Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2574
Peer-review started: March 19, 2020
First decision: April 7, 2020
Revised: April 24, 2020
Accepted: June 4, 2020
Article in press: June 4, 2020
Published online: June 26, 2020
Processing time: 97 Days and 2.3 Hours
In a phase III trial of lenvatinib as first-line treatment for advanced unresectable hepatocellular carcinoma (uHCC), the drug proved non-inferior to sorafenib in terms of the overall survival, but offered better progression-free survival. However, the effects of lenvatinib in uHCC patients with a tumor thrombus in the main portal vein and/or a high tumor burden (tumor occupancy more than 50% of the total liver volume), remain unclear, because these were set as exclusion criteria in the aforementioned trial.
A 53-year-old man (case 1) and 66-year-old woman (case 2) with uHCC presented to us with a tumor thrombus in both the main portal vein and inferior vena cava, a high tumor burden accompanied by a tumor diameter greater than > 100 mm, and distant metastasis, with the residual liver function classified as grade 2A according to the modified Albumin–Bilirubin grading. We started both patients on lenvatinib. The therapeutic effect, as evaluated by the modified Response Evaluation Criteria in Solid Tumors, was rated as partial response in both case 1 and case 2 (at 8 wk and 4 wk after the start of lenvatinib administration, respectively). The therapeutic effect was sustained for 6 mo in case 1 and 20 mo in case 2. Fever occurred as an adverse event in both case 1 and 2, and hyperthyroidism and thrombocytopenia in only case 2, neither of which, however, necessitated treatment discontinuation.
Even in hepatocellular carcinoma patients with poor prognostic factors, if the liver function is well-preserved, lenvatinib is effective and safe.
Core tip: We present two cases of unresectable hepatocellular carcinoma with a tumor thrombus in the main portal vein and a high tumor burden accompanied by a tumor diameter > 100 mm. Despite the aforementioned poor prognostic factors, due to the well-preserved liver function, we elected to treat both patients with lenvatinib in the hope of obtaining tumor shrinkage, based on the REFLECT trial. Lenvatinib was demonstrated to be safe, and good therapeutic responses were obtained. Thus, even in the presence of poor prognostic factors, if the liver function is well-preserved, lenvatinib can be effective and safe in patients with unresectable hepatocellular carcinoma.
- Citation: Komiyama S, Numata K, Moriya S, Fukuda H, Chuma M, Maeda S. Lenvatinib for large hepatocellular carcinomas with portal trunk invasion: Two case reports. World J Clin Cases 2020; 8(12): 2574-2584
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2574.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2574
According to GLOBOCAN 2018, liver cancer is the sixth most commonly diagnosed cancer around the world, and ranks fourth as a cause of death from cancer, with about 841000 newly diagnosed cases and 782000 deaths reported worldwide annually[1]. The SHARP trial demonstrated the efficacy of first-line systemic chemotherapy with sorafenib in Child-Pugh class A (CP-A) patients with primary advanced hepatocellular carcinoma (HCC) and Barcelona Clinic Liver Cancer (BCLC) stage B/C[2]. The REFLECT trial showed the non-inferiority of lenvatinib to sorafenib in terms of the duration of survival[3]; however, the trial also showed that lenvatinib was significantly superior to sorafenib in terms of the progression-free survival and overall response rate (ORR) in the trial; therefore, lenvatinib is often administered in preference to sorafenib as first-line chemotherapy for patients with advanced HCC who are not suitable candidates for locoregional treatment.
Patients with BCLC stage C HCC have heterogeneous background factors. In a retrospective study of BCLC stage C HCC patients treated with various therapeutic regimens, a serum Alpha-Fetoprotein (AFP) level of > 200 ng/mL, tumor diameter of > 50 mm, and presence of macrovascular invasion prior to the start of treatment were identified as poor prognostic factors[4]. In another retrospective analysis of patients with BCLC stage C HCC treated with various therapeutic regimens, a tumor diameter of ≥ 100 mm, presence of a tumor thrombus in the main portal vein (Vp4), presence of distant metastasis, and poor residual liver function were identified as independent poor prognostic factors[5]. In addition, a subgroup analysis of the SHARP trial also identified portal vein invasion and extrahepatic metastasis as poor prognostic factors in patients with HCC. However, according to both the SHARP trial and one retrospective analysis, treatment with sorafenib improved the overall survival, as compared to placebo or no treatment, in advanced CP-A HCC patients with a tumor thrombus in the main portal vein and/or extrahepatic metastasis, both of which correspond to BCLC stage C disease[2,6].
On the other hand, presence of a tumor thrombus in the main portal vein and a high tumor burden (tumor occupancy > 50% of the total liver volume) were listed as exclusion criteria in the REFLECT trial. Therefore, the American Association for the study of Liver Disease guideline and European Association for the Study of the Liver guideline recommend that advanced HCC patients with a tumor thrombus in the main portal vein and/or a high tumor burden be excluded from the indications for lenvatinib administration[7,8]. Thus, sorafenib is often considered to be the agent of first choice for the treatment of patients with primary CP-A HCC with a tumor thrombus in the main portal vein and/or a high tumor burden. However, the National Comprehensive Cancer Network guideline recommends lenvatinib as the first-line chemotherapy for CP-A HCC patients, without limitations imposed by the background factors[9]. While the safety of lenvatinib in advanced BCLC stage C HCC patients with portal vein invasion remains unclear, there is a case report of long-term disease control having been achieved with lenvatinib administration, after controlling for adverse events, in an HCC patient with portal vein invasion[10].
Herein, we report two patients with advanced BCLC stage C HCC, both of whom had a tumor thrombus in the main portal vein, a high tumor burden, and well-preserved liver function, in whom lenvatinib administration caused necrosis of the main portal vein tumor thrombus, and yielded good treatment outcomes, without the occurrence any adverse events necessitating treatment discontinuation.
Case 1 was a 53-year-old man who had visited previous hospital with a 2-mo history of right hypochondrial pain, and case 2 presented with the chief complaint of lower abdominal pain.
Case 1 presented with the complaint of right back pain that did not improve even after rest, and case 2 presented with prolonged lower abdominal pain that persisted even after treatment with medicines for enteritis given by the previous doctor.
Case 1 had never been diagnosed as having hepatitis until he visited our hospital. Case 2 was diagnosed as having chronic hepatitis C as she was being evaluated for enteritis at previous hospital. Other than the above, both patients denied having any history of diabetes or surgery, being on any medications or having any food allergies. Their vaccination history was unknown.
Both patients had a history of smoking, and case 1 gave a history of drinking for more than 30 years. Neither patient had any family history of liver disease, history of blood transfusion/blood product use, or any recent history of use of hepatotoxic drugs.
The ECOG-PS of both patients was 1 and their Karnofsky Performance Scale score was 90. Physical examination revealed no yellow staining of the skin or sclera in either patient. Abdominal examination revealed a flat abdomen and mild tenderness in the upper abdomen in case 1, and mild tenderness in the lower abdomen in case 2.
Blood tests at our hospital in case 1 revealed positive results for anti-HBs antigen and anti-HBe antibody, and a negative result for anti-HBe antigen, and the patient was diagnosed as having chronic hepatitis B. The serum HBV DNA titer was 5.0 log IU/mL. Other laboratory test results were as follows: Serum total bilirubin 17.10 μmol/L, serum albumin 40 g/L, serum alanine aminotransferase 405 U/L, serum aspartate aminotransferase 86 U/L, serum alkaline phosphatase 566 U/L, serum alpha-fetoprotein 6487 ng/mL, and serum protein induced by Vitamin K absence or antagonists-II 11900 mAU/mL. The laboratory examination revealed no significant abnormalities other than the above. His residual liver function was classified as CP-A (5 points) and modified Albumin–Bilirubin (mALBI) grade 2A (-2.59 points)[11,12].
Laboratory tests at our hospital in case 2 revealed the following: Serum total bilirubin 22.23 μmol/L, serum albumin 39 g/L, serum alanine aminotransferase 44 U/L, serum aspartate aminotransferase 121 U/L, serum alkaline phosphatase 318 U/L, serum alpha-fetoprotein 18.4 ng/mL, and serum protein induced by Vitamin K absence or antagonists-II 3110 mAU/mL. The serum test for anti-HCV antigen was positive, and the HCV RNA titer was 5.8 log IU/mL. The laboratory examination revealed no significant abnormalities other than the above. Her residual liver function was classified as CP-A (6 points) and mALBI grade 2A (-2.43 points)[11,12].
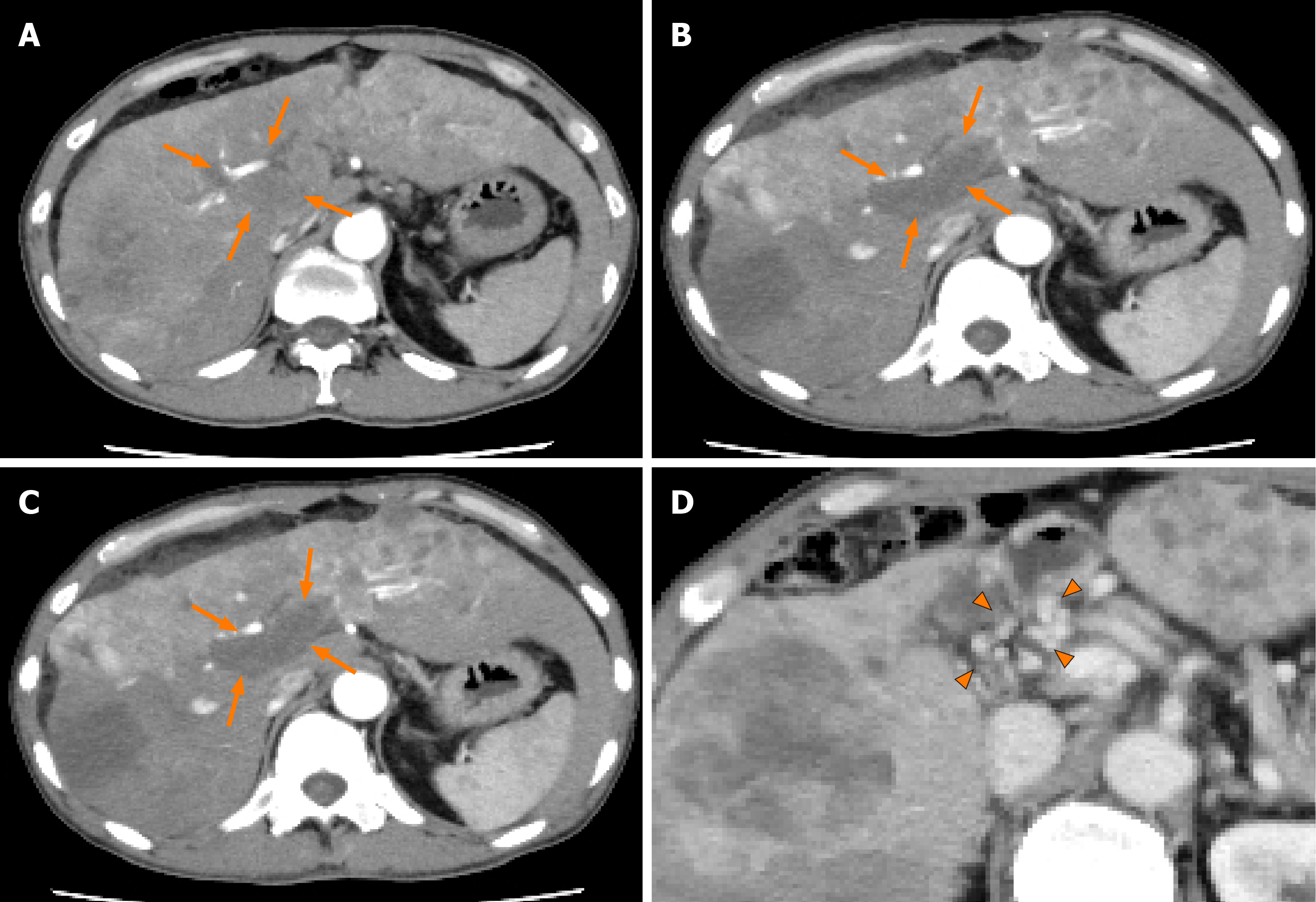
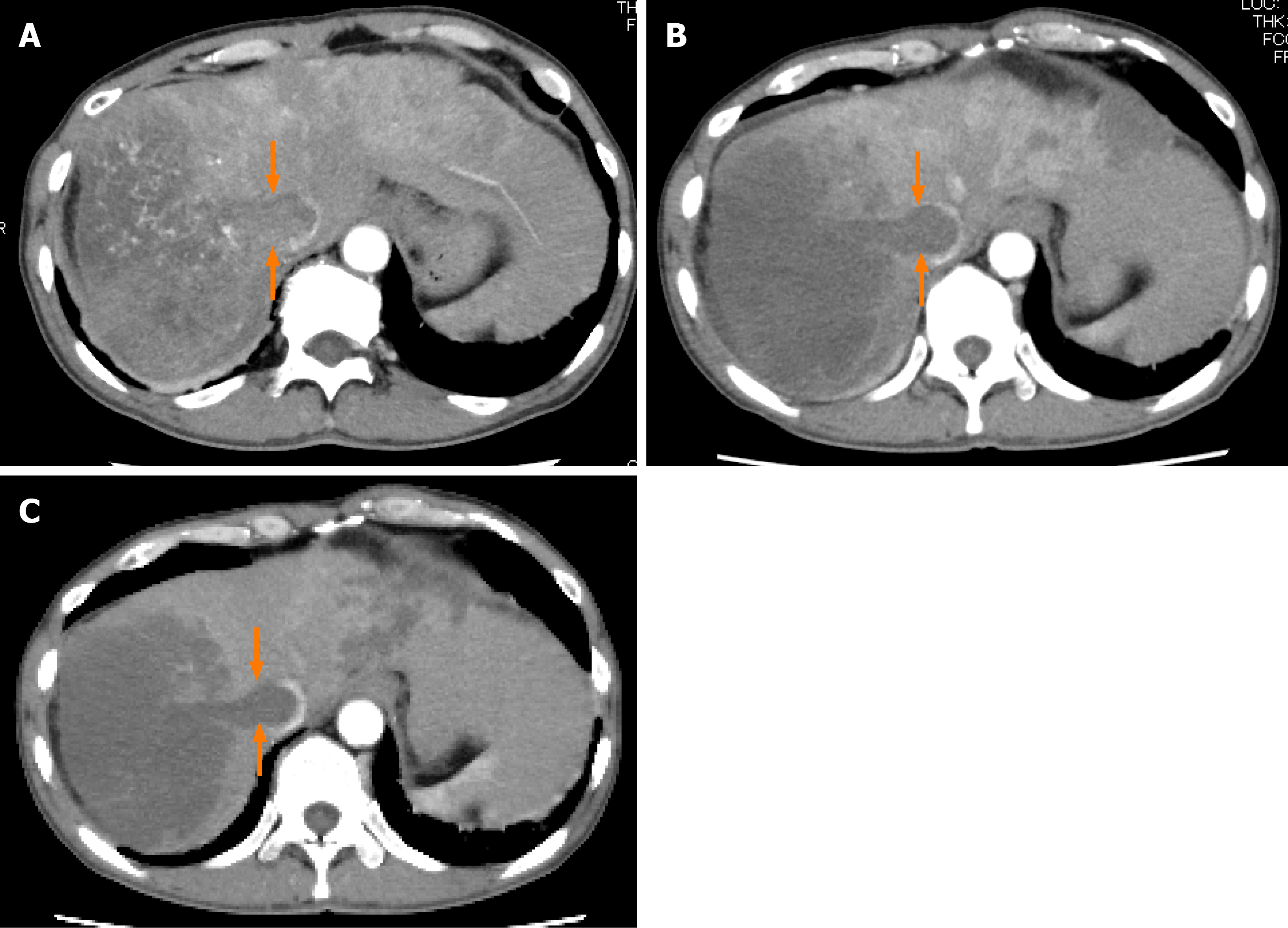
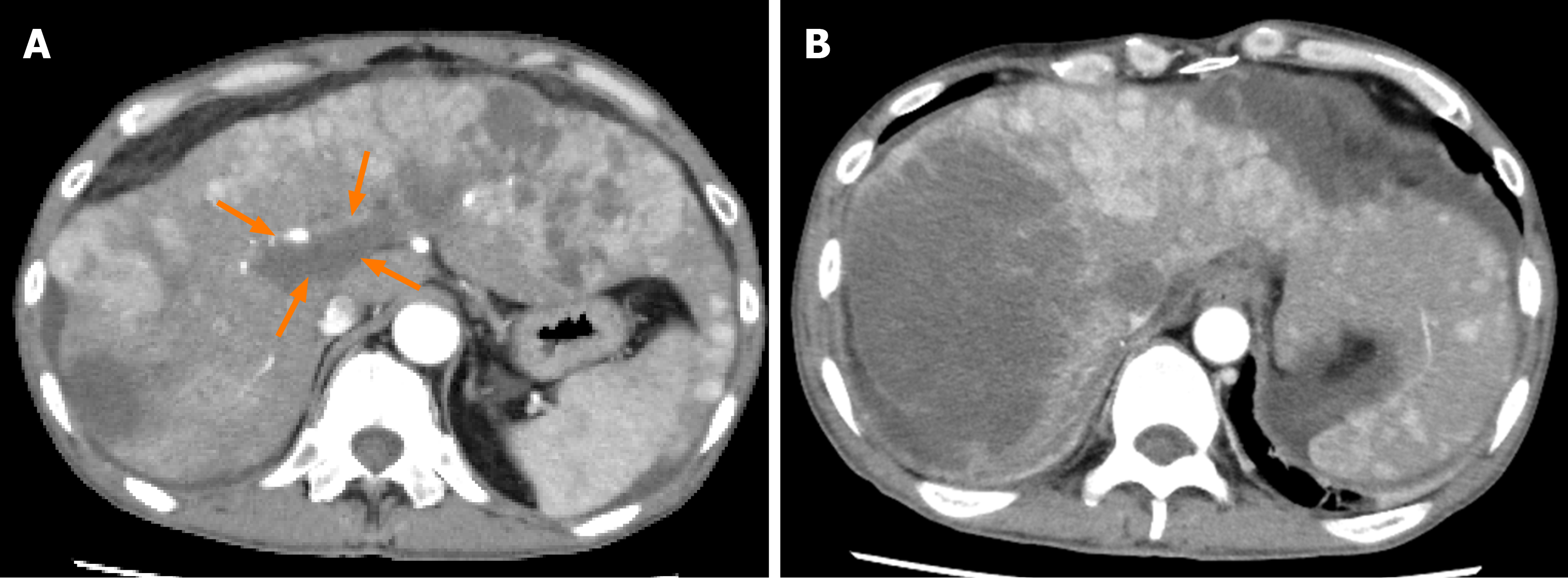
Case 1: Abdominal ultrasonography at the previous hospital showed multiple tumors in both hepatic lobes. Dynamic contrast-enhanced computed tomography (CT) at our hospital revealed scattered small nodules in both lung fields and multiple tumors in both hepatic lobes. The tumor located in the right hepatic lobe measured 130 mm in maximum diameter. This tumor was associated with a tumor thrombus in the main portal vein (Vp4) (Figure 1A) and inferior vena cava (Vv3) (Figure 2A), and a high tumor burden. The main portal vein was completely obstructed, and there were no apparent collateral veins. Dynamic contrast-enhanced CT showed early enhancement and subsequent washout of the entire hepatic tumor.
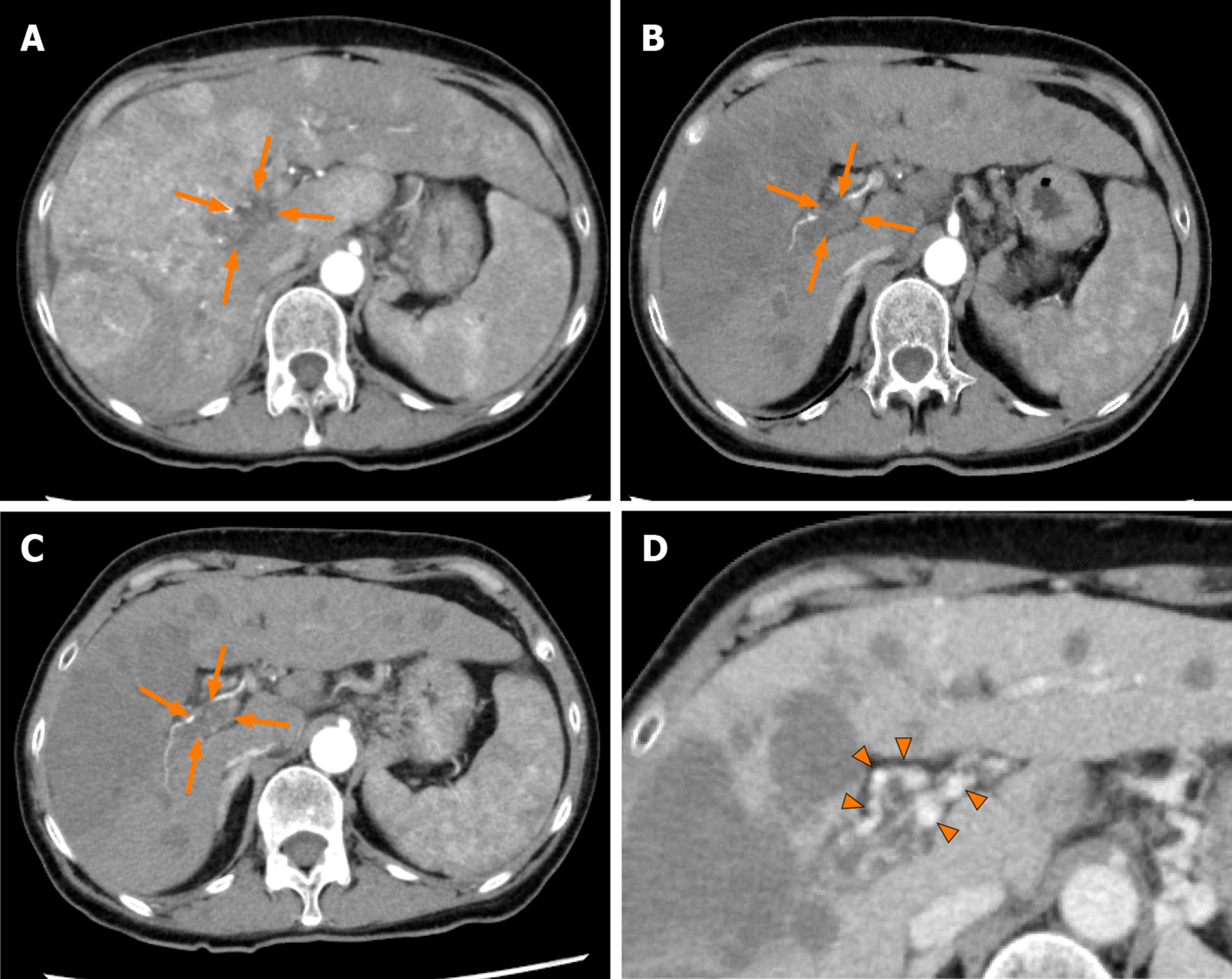
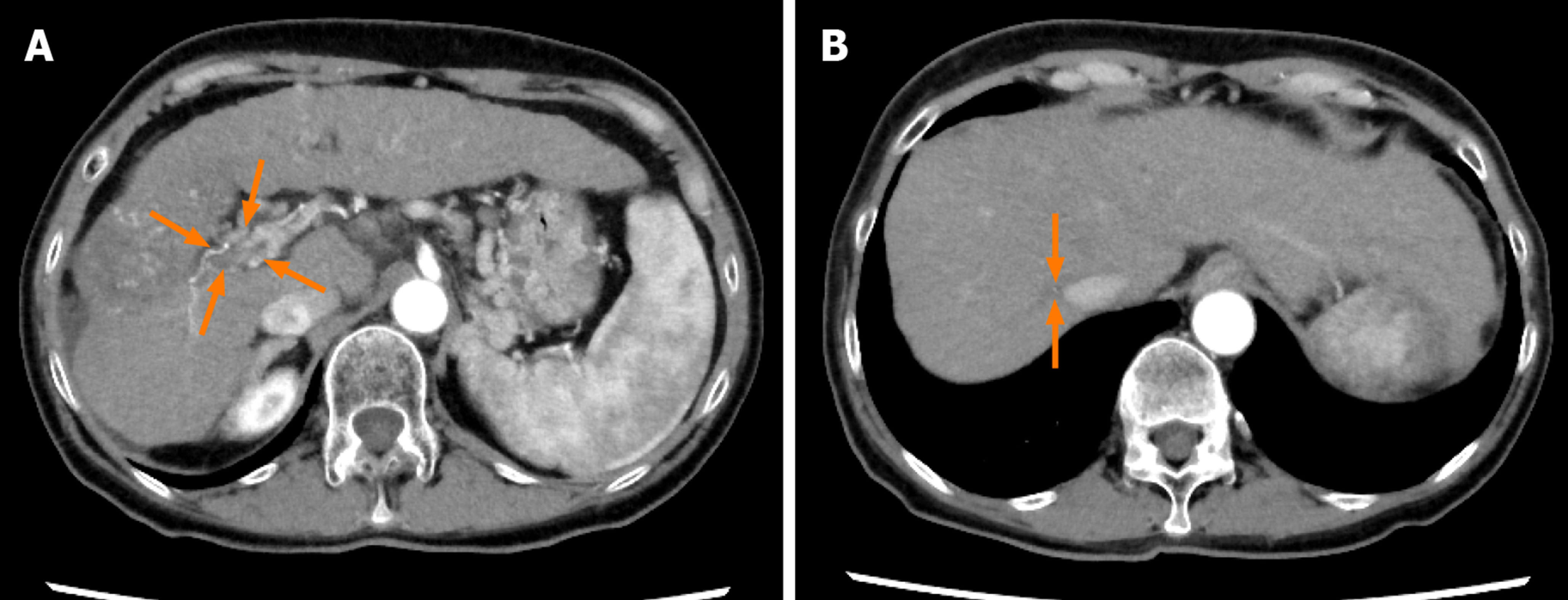
Case 2: Dynamic contrast-enhanced CT revealed a small nodule in the upper lobe of the left lung and left adrenal gland, and multiple tumors in both the hepatic lobes. A tumor located in the right hepatic lobe measured 190 mm in maximum diameter. This tumor was associated with a tumor thrombus in the main portal vein (Vp4) (Figure 3A) and inferior vena cava (Vv3) (Figure 4A), and a high tumor burden. The main portal vein was almost completely obstructed, and there were no apparent collateral veins. Dynamic contrast-enhanced CT showed early enhancement and subsequent washout of the entire hepatic tumor.
Both patients were diagnosed as having HCC with a tumor thrombus in the main portal vein and intrahepatic and distant metastases; furthermore, the HCC in both patients was classified as BCLC stage C, and cT4N0M1, stage IVB, according to the UICC classification 8th edition.
According to the American Association for the study of Liver Disease guideline, we initiated both patients on systemic chemotherapy.
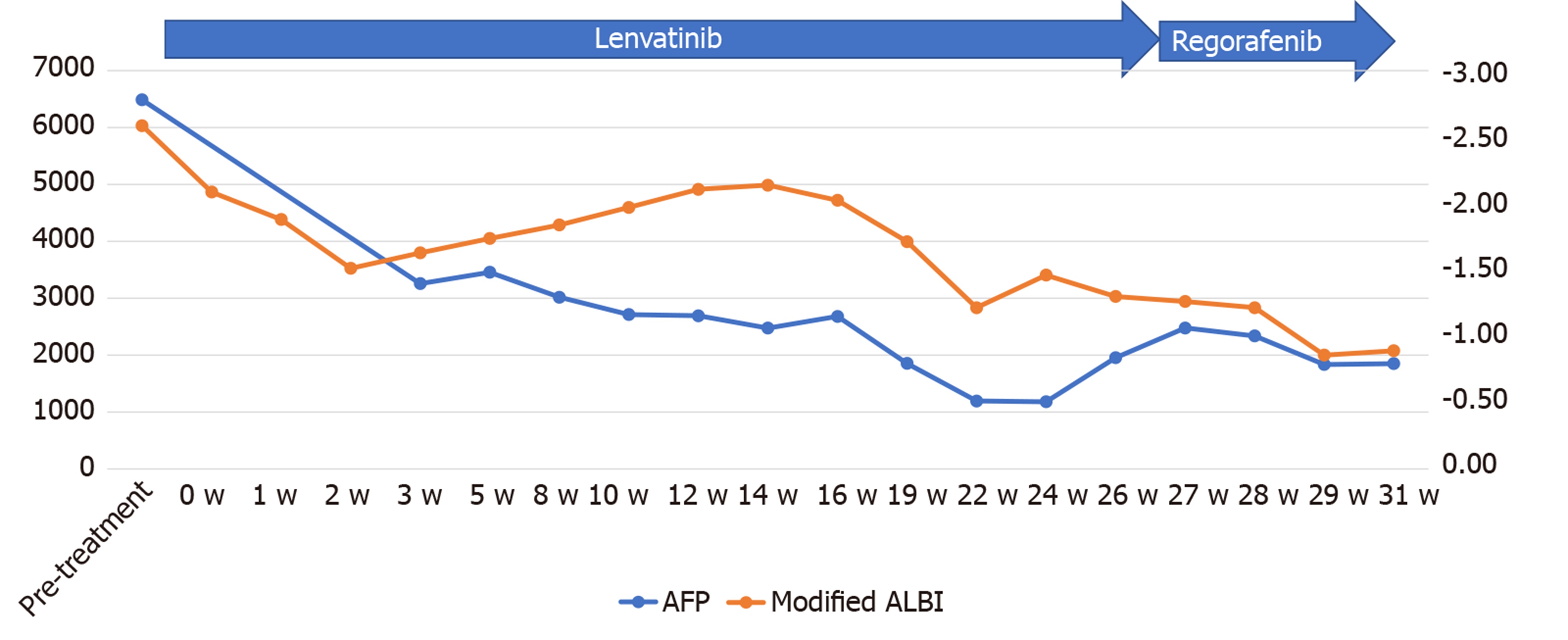
Informed consent for treatment was obtained from the patient and his relatives. His body weight at his first visit to our department was 56 kg. We started the patient on lenvatinib 8 mg/d. At one week after the start of lenvatinib administration, he developed high fever (body temperature 40 °C). Dynamic contrast-enhanced CT was performed at 2 wk after the start of lenvatinib administration to determine if the fever represented an adverse event or was associated with tumor necrosis (Figures 1B and 2B). There were no interval changes in the size or number of the HCCs, however, decreased hypervascularity of the HCC associated with the tumor thrombus in both the main portal vein and the inferior vena cava was observed in the arterial-phase images (Figures 1B and 2B). Furthermore, the serum AFP level had decreased to 3259 ng/mL. Based on the findings, we regarded the fever as having arisen from tumor necrosis and decided to continue the lenvatinib administration without any dose reduction. At 8 wk after the start of lenvatinib administration, the maximum tumor response was rated as partial response (PR) according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (Figures 1C and 2C)[13]. During treatment, the serum AFP level continued to decline, and the mALBI score continued to improve from 2 wk onward after the start of lenvatinib administration (Figure 5). At 14 wk after the start of lenvatinib administration, the portal vein tumor thrombus decreased in size and collateral veins around the portal vein could be clearly visualized on dynamic contrast-enhanced CT (Figure 1D). There were no apparent hepatic encephalopathy episodes or adverse events other than grade 2 fever (Common Terminology Criteria for Adverse Events version 4.0 [CTCAE v4.0]).
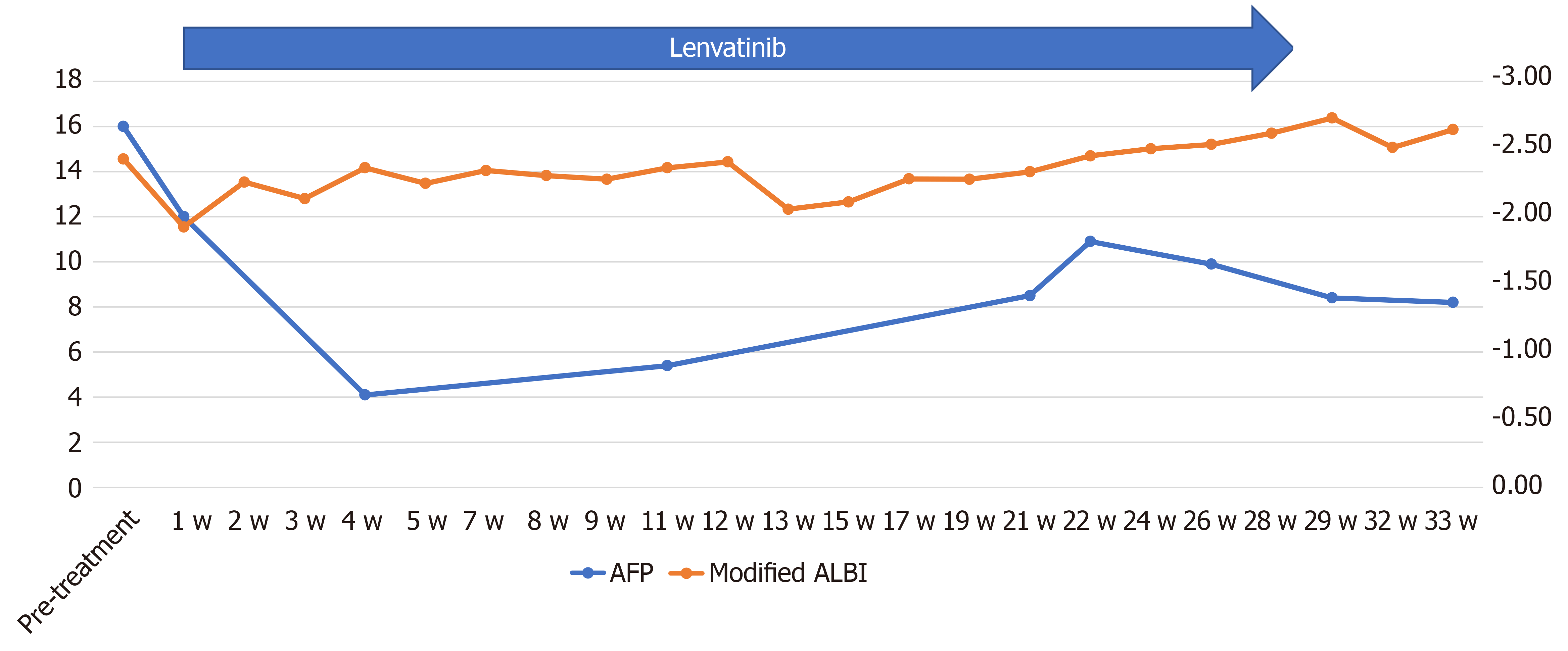
Informed consent was obtained from the patient and her relatives. The body weight of the patient at her first visit to our department was 45 kg. She showed evidence of a high tumor burden, and we started her on treatment with lenvatinib 4 mg/d. At one week after the start of lenvatinib administration, she developed high fever (grade 1) and she recovered without specific therapy. Dynamic contrast-enhanced CT was performed at 4 wk after the start of lenvatinib administration, which revealed moderate shrinkage of the HCCs, and marked decrease in the hypervascularity of the largest liver tumor and tumor thrombus in the inferior vena cava in the arterial-phase images (Figures 3B and 4B). At that point, the response was rated as PR according to mRECIST[13]. Furthermore, the serum AFP level decreased to within normal limits at 2 wk after the start of lenvatinib administration. The mALBI score worsened once after the start of treatment, but recovered and, in fact, improved by 5 wk after the start of treatment (Figure 6). In response to this, we increased the lenvatinib dose to 8 mg/d. At 9 wk after the start of lenvatinib administration, the response was rated as PR according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). At the same time, both the portal vein tumor thrombus and inferior vena cava thrombus decreased in size, and collateral veins around the portal vein could be clearly visualized (Figures 3C, D and 4C).
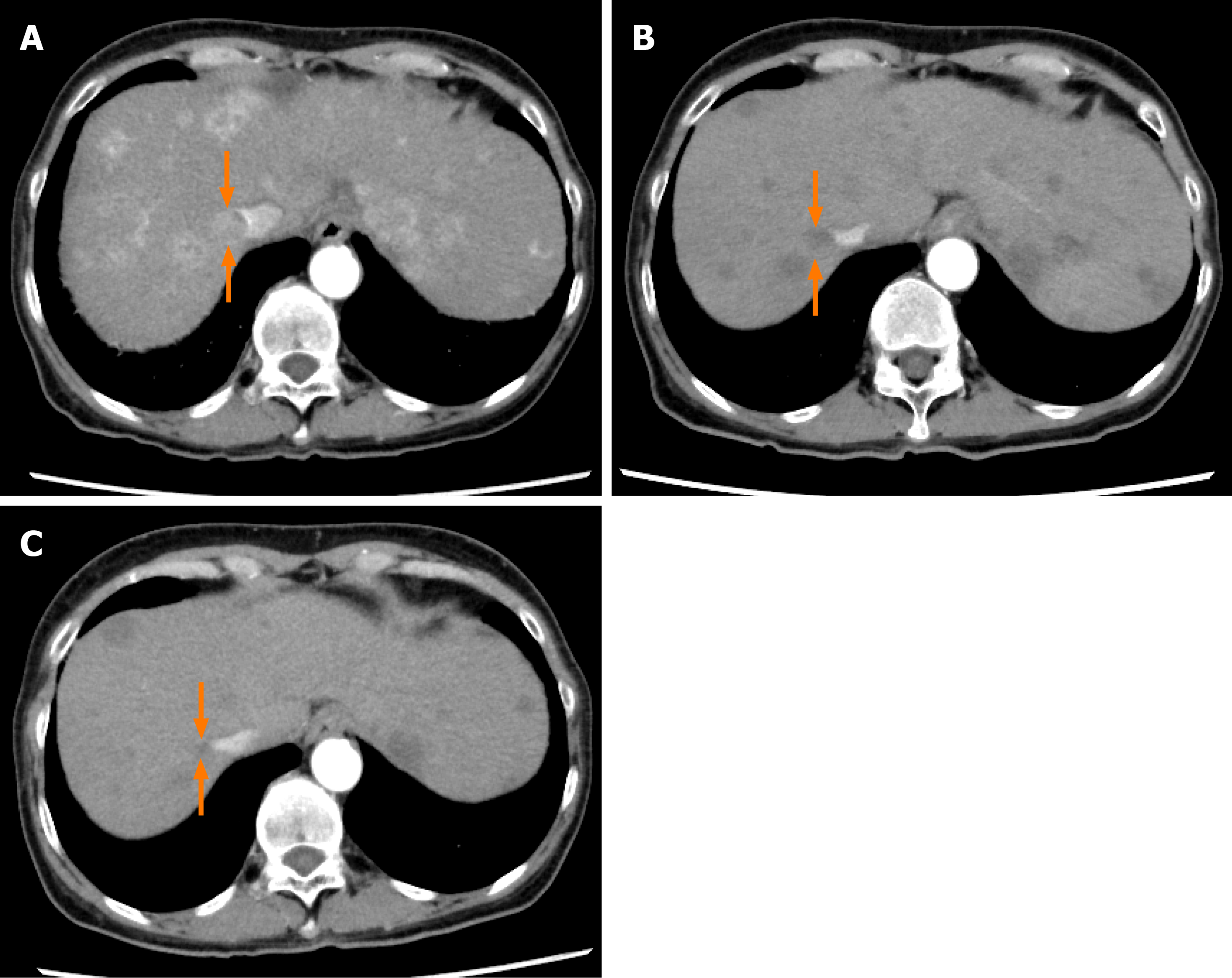
The serum AFP level increased, and the mALBI score worsened at 6 mo after the start of lenvatinib administration. Dynamic contrast-enhanced CT after 6 mo of lenvatinib administration revealed the appearance of new intrahepatic metastases and enlargement of both the primary liver lesion and the pulmonary nodules, with the emergence of ascites around the liver, and the treatment response was rated as progressive disease according to mRECIST (Figure 7A and 7B). After informed consent was obtained from the patient and his relatives, the treatment was switched to regorafenib at the starting dose of 120 mg/d. At 2 wk after the start of regorafenib administration, the patient reported reduced dietary intake, which was suspected as being due to progression of the HCC. Thereafter, the patient desired to receive treatment at his native place and the treatment at our hospital ended at 8 mo after the start of lenvatinib administration.
The dose of lenvatinib was reduced to 4 mg/d at 19 wk after the start of lenvatinib administration because the patient developed grade 1 thrombocytopenia. At 21 wk after the start of lenvatinib administration, the patient developed grade 2 hyperthyroidism. Thiamazole treatment was started and the patient recovered by 21 wk after the start of treatment. The treatment response of PR according to mRECIST and RECIST v1.1 was maintained until 80 wk after the start of lenvatinib administration (Figure 8A and B).
Although our patients had primary advanced HCC with a high tumor burden and a tumor thrombus in the main portal vein (Vp4), lenvatinib was successfully administered without the appearance of any adverse events necessitating treatment discontinuation. Moreover, the therapeutic responses were rated as PR according to mRECIST, and the antitumor effect was sustained for a long time, with declining serum AFP levels. Thus, even in patients with advanced HCC with a high tumor burden and a tumor thrombus in the main portal vein (Vp4), if the liver function is well-preserved, lenvatinib can be safe and effective.
Patients with BCLC stage C HCC have heterogeneous background factors, and a high serum AFP level, large tumor size, presence of macrovascular invasion, presence of distant metastasis and poor residual liver function prior to the start of treatment are reported as poor prognostic factors in BCLC stage C HCC patients[5-8]. Although both our patients had multiple poor prognostic factors, including a tumor diameter of ≥ 100 mm, tumor thrombus in the main portal vein, and distant metastasis, and case 1 had a high serum concentration of AFP, we elected to treat the patients with lenvatinib, because in the REFLECT trial, lenvatinib treatment was shown to yield a superior progression-free survival as compared sorafenib in patients with well-preserved liver function. In our cases, the residual liver function was classified as CP-A and mALBI grade 2A; the mALBI grade is calculated from the serum bilirubin and albumin levels and has been proposed as a new liver function indicator in addition to the Child-Pugh score. In a clinical trial of sorafenib for patients with advanced HCC with well-preserved liver function (Child-Pugh score 5-7) who were unsuitable candidates for loco-regional therapy[14], a subgroup analysis revealed a significant difference in the overall survival and a different tendency in decline of the residual liver function after sorafenib administration between patients classified as mALBI grade 1-2A and 2B before the start of treatment[15]. Therefore, it was proposed that the eligibility judgment for sorafenib administration be made by the mALBI grade. In addition, lenvatinib administration has been reported to yield significantly higher response rates and lower treatment discontinuation due to adverse events in advanced HCC patients with Child–Pugh score 5 and ALBI grade 1 than in other groups[16]. It is expected that the mALBI grade could also become established as a reliable basis for determining the eligibility for treatment with lenvatinib.
On the other hand, our case 1 developed grade 2 fever one week after the start of lenvatinib administration. To determine if the fever arose as an adverse event, such as drug allergy, or from tumor necrosis, dynamic contrast-enhanced CT was performed; based on the results of this imaging examination, we determined tumor necrosis as the cause of the fever and followed up the patient about once a week. In addition, as both the patients had macrovascular invasion, we were concerned about hepatic dysfunction occurring due to decreased hepatic blood flow, which however, did not occur. One possible reason for this could be that the collateral veins around the portal vein, called cavernous transformation of the portal vein, maintained the hepatic blood flow. Cavernous transformation of the portal vein has been reported in HCC patients with a tumor thrombus in the main portal vein and reported as collateral veins of the biliary and gastric branches of the portal vein[17]. In advanced HCC patients with multiple poor prognostic factors, it is essential to pay attention to the possibility of emergence of adverse events and hepatic dysfunction after the initiation of lenvatinib treatment.
We started case 1 on regorafenib treatment as second-line therapy after failure of lenvatinib treatment. Regorafenib is pharmacologically more potent than sorafenib due to differences in the molecular structure[18]; therefore, we decided to administer regorafenib while paying attention to possible adverse effects.
In both our patients, dynamic contrast-enhanced CT showed decrease of the tumor hypervascularity as early as by 4 wk after the start of lenvatinib administration, and the therapeutic response of PR according to mRECIST was achieved within 8 wk after the start of lenvatinib administration; the antitumor effect was sustained for a long period, with declining serum AFP levels. Hiraoka et al[19] also reported an early therapeutic response as follows, from a study of lenvatinib treatment in patients with advanced unresectable hepatocellular carcinoma: The ORR was 40.7% and the disease control rate was 85.2%, according to mRECIST, at 4 wk after the start of lenvatinib administration, and the ORR and disease control rate in tyrosine-kinase inhibitor-naïve patients were 50.0% and 87.5%, respectively[19]. Sho et al[20] and Kuzuya et al[21] reported that high early therapeutic response was obtained in advanced HCC patients treated with lenvatinib, including CP-B patients, which was set as an exclusion criterion in the REFLECT trial[20,21]. It has been suggested that in cases where lenvatinib proves to be effective, the tumor hypervascularity begins to decrease and the treatment effect is obtained in the early period after the start of lenvatinib administration. In addition, in our case 1, the serum AFP level decreased by 50% by 2 wk after the start of lenvatinib administration. Hiraoka et al[22] reported that the serum AFP level decreased significantly in advanced HCC patients in which disease control was obtained by 4 wk after the start of lenvatinib administration[22]. Kodama et al[23] also reported a relationship between higher objective response based on imaging findings and early decline of the serum AFP levels[23]. It is possible that early evaluation of the tumor hypervascularity and measurement of the serum AFP level after the start of lenvatinib administration represent predictors of the treatment response to the drug.
We report the cases of two advanced BCLCL stage C HCC patients with a tumor thrombus in the main portal vein and a high tumor burden, in whom lenvatinib treatment, administered with attention paid to the possible development of adverse events, was found to be effective and safe. Further accumulation of cases is necessary to endorse and establish the efficacy and safety of lenvatinib for advanced BCLC stage C HCC patients with a tumor thrombus in the main portal vein and a high tumor burden.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu TL, Jin ZH, Sempokuya T S-Editor: Zhang L L-Editor: A E-Editor: Liu MY
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55699] [Article Influence: 7957.0] [Reference Citation Analysis (132)] |
| 2. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10238] [Article Influence: 602.2] [Reference Citation Analysis (2)] |
| 3. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3799] [Article Influence: 542.7] [Reference Citation Analysis (1)] |
| 4. | Ponziani FR, Spinelli I, Rinninella E, Cerrito L, Saviano A, Avolio AW, Basso M, Miele L, Riccardi L, Zocco MA, Annicchiarico BE, Garcovich M, Biolato M, Marrone G, De Gaetano AM, Iezzi R, Giuliante F, Vecchio FM, Agnes S, Addolorato G, Siciliano M, Rapaccini GL, Grieco A, Gasbarrini A, Pompili M. Reverse time-dependent effect of alphafetoprotein and disease control on survival of patients with Barcelona Clinic Liver Cancer stage C hepatocellular carcinoma. World J Hepatol. 2017;9:1322-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Lee DW, Yim HJ, Seo YS, Na SK, Kim SY, Suh SJ, Hyun JJ, Jung SW, Jung YK, Koo JS, Kim JH, Yeon JE, Lee SW, Byun KS, Um SH. Prognostic assessment using a new substaging system for Barcelona clinic liver cancer stage C hepatocellular carcinoma: A nationwide study. Liver Int. 2019;39:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Fan W, Wang Y, Lu L, Fu S, Yang J, Huang Y, Yao W, Li J. Sorafenib With and Without Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma With Main Portal Vein Tumor Thrombosis: A Retrospective Analysis. Oncologist. 2015;20:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3219] [Article Influence: 459.9] [Reference Citation Analysis (1)] |
| 8. | European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6013] [Article Influence: 859.0] [Reference Citation Analysis (3)] |
| 9. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology Hepatobiliary cancers. version 1. 2020. In: National Comprehensive Cancer Network Website. Available from: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. |
| 10. | Takeda H, Nishijima N, Nasu A, Komekado H, Kita R, Kimura T, Kudo M, Osaki Y. Long-term antitumor effect of lenvatinib on unresectable hepatocellular carcinoma with portal vein invasion. Hepatol Res. 2019;49:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 1995] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 12. | Hiraoka A, Kumada T, Tsuji K, Takaguchi K, Itobayashi E, Kariyama K, Ochi H, Tajiri K, Hirooka M, Shimada N, Ishikawa T, Tachi Y, Tada T, Toyoda H, Nouso K, Joko K, Hiasa Y, Michitaka K, Kudo M. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer. 2019;8:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 13. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3287] [Article Influence: 219.1] [Reference Citation Analysis (36)] |
| 14. | Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Saito T, Motoyama T, Suzuki E, Tawada A, Kanai F, Yokosuka O. Sorafenib treatment in Child-Pugh A and B patients with advanced hepatocellular carcinoma: safety, efficacy and prognostic factors. Invest New Drugs. 2015;33:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Ogasawara S, Chiba T, Ooka Y, Suzuki E, Kanogawa N, Saito T, Motoyama T, Tawada A, Kanai F, Yokosuka O. Liver function assessment according to the Albumin-Bilirubin (ALBI) grade in sorafenib-treated patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Ueshima K, Nishida N, Hagiwara S, Aoki T, Minami T, Chishina H, Takita M, Minami Y, Ida H, Takenaka M, Sakurai T, Watanabe T, Morita M, Ogawa C, Hiraoka A, Johnson P, Kudo M. Impact of Baseline ALBI Grade on the Outcomes of Hepatocellular Carcinoma Patients Treated with Lenvatinib: A Multicenter Study. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 17. | Song B, Min P, Oudkerk M, Zhou X, Ge Y, Xu J, Chen W, Chen X. Cavernous transformation of the portal vein secondary to tumor thrombosis of hepatocellular carcinoma: spiral CT visualization of the collateral vessels. Abdom Imaging. 2000;25:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1015] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 19. | Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Itobayashi E, Shimada N, Tajiri K, Tsuji K, Ishikawa T, Ochi H, Hirooka M, Tsutsui A, Shibata H, Tada T, Toyoda H, Nouso K, Joko K, Hiasa Y, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group and the HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: Multicenter analysis. Hepatol Res. 2019;49:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Sho T, Suda G, Ogawa K, Kimura M, Shimazaki T, Maehara O, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Umemura M, Kawagishi N, Natsuizaka M, Nakai M, Morikawa K, Furuya K, Baba M, Yamamoto Y, Kobayashi T, Meguro T, Saga A, Miyagishima T, Yokoo H, Kamiyama T, Taketomi A, Sakamoto N. Early response and safety of lenvatinib for patients with advanced hepatocellular carcinoma in a real-world setting. JGH Open. 2020;4:54-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res. 2020;50:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Hiraoka A, Kumada T, Kariyama K, Takaguchi K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada N, Shibata H, Ishikawa T, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Itokawa N, Imai M, Joko K, Hiasa Y, Michitaka K; Real-life Practice Experts for HCC (RELPEC) Study Group, HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan). Clinical features of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions: Multicenter analysis. Cancer Med. 2019;8:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 23. | Kodama K, Kawaoka T, Namba M, Uchikawa S, Ohya K, Morio K, Nakahara T, Murakami E, Yamauchi M, Hiramatsu A, Imamura M, Chayama K, Aikata H. Correlation between Early Tumor Marker Response and Imaging Response in Patients with Advanced Hepatocellular Carcinoma Treated with Lenvatinib. Oncology. 2019;97:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |