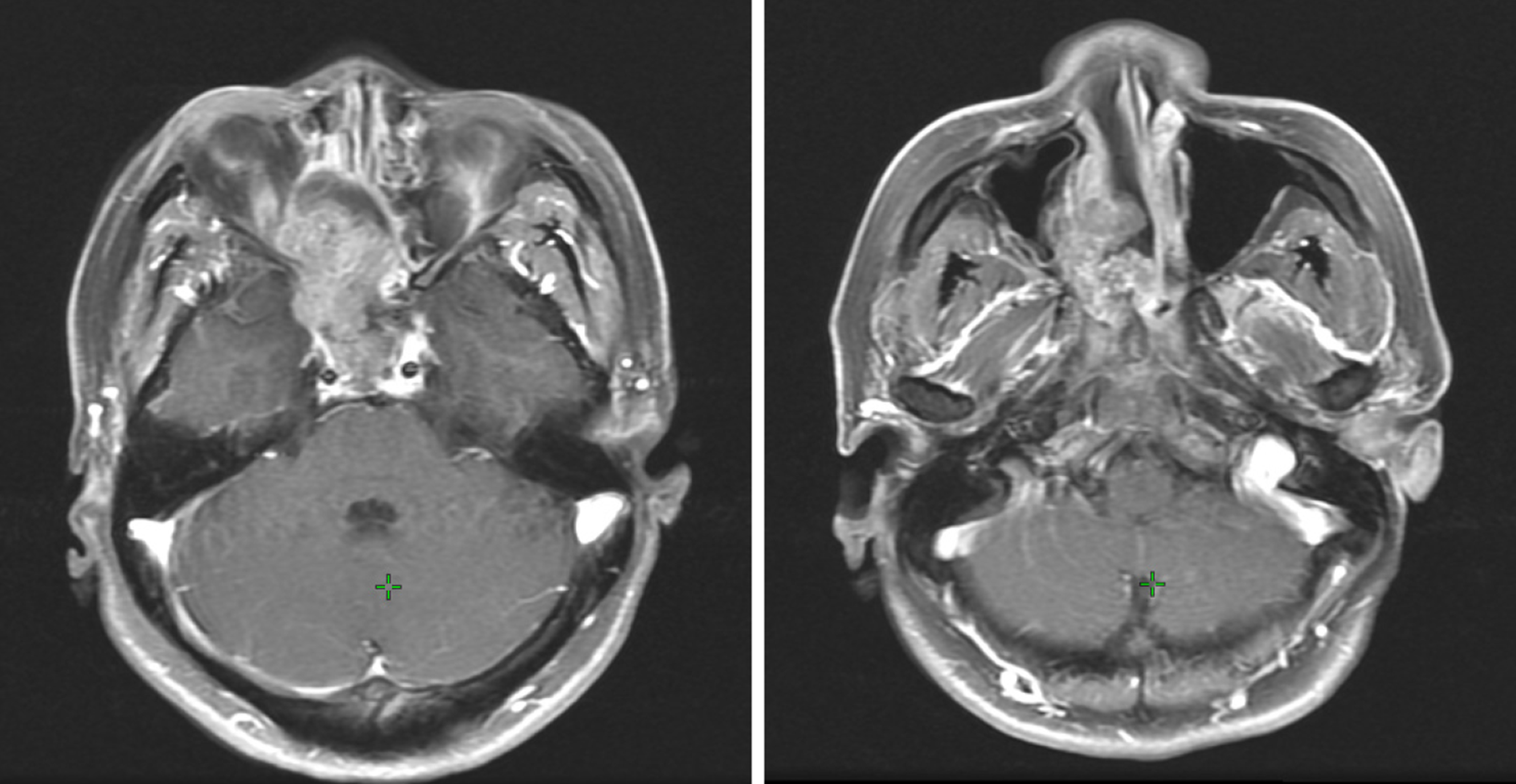
A computed tomography (CT) scan revealed a 4.1 cm × 2.85 cm × 4.3 cm heterogenous mass of the right nasal and paranasal region, which invaded the nasal septum, right inferior middle turbinate, and right lateral wall of maxillary sinus bone, expanded to the right orbital base, and compressed the right internal rectus muscle and the right optic nerve (Figure 1). The neck and abdominal ultrasound showed no metastasis signs.
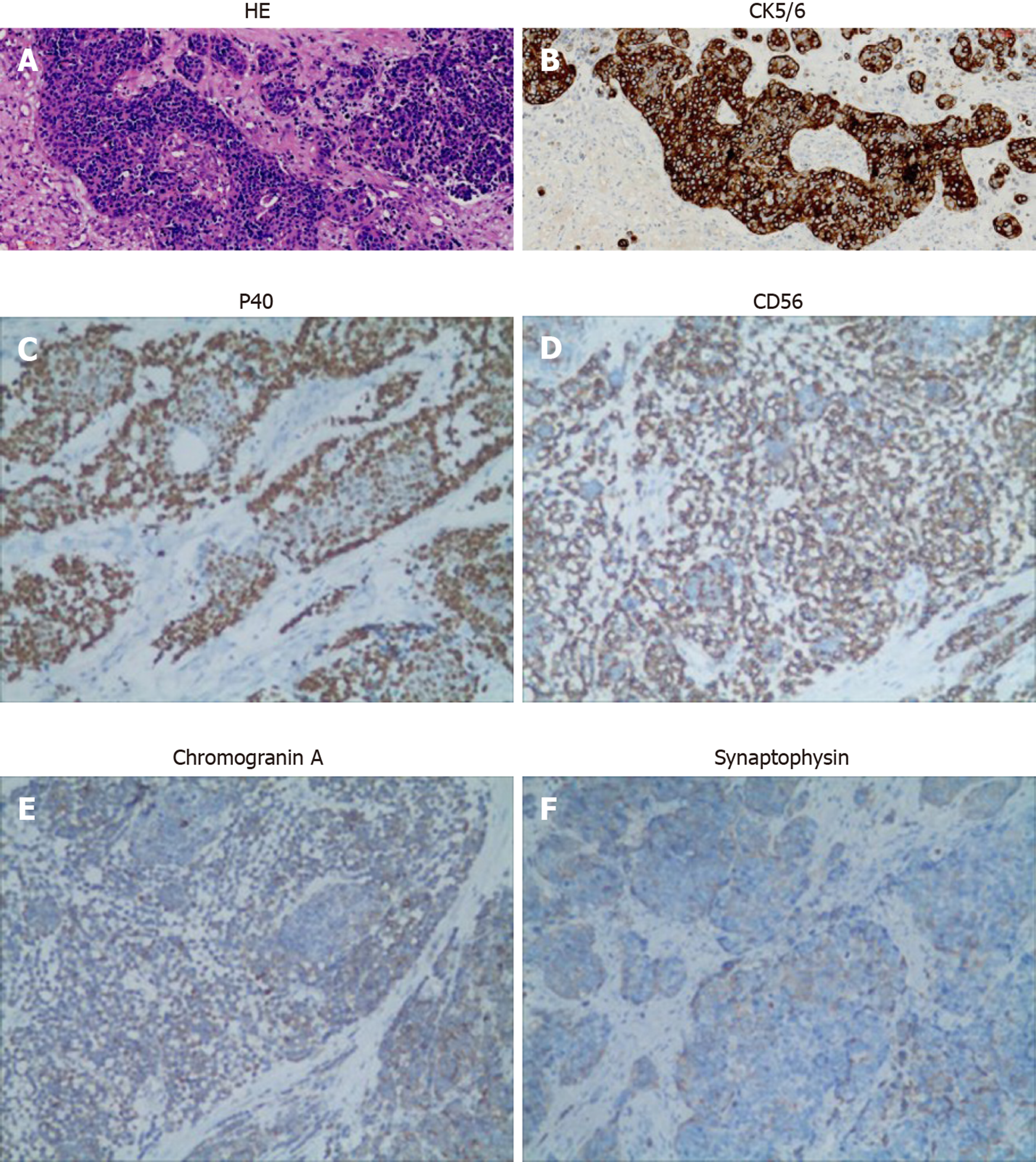
Biopsy pathology of nasal mass specimen revealed poorly differentiated SCC with necrosis. Postoperative pathology of the specimen revealed poorly differentiated SCC with neuroendocrine carcinoma as a collision tumor (Figure 2A). CK5/6 was diffusely positive in the squamous differentiation area and negative in neuroendocrine differentiation cells (Figure 2B). P40 was also diffusely positive in the squamous differentiation area (Figure 2C). CD56 was positive in neuroendocrine components (Figure 2D and E). Chromogranin A and synaptophysin expression was negative (Figure 2E and F).
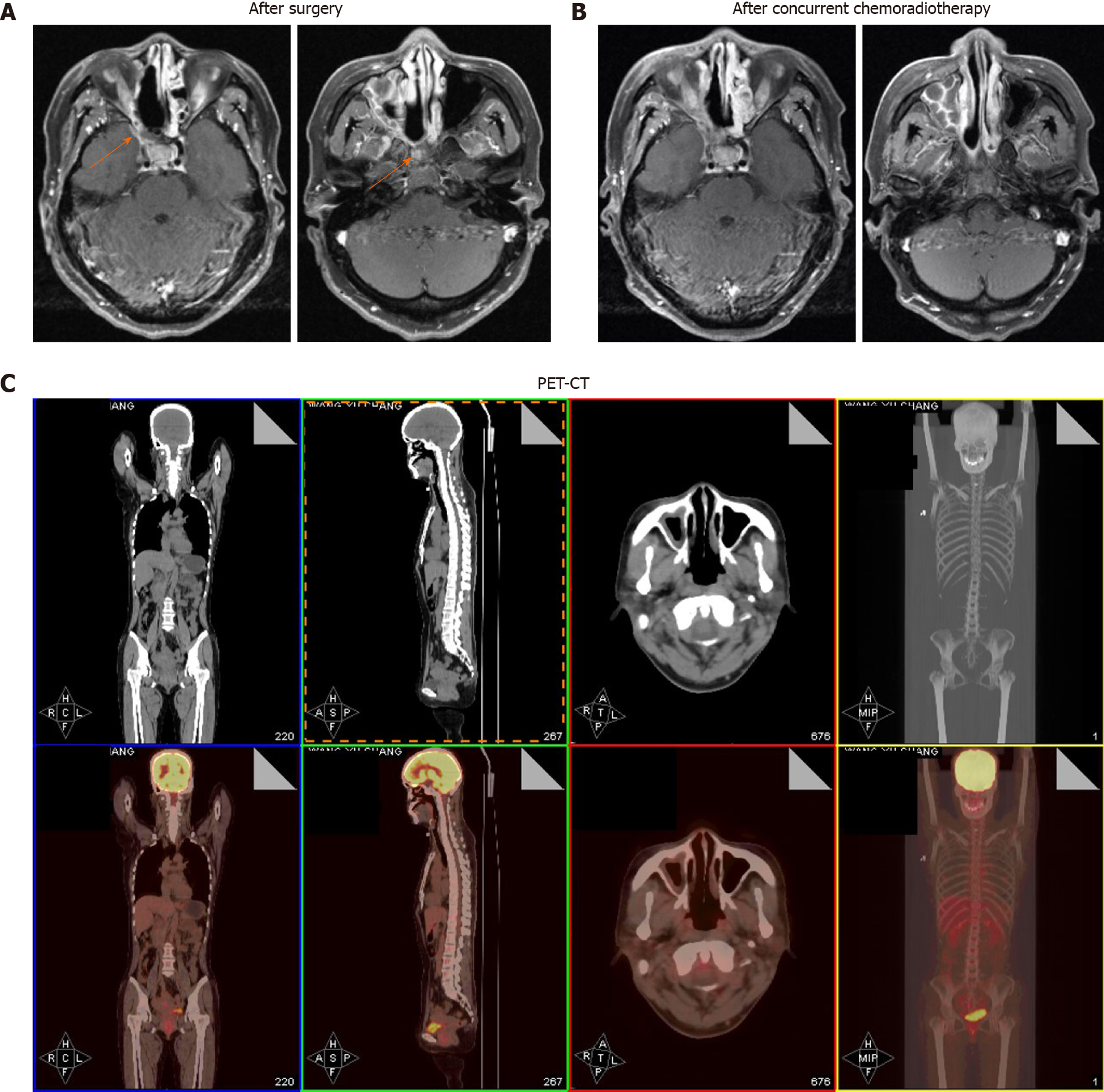
She received operation in August 2018. A CT scan after the surgery revealed residual lesions in the primary tumor region (Figure 3A and B). Then concurrent chemoradiotherapy was performed with radiotherapy 68.0 Gy and chemotherapy of lobaplatin and VP-16 for four cycles. The chemotherapy regimen was lobaplatin (55 mg, day 1) and VP-16 (100 mg/m2, day 1-3). Follow-up at 3 mo after completion of the full course of treatment showed that the patient experienced a complete response (Figure 3A and B).
Positron emission tomography-CT taken in August 2019 (12 mo after diagnosis) revealed no signs of recurrence or metastasis (Figure 3C).
Different terms, including collision/mixed/composite/combined tumor, have been employed to describe tumor consisting of two malignant components. The more troublesome is that the definition of collision tumor has been a matter of debate for years. In 2000, the World Health Organization (WHO) classifications of tumors of endocrine organs suggested that collision tumors are those in which either component occupies at least 30% of the tumor tissues[3,4]. However, it might be an arbitrary criterion since the 30% cutoff has not been proven by reliable clinical data. The more malignant component in collision tumor, even if its share is less than 30%, tends to alter the biological behaviors. Furthermore, in the WHO classification of thyroid and lung tumors[5,6], mixed NEC and non-NEC tumors were not defined by a minimum percentage. We agree to the proposition of Yu et al[7] that collision carcinoma should meet the following conditions: Two malignant neoplasms of different pathological types derive simultaneously from the same location of human body and appear like a mass to the naked eye, with no evidence that one neoplasm migrates from the other.
According to our definition of collision tumor, we reviewed 26 well-documented cases of collision tumor of NEC and SCC in the head and neck from 1978 to 2019 (key words: Collision or mixed or composite or combined carcinoma, and the head and neck). Combining the data with our patient, the resulting group of 27 patients is large enough to allow some meaningful analysis. Of all cases, 23 were male and 4 were female. The sex ratio of men to women is 5.75:1, which displays a significant difference. The mean age of the 25 patients was 58.37 years (range: 32-83 years). Their clinical pathological features, treatments, and outcomes are summarized in Table 1. As to tumor sites, 20 (74.1%) originated from the larynx, 4 (14.8%) from the nasal and paranasal cavity, 1 from the palatine tonsil, and 1 from the soft palate. Fourteen (55.5%) patients were heavy smokers, which keeps consistent with the association between cigarette usage and head-neck cancer. Of all cases, 7 received surgery (S) alone; 7 received chemotherapy (C) and radiotherapy (R); 6 received S + R + C; 5 received S + R; 1 received S + C; and 1 received no treatment for high performance status (grade 3). Follow-up information was available for 23 patients (Table 1). Eight patients died of diseases (tumor) from 3.5 to 39 mo after diagnosis (mean survival, 24.1 mo), and 8 were clinically disease-free, from 6 to 60 mo (mean survival: 18.9 mo) following diagnosis. Seven patients were living with unresectable tumor, from 12 to 39 mo (mean survival, 28.8 mo), after diagnosis. There were only 2 patients who developed local relapse, and coincidentally, both of them received C + R treatment but no surgery. Four patients had recurrent regional lymph nodes metastases, 7 developed remote metastasis, including 3 affecting the bone, 1 lung, 1 lung and bone, 1 bone and liver, and 1 right breast mass.
| Ref. | Year | Sex/age | Site | Smoke | Treatment | Follow-up | Recurrence type |
| Eusebi et al[9] | 1978 | M/63 | Larynx | Yes | S | DOD (24) | Regional |
| Gnepp et al[10] | 1983 | M/57 | Larynx | Yes | S + R | DOD (3.5) | Bone |
| Gnepp et al[10] | 1983 | M/63 | Larynx | Yes | S | DOD (24) | Regional |
| Mills et al[11] | 1983 | M/49 | Larynx | Yes | S + R | AFD (6) | |
| Ferlito et al[8] | 1985 | M/57 | Larynx | Yes | S + R | DOD (3.5) | Bone |
| Ferlito et al[8] | 1985 | M/56 | Larynx | Yes | C + R | AFD (60) | |
| Ferlito et al[8] | 1985 | M/45 | Larynx | Yes | C + R | DOD (21) | |
| Ferlito et al[8] | 1985 | M/47 | Larynx | Yes | C + R | AWD (39) | Lung |
| Ferlito et al[8] | 1985 | M/54 | Larynx | Yes | S | AFD (24) | |
| Chen et al[12] | 1986 | M/55 | Larynx | No | S + R + C | DOD (9) | Bone, lung |
| Chen et al[12] | 1986 | M/56 | Larynx | No | S + C | AWD (13) | Regional |
| Cosby et al[13] | 1988 | M/56 | Larynx | Yes | S + R + C | uk | |
| Gianoli et al[14] | 1992 | M/83 | Larynx | Yes | S | AFD (8) | |
| Yücel et al[15] | 2000 | M/32 | Larynx | No | S + R + C | AWD (12) | Regional |
| Jaiswal et al[16] | 2004 | M/41 | Larynx | Yes | C + R | AFD (8) | |
| Barbeaux et al[17] | 2006 | M/61 | Larynx | No | C + R | AWD (44) | Local |
| Barbeaux et al[17] | 2006 | F/54 | Larynx | No | C + R | AWD (36) | Breast |
| Aggarwal et al[18] | 2010 | M/59 | Larynx | Yes | S + R | uk | |
| Kołodziej et al[19] | 2010 | M/59 | Larynx | No | S | uk | |
| Barham et al[2] | 2013 | F/83 | Nasal cavity | No | S | AWD (13) | Bone |
| Kayakabe et al[20] | 2014 | F/80 | Nasal cavity | No | None | DOD (5) | |
| Franchi et al[21] | 2015 | M/75 | Maxillary sinus | Yes | S + R | AFD (20) | |
| Yamagata et al[22] | 2016 | M/65 | The floor of the mouth | No | C + R | AWD (12) | Local |
| Nakano et al[23] | 2017 | M/59 | Palatine tonsil | Yes | S + R + C | DOD (12) | Liver, bone |
| Udompatanakorn et al[24] | 2018 | M/59 | Soft palate | No | S | uk | |
| Yu et al[7] | 2019 | M/61 | Larynx | No | S + R + C | AFD (13) | |
| Our case | 2019 | F/47 | Nasal cavity and paranasal sinus | No | S + R + C | AFD (12) |
One difficulty existing in collision tumor is to establish accurate preoperative diagnosis. To obtain two malignant neoplasms by single biopsy is like to kill two birds with one stone. A definite diagnosis most depends on careful microscopic observation of surgical specimens, especially with the application of immunohistochemistry. Another problem is that the outcome of collision tumor of SCC and NEC appear very poor. As far as we know, there have been two reports of collision carcinoma involving NEC and SCC in the nasal and paranasal region. However, it is the first case who achieved a complete response. Ferlito et al[8] raised the point that radical surgery takes little effect in improving local tumor control of collision tumor of the larynx. Besides, laryngectomy will result in voice loss and control only the primary lesion. Therefore, a combination of systematic chemotherapy and radiotherapy is recommended for small cell carcinoma. National Comprehensive Cancer Network Guidelines of Neuroendocrine Tumors (version 2015) suggest that patients with locoregional unresectable neuroendocrine tumors receive R + C; moreover, therapeutic manner of small cell carcinoma of the lung, which combined R with systemic chemotherapy, is worth learning.
Due to the rarity of collision carcinoma of NEC and SCC in the head and neck, the treatments should be carefully designed for two different malignancies. Our case indicated that high attention should be paid to the malignant degree of both components and individual characteristics, and certain patients may benefit from postoperative concurrent radiotherapy and chemotherapy.
In a word, we have described a case of collision carcinoma of SCC and NEC in the nasal cavity and paranasal sinus. The existed literature of collision tumor in the head and neck region has been analyzed to understand this disease. Treatments including surgery and chemoradiotherapy may lead to a good survival for patients. More cases of collision carcinoma need to be reported and summarized.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang AHC, Tsuji Y S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Coca-Pelaz A, Triantafyllou A, Devaney KO, Rinaldo A, Takes RP, Ferlito A. Collision tumors of the larynx: A critical review. Am J Otolaryngol. 2016;37:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Barham HP, Said S, Ramakrishnan VR. Colliding tumor of the paranasal sinus. Allergy Rhinol (Providence). 2013;4:e13-e16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Solcia E, Kloppel G, Sobin LH. Histological typing of endocrine tumours. WHO International Histological Classification of Tumours, 2nd edition. Berlin: Springer, 2000. |
| 4. | Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | DeLellis RA, Lloyd RA, Heitz PU, Eng C. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press, 2004. |
| 6. | Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. World Health Organization Classification of Tumours. Pathology Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2015. |
| 7. | Yu Q, Chen YL, Zhou SH, Chen Z, Bao YY, Yang HJ, Yao HT, Ruan LX. Collision carcinoma of squamous cell carcinoma and small cell neuroendocrine carcinoma of the larynx: A case report and review of the literature. World J Clin Cases. 2019;7:242-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 8. | Ferlito A, Recher G, Caruso G. Primary combined small cell carcinoma of the larynx. Am J Otolaryngol. 1985;6:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Eusebi V, Betts CM, Giangaspero F. Primary oat-cell carcinoma of the larynx. Virchows Arch A Pathol Anat Histol. 1978;380:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Gnepp DR, Ferlito A, Hyams V. Primary anaplastic small cell (oat cell) carcinoma of the larynx. Review of the literature and report of 18 cases. Cancer. 1983;51:1731-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Mills SE, Cooper PH, Garland TA, Johns ME. Small cell undifferentiated carcinoma of the larynx. Report of two patients and review of 13 additional cases. Cancer. 1983;51:116-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Chen DA, Mandell-Brown M, Moore SF, Johnson JT. "Composite" tumor--mixed squamous cell and small-cell anaplastic carcinoma of the larynx. Otolaryngol Head Neck Surg. 1986;95:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Cosby WN, Babin RW. Simultaneous oat cell and squamous cell carcinoma of the larynx. Mil Med. 1988;153:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Gianoli GJ, Butcher RB, Martin EJ. Composite tumor of the larynx. Ear Nose Throat J. 1992;71:81-82, 85-87. [PubMed] |
| 15. | Yücel OT, Sökmensüer C, Gedikoglu G, Ayas K. Combined small cell and squamous cell carcinoma of the larynx: short communication. Tumori. 2000;86:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Jaiswal VR, Hoang MP. Primary combined squamous and small cell carcinoma of the larynx: a case report and review of the literature. Arch Pathol Lab Med. 2004;128:1279-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Barbeaux A, Duck L, Weynand B, Desuter G, Hamoir M, Gregoire V, Baurain JF, Machiels JP. Primary combined squamous and small cell carcinoma of the larynx: Report of two cases and discussion of treatment modalities. Eur Arch Otorhinolaryngol. 2006;263:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Aggarwal G, Jackson L, Sharma S. Primary combined small cell carcinoma of larynx with lateralized histologic components and corresponding side-specific neck nodal metastasis: report of a unique case and review of literature. Int J Clin Exp Pathol. 2010;4:111-117. [PubMed] |
| 19. | Kołodziej P, Ostasiewicz P, Ziółkowski P. Combined small cell and squamous cell carcinoma of the larynx. Contemp Oncol (Pozn). 2012;16:350-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Kayakabe M, Takahashi K, Okamiya T, Segawa A, Oyama T, Chikamatsu K. Combined small cell carcinoma of the sinonasal tract associated with syndrome of inappropriate secretion of antidiuretic hormone: A case report. Oncol Lett. 2014;7:1253-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Franchi A, Rocchetta D, Palomba A, Degli Innocenti DR, Castiglione F, Spinelli G. Primary combined neuroendocrine and squamous cell carcinoma of the maxillary sinus: report of a case with immunohistochemical and molecular characterization. Head Neck Pathol. 2015;9:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Yamagata K, Terada K, Uchida F, Kanno N, Hasegawa S, Yanagawa T, Bukawa H. A Case of Primary Combined Squamous Cell Carcinoma with Neuroendocrine (Atypical Carcinoid) Tumor in the Floor of the Mouth. Case Rep Dent. 2016;2016:7532805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Nakano T, Motoshita J, Tanaka R, Okabe M, Tamae A, Shiratsuchi H, Yasumatsu R, Nakashima T, Nakagawa T. Primary combined small cell carcinoma and squamous cell carcinoma of the oropharynx with special reference to EGFR status of small cell carcinoma component: Case report and review of the literature. Auris Nasus Larynx. 2017;44:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Udompatanakorn C, Yada N, Ishikawa A, Miyamoto I, Sato Y, Matsuo K. Primary Neuroendocrine Carcinoma Combined with Squamous Cell Carcinoma of the Soft Palate: A Case Report and Review of Literature. Open J Stomatol. 2018;8:90-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |