Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2530
Peer-review started: February 24, 2020
First decision: March 27, 2020
Revised: May 9, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 26, 2020
Processing time: 120 Days and 21.7 Hours
Previous publications indicated that genetic predisposition might play important roles in the onset of osteonecrosis of the femoral head (ONFH) in systemic lupus erythematosus (SLE). Some gene loci such as complement C3d receptor 2 (CR2), nitric oxide synthase 3 (NOS3), collagen type II alpha 1 chain (COL2A1), protein tyrosine phosphatase non-receptor type 22 (PTPN22), and transient receptor potential cation channel subfamily V member 4 (TRPV4) were reported to be involved in this process.
To investigate whether the risk of ONFH in SLE is associated with single nucleotide variations (SNVs) in these five genes.
SNVs in the CR2, NOS3, COL2A1, PTPN22, and TRPV4 genes were examined by using FastTarget and Illumina Miseq sequencing technologies in 49 cases of SLE with ONFH. Burrows–wheeler aligner was used to align the sequencing reads to hg19, and GATK and Varscan programs were used to perform SNV calling. PolyPhen-2, SIFT, and MutationTaster were used to assess the functional effects of non-synonymous SNVs.
Six of the 49 patients were confirmed to have low frequency SNVs, including one patient with SNVs in NOS3 (exon 6: c.814G>A: p.E272K and exon 7: c.814G>A: p.E272K.), four in COL2A1 (rs41263847: exon 29: c.1913C>T: p.T638I, exon 28: c.1706C>T: p.T569I, and rs371445823: exon 8: c.580G>A: p.A194T, exon 7: c.373G>A: p.A125T), and one in CR2 (rs45573035: exon 2: c.200C>G: p.T67S).
The onset of ONFH in SLE might be associated with the identified SNVs in NOS3, COL2A1, and CR2.
Core tip: Genetic predisposition might play important roles in the onset of osteonecrosis of the femoral head (ONFH) in systemic lupus erythematosus (SLE). Some gene loci such as complement C3d receptor 2 (CR2), nitric oxide synthase 3 (NOS3), collagen type II alpha 1 chain (COL2A1), protein tyrosine phosphatase non-receptor type 22 (PTPN22), and transient receptor potential cation channel subfamily V member 4 (TRPV4) were reported to be involved in this process. We investigated whether the risk of ONFH in SLE is associated with single nucleotide variations (SNVs) in these five genes by using FastTarget and Illumina Miseq sequencing technologies in 49 cases. Six patients were confirmed to have low frequency SNVs, including one in NOS3, four in COL2A1, and one in CR2. The onset of ONFH in SLE might be associated with the identified SNVs in NOS3, COL2A1, and CR2.
- Citation: Sun HS, Yang QR, Bai YY, Hu NW, Liu DX, Qin CY. Gene testing for osteonecrosis of the femoral head in systemic lupus erythematosus using targeted next-generation sequencing: A pilot study. World J Clin Cases 2020; 8(12): 2530-2541
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2530.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2530
Both osteonecrosis of the femoral head (ONFH) and systemic lupus erythematosus (SLE) are multifactorial and complex diseases caused by both genetic alterations and environmental exposures[1]. Risk factors for secondary ONFH are known to involve the intake of corticosteroids, autoimmune disease, alcohol abuse, and radiation. Epidemiological studies suggest that SLE is the most common autoimmune disease to be the primary cause of steroidinduced ONFH[2]. However, only some patients with SLE receiving steroid administration ultimately develop ONFH, and there are a number of patients with SLE with ONFH who have no experience of corticosteroid treatment[3]. These reports indicated that other reasons, such as genetic variations between individuals, might also be involved in the onset of ONFH in SLE. Indeed, gene polymorphisms that affect coagulation, metabolic factors, mechanical stresses, immunologic factors, and fibrinolytic systems have been identified[4,5] and some of these genes have been suggested to be involved in SLE with ONFH.
Complement receptor type 2 (CR2) is a transmembrane glycoprotein expressed in mature B and follicular dendritic cells. CR2 plays a role in complement activation, antigen targeting, and B cell activation. Endothelial nitric oxide synthase (NOS3) regulates bone formation and osteoblast function. It has been reported that in Korean patients with SLE, CR2 and NOS3 contribute to ONFH susceptibility[6,7]. Collagen type II alpha-1 gene (COL2A1) is a causal gene of skeletal dysplasia and epiphyseal dysplasia. COL2A1 mutations cause familial idiopathic ONFH[8]; however, the relationship was not found in Japanese idiopathic ONFH patients[9]. PTPN22 encodes protein tyrosine phosphatase nonreceptor 22, a lymphoid protein, mutations in which might promote T cell activation and thus cause autoimmune diseases, such as rheumatoid arthritis, SLE, and juvenile idiopathic arthritis[10,11]. Transient receptor potential vanilloid 4 (TRPV4) forms a calciumpermeable non-selective cation channel and plays a role in vasoregulation and osteoclast differentiation. A novel TRPV4 mutation and altered calcium homeostasis have been observed in ONFH[12].
In this study, we investigated whether patients with SLE with ONFH have a genetic predisposition to ONFH in SLE, the identification of which might lead to more efficient diagnosis, evaluation, and even prevention of the disease. Using FastTarget and Illumina Miseq sequencing technologies, we analyzed single nucleotide variations (SNVs) in CR2, NOS3, COL2A1, PTPN22, and TRPV4 genes of patients with SLE with ONFH.
We enrolled 49 patients with SLE with ONFH (4 males and 45 females; mean age: 33.57 ± 11.24 years) visiting the Department of Rheumatology and Immunology of Shandong Provincial Hospital Affiliated to Shandong University. All patients met the criteria of the American College of Rheumatology as revised in 1997[13]. Individuals provided samples of peripheral blood, 2 mL of which was used for genomic DNA isolation using a DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) following the manufacturer's standard procedure. The local Ethics Review Board approved the current study, and informed consent was provided by all participants.
Primers were designed using Primer 3. To cover all the coding sequences and most of the untranslated regions of the five genes that might be responsible for ONFH in SLE, 243 oligonucleotide pairs were produced. A first round of primer design using the most stringent conditions (e.g., no single nucleotide polymorphisms in the primer annealing region, an amplicon comprising 200-270 bp, and a GC content of 30%-80%) allowed us to put the 243 oligonucleotide pairs into 15 multiplex PCR panels to amplify the target regions from the five genes. An ABI 2720 Thermal Cycler (Life Technologies Corporation, Carlsbad, CA, United States) was used to carry out the amplification reactions using following cycling program: 95°C for 2 min; 11 cycles of 94 °C for 20 s, 63.5 °C for 40 s, and 72 °C for 1 min; 24 cycles of 94 °C for 20 s, 65 °C for 30 s, and 72 ºC for 1 min; and final incubation at 72 °C for 2 min. An 8 bp barcode was incorporated into each PCR product, and then all the libraries for each sample were pooled. Cluster generation and hybridization of the sequencing primers were followed by nucleotide sequencing carried out on a MiSeq Benchtop Sequencer (Illumina, Inc, San Diego, CA, United States) in one single lane, following the manufacturer's standard protocols. For each sequencing read, 300 cycles were carried out to produce paired-end reads including 300 bp at each end and the 8-bp index tag.
The Burrows–wheeler aligner[14] method was used to align the sequencing reads to human genome version hg19. The GATK[15] and Varscan programs[16] were used to call the SNVs, the data for which were then combined. SNVs were annotated using the Annovar program[17]. PolyPhen-2, SIFT, and MutationTaster[18-20] were used to assess the functional effects of non-synonymous SNVs. Significantly non-benign non-synonymous SNVs were identified as those with a Polyphen-2 score of > 0.85, a SIFT score of < 0.05, or a MutationTaster score of > 0.85. Perl scripts then filtered the SNVs against those of dbSNP135 to segregate benign polymorphisms from potentially deleterious variants. Benign polymorphisms were considered as those SNVs present in dbSNP135 with a minor allele frequency of ≥ 1% in a Chinese population from the 1000 genome database, and were removed from subsequent analysis.
The two-tailed Student’s t-test and Fisher’s exact test or χ2 test were used when appropriate. Differences were considered significant if the P value was less than 0.05. The odds ratio and 95% confidence interval (95%CI) were calculated.
To identify SNVs, VarScan (http://varscan.sourceforge.net/) and GATK HaplotypeCaller (https://software.broadinstitute.org/gatk/best-practices//) were used to analyze single nucleotide polymorphisms and insertion-deletions (InDels) in the samples. A total of 92 SNVs and 17 InDels were identified using both approaches, with 4 SNVs and 1 InDel being identified only by VarScan, and another 16 SNVs and 2 InDels only by GATK HaplotypeCaller.
The identified SNVs and InDels were then grouped by the corresponding regions of the genome, including: Intron (40.15%), exon (21.21%), intergenic (13.64%), ncRNA (non-coding)_intron (7.58%), 3′ untranslated region (UTR; 5.3%), splicing site (10 bp around a splice junction) (4.55%), upstream (1 kb upstream of the transcription start site; 3.03%); downstream (between one gene’s upstream and another gene’s downstream; 1.52%), 5′ UTR (1.52%), exonic splicing (0.76%), and ncRNA (non-coding)_splicing (0.76%). In this study, 15 SNVs were nonsynonymous (51.72%), while the other 14 SNVs were synonymous (48.28%). Gene transition (Ts) and transvertion (Tv) (both as annotated by dbSNP) of the SNVs were also analyzed. There were 61 (54.46%) Ts events and 29 (25.89%) Tv events, giving a Ts:Tv ratio of 2.1034. There were 15 (13.39%) Novel_Ts events (not annotated by dbSNP) and 7 (6.25%) Novel_Tv events (not annotated by dbSNP), giving a Novel_Ts:Tv ratio of 2.1429.
For all the identified InDels, 2 insertions comprised 1-5 bp (10%), while 17 deletions comprised 1-5 bp (85%) and 1 deletion was 6-10 bp (5%). The genomic distributions of the SNVs for each sample are shown in Table 1.
| Sample | Het_SNV | Hom_SNV | Novel_SNV | Het_InDel | Hom_InDel | Novel_InDel |
| G1 | 25 | 22 | 2 | 4 | 0 | 7 |
| G2 | 29 | 11 | 0 | 7 | 0 | 7 |
| G3 | 21 | 9 | 1 | 5 | 0 | 7 |
| G4 | 9 | 14 | 1 | 4 | 0 | 7 |
| G5 | 17 | 12 | 2 | 6 | 0 | 5 |
| G6 | 26 | 15 | 1 | 3 | 0 | 7 |
| G7 | 14 | 24 | 1 | 4 | 0 | 7 |
| G8 | 14 | 11 | 2 | 4 | 0 | 7 |
| G9 | 25 | 21 | 0 | 3 | 0 | 5 |
| G10 | 21 | 9 | 1 | 4 | 0 | 6 |
| G11 | 19 | 25 | 0 | 5 | 1 | 6 |
| G12 | 30 | 12 | 0 | 4 | 0 | 4 |
| G13 | 17 | 14 | 1 | 7 | 0 | 7 |
| G14 | 26 | 12 | 0 | 3 | 0 | 5 |
| G15 | 17 | 14 | 0 | 4 | 0 | 6 |
| G16 | 31 | 11 | 4 | 4 | 0 | 8 |
| G17 | 16 | 19 | 1 | 7 | 0 | 6 |
| G18 | 18 | 24 | 1 | 7 | 0 | 7 |
| G19 | 18 | 22 | 2 | 5 | 0 | 4 |
| G20 | 22 | 13 | 1 | 5 | 0 | 6 |
| G21 | 5 | 17 | 4 | 4 | 0 | 8 |
| G22 | 12 | 11 | 2 | 5 | 0 | 6 |
| G23 | 20 | 26 | 1 | 3 | 1 | 5 |
| G24 | 31 | 14 | 2 | 5 | 0 | 6 |
| G25 | 19 | 13 | 2 | 5 | 0 | 7 |
| G26 | 16 | 20 | 1 | 6 | 0 | 4 |
| G27 | 6 | 16 | 2 | 4 | 0 | 6 |
| G28 | 14 | 24 | 1 | 3 | 0 | 4 |
| G29 | 45 | 8 | 0 | 6 | 0 | 6 |
| G30 | 22 | 13 | 1 | 7 | 0 | 6 |
| G31 | 20 | 23 | 3 | 5 | 0 | 6 |
| G32 | 31 | 15 | 0 | 6 | 0 | 5 |
| G33 | 23 | 13 | 0 | 4 | 0 | 4 |
| G34 | 31 | 7 | 2 | 5 | 0 | 6 |
| G35 | 17 | 13 | 2 | 6 | 0 | 6 |
| G36 | 19 | 10 | 0 | 4 | 0 | 5 |
| G37 | 19 | 25 | 1 | 5 | 1 | 7 |
| G38 | 26 | 20 | 1 | 6 | 0 | 6 |
| G39 | 26 | 13 | 2 | 4 | 0 | 8 |
| G40 | 21 | 10 | 1 | 5 | 0 | 6 |
| G41 | 30 | 12 | 1 | 6 | 0 | 7 |
| G42 | 26 | 17 | 1 | 4 | 0 | 7 |
| G43 | 31 | 14 | 1 | 7 | 0 | 7 |
| G44 | 18 | 13 | 0 | 4 | 0 | 6 |
| G45 | 16 | 21 | 0 | 6 | 0 | 7 |
| G46 | 5 | 17 | 4 | 4 | 0 | 6 |
| G47 | 19 | 25 | 0 | 4 | 1 | 6 |
| G48 | 21 | 9 | 2 | 5 | 0 | 4 |
| G49 | 9 | 14 | 0 | 5 | 0 | 4 |
Among the 49 patients, six (12.25%) were confirmed to have low frequency functional mutations, including one patient with a mutation in the NOS3 gene, four patients with a mutation in the COL2A1 gene, and one patient with a mutation in the CR2 gene; and the first priority (Take the highest priority of SNVs if the mutation is dominant or homozygous; take the lower one from the top two highest priority SNVs if it is a heterozygous recessive pattern) of these gene mutations were third, first1, and second, respectively. However, we failed to detect significant differences in the frequency of these mutations in comparison with controls (Table 2).
| Gene | First priority | SNV count | Sample count | Mutation (0|1|2) | Mutation 1 | GENESKY ControlDB SNV Count | GENESKY ControlDB Mutation (0|1|2) | P1 | P2 | P3 | P4 |
| NOS3 | Third | 1 | 1 | 44|1|0 | G19 | 14 | 203|15|2 | 0.399 | 0.3257 | 1 | 0.2235 |
| COL2A1 | First1 | 2 | 4 | 45|4|0 | G34, G42, G6, G4 | 10 | 193|26|1 | 0.6773 | 0.6202 | 1 | 0.4848 |
| CR2 | Second | 1 | 1 | 48|1|0 | G2 | 5 | 209|11|0 | 0.6625 | 0.7007 | 1 | 0.7038 |
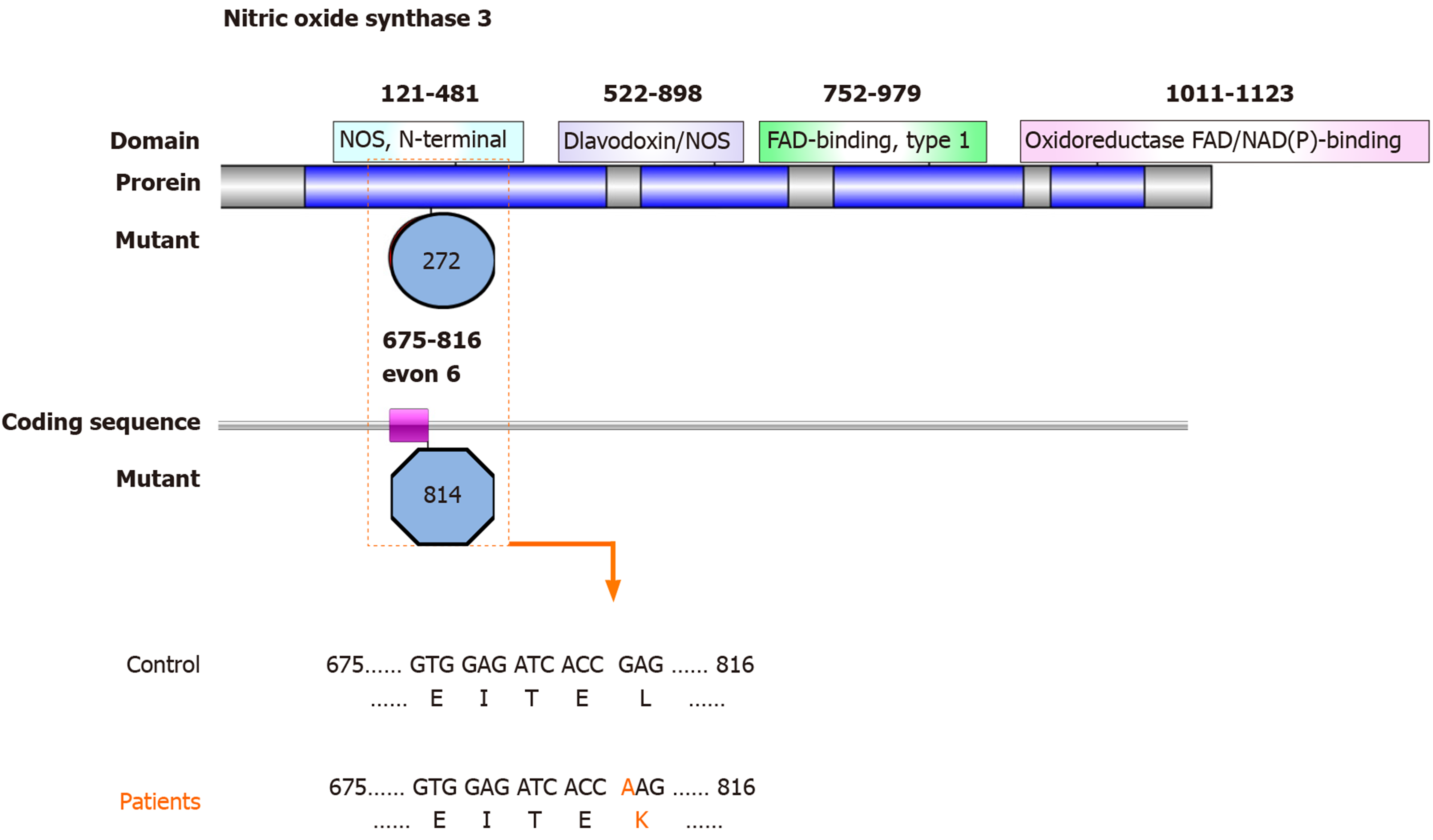
The low frequency functional mutations in NOS3 are shown as follows: NM_001160109: exon 6: c.814G>A: p.E272K, NOS3: NM_000603: exon 7: c.814G>A: p.E272K, NOS3: NM_001160110: exon 6: c.814G>A: p.E272K, and NOS3: NM_001160111: exon 6: c.814G>A: p.E272K (Figure 1), which had never been reported previously. Most of these mutations here comprised nonsynonymous SNVs and were predicted as tolerated (SIFT Score Pred), possibly damaging (POLYPHEN Score Pred), and disease_causing damaging (MutationTaster Score Pred) (Table 3).
| Gene | NOS3 | COL2A1 | COL2A1 | CR2 |
| First priority | Third | Second | First1 | Second |
| SNP ID | rs41263847 | rs371445823 | rs45573035 | |
| Ref allele | G | G | C | C |
| Alt allele | A | A | T | G |
| Chrs | 7 | 12 | 12 | 1 |
| Position | 1.51E+08 | 48377898 | 48390360 | 2.08E+08 |
| Strand orientation | + | − | − | + |
| Gene region | Exonic | Exonic | Exonic | Exonic |
| Function | Nonsynonymous SNV | Nonsynonymous SNV | Nonsynonymous SNV | Nonsynonymous SNV |
| SIFT score | 0.128 | 0.24 | 0.061 | 0.958 |
| SIFT Score Pred | T | T | T | T |
| POLYPhen V2 Score | 0.845 | 0.356 | 0.938 | 0 |
| POLYPhen V2 Score pred | P | B | P | B |
| MutationTaster | 1 | 1 | 1 | 1 |
| MutationTaster Pred | D | D | D | N |
| Cadd | 3.677832 | 2.954573 | 3.523652 | −1.25999 |
| Dann | 0.999 | 0.995 | 0.996 | 0.129 |
| Eigen | 0.1917 | −0.0992 | 0.1764 | −1.5893 |
| Kaviar_20150923 | 0.002186 | 1.29E-05 | 0.00066 | |
| 1000g_chbs | 0.0203 | 0.0254 | ||
| esp6500 | 0.000077 | 0.000077 | ||
| ExAC03 | 0.0026 | 1.65E-05 | 0.0007 | |
| ExAC03_EAS | 0.0307 | 0 | 0.0097 |
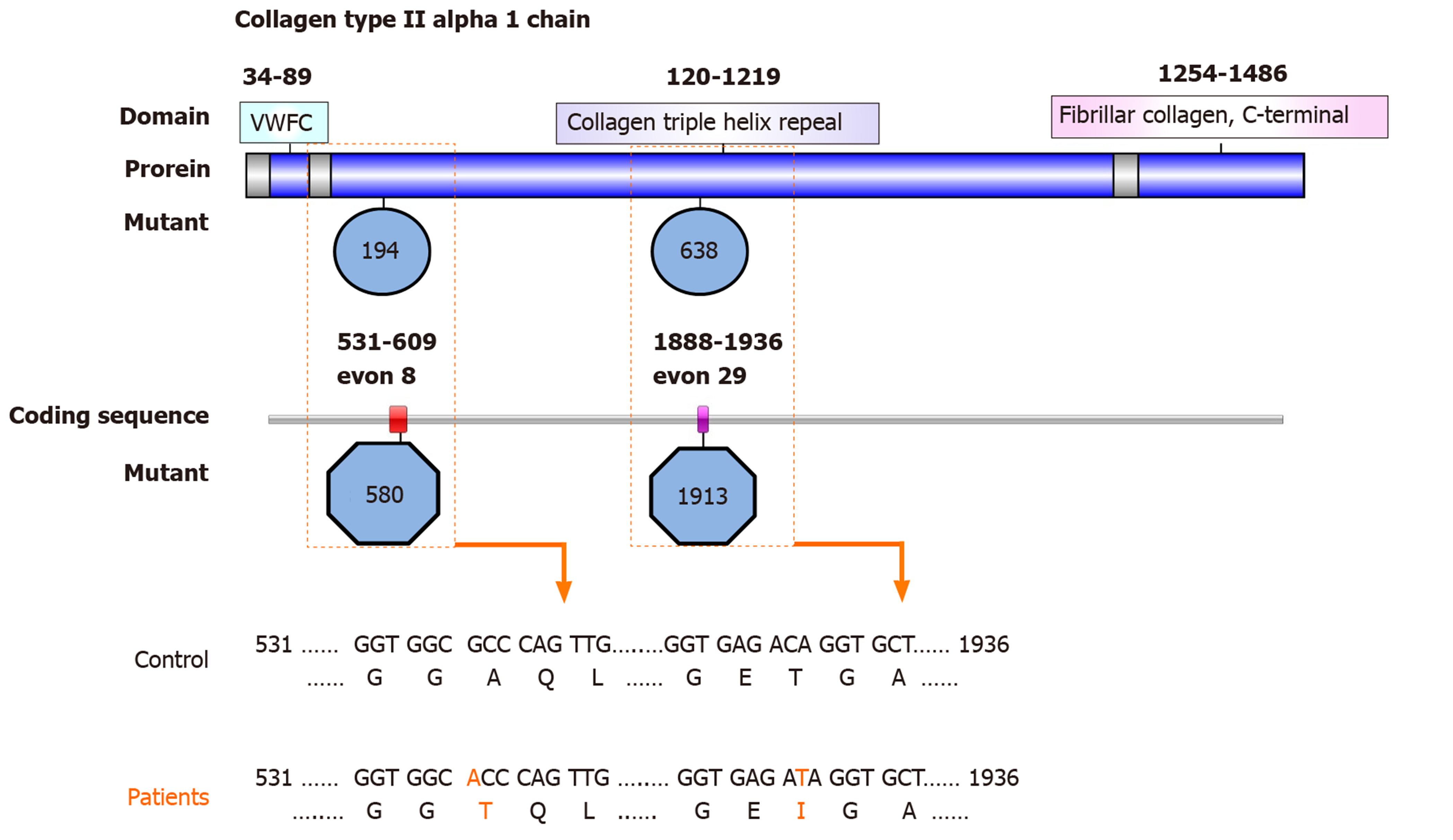
We revealed two rare functional mutations of COL2A1, both of which are nonsynonymous SNVs. One is rs41263847: COL2A1: NM_001844: exon 29: c.1913C>T: p.T638I; COL2A1: NM_033150: exon 28: c.1706C>T: p.T569I, the first priority of which is second; and was predicted as tolerated (SIFT Score Pred), benign (POLYPHEN Score Pred), and disease_causing (MutationTaster Score Pred ) damaging. The other is rs371445823 COL2A1: NM_001844: exon 8: c.580G>A: p.A194T, COL2A1: NM_033150: exon 7: c.373G>A: p.A125T (Figure 2), the first priority of which is first1; and was predicted as tolerated (SIFT Score Pred), possibly damaging (POLYPHEN Score Pred), and disease_causing damaging (MutationTaster Score Pred ) (Table 3).
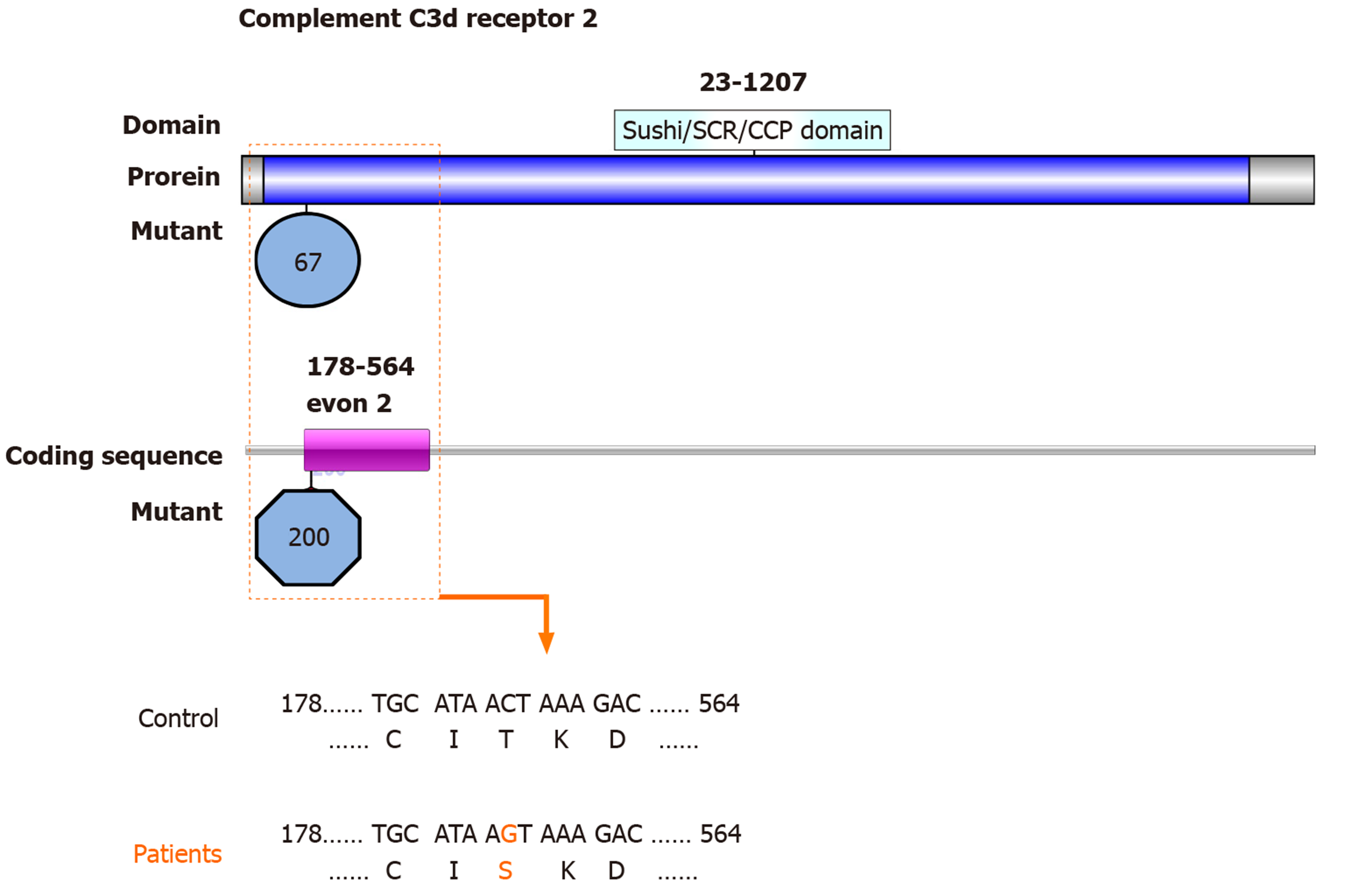
One SNV identified in the CR2 gene is also a nonsynonymous SNV with the first priority being second: rs45573035: CR2: NM_001006658: exon 2: c.200C>G: p.T67S, CR2: NM_001877: exon 2: c.200C>G: p.T67S (Figure 3). The variations were predicted as tolerated (SIFT Score Pred), possibly damaging (POLYPHEN Score Pred), and polymorphism damaging (MutationTaster Score Pred) (Table 3).
The phenotypic features of patients with SLE with ONFH are listed in Table 4. The patient with mutations in NOS3 was a 36-year-old woman who had suffered from SLE for 4 years and ONFH for 1 mo, who had skin rashes and arthritis. For the patient with mutations in CR2, she also had skin rashes and arthritis, and a renal disorder. The four patients with COL2A1 mutations (G4, G6, G34, and G42) had a relatively low SLEDAI (Systemic Lupus Erythematosus Disease Activity Index) and none of them had interstitial pneumonia, renal disorders, or neurological disorders; however, three of them had arthritis and anemia. The C3 and C4 levels, and 24-hour urinary protein levels were normal in all six patients.
| Patients (n = 49) | G19 | G4 | G6 | G34 | G42 | G2 | |
| Age (yr) | 34.1 ± 11.2 | 36 | 34 | 45 | 36 | 33 | 44 |
| Sex (female/male) | 45/4 | Female | Female | Female | Female | Female | Female |
| Disease duration of SLE (mo) | 61.8 ± 49.8 | 48 | 48 | 204 | 168 | 84 | 72 |
| Disease duration of ONFH (mo) | 14.2 ± 18.8 | 1 | 2 | 2 | 24 | 1 | 48 |
| Fever, n (%) | 4 (8.2) | N | N | Y | Y | N | N |
| Skin rashes, n (%) | 28 (57.1) | Y | Y | N | N | N | Y |
| Photosensitivity, n (%) | 8 (16.3) | N | Y | N | N | N | N |
| Raynaud phenomenon, n (%) | 11 (22.4) | N | N | Y | N | Y | N |
| Oral ulcer, n (%) | 5 (10.2) | N | N | Y | N | N | N |
| Arthritis, n (%) | 37 (75.5) | Y | N | Y | Y | Y | Y |
| Polyserositis, n (%) | 12 (24.5) | N | Y | N | N | N | N |
| Interstitial Pneumonia, n (%) | 8 (16.3) | N | N | N | N | N | N |
| Renal disorder, n (%) | 21 (42.9) | N | N | N | N | N | Y |
| Neurological disorder, n (%) | 6 (12.2) | N | N | N | N | N | N |
| Anemia, n (%) | 27 (55.1) | N | Y | Y | N | Y | N |
| Thrombocytopenia, n (%) | 6 (12.2) | N | N | N | N | Y | N |
| Leukopenia, n (%) | 8 (16.3) | N | N | Y | N | N | N |
| dsDNA, n (%) | 28 (57.1) | N | N | Y | N | N | N |
| AnuA, n (%) | 25 (51.0) | N | N | Y | N | N | N |
| Smith, n (%) | 19 (38.8) | N | N | N | N | N | N |
| AHA, n (%) | 16 (32.7) | N | N | Y | N | N | N |
| rRNP, n (%) | 8 (16.3) | N | N | Y | N | N | N |
| ESR (mm/h), n (%) | 46 (83.7) | 21 | 16 | 34 | 76 | 24 | 59 |
| Low C3, n (%) | 11 (22.4) | N | N | N | N | N | N |
| Low C4, n (%) | 10 (20.4) | N | N | N | N | N | N |
| 24-hour urine protein n (%) | 32 (65.3) | N | N | N | N | N | N |
| SLEDAI | 1-21 (8.9 ± 4.1) | 6 | 4 | 8 | 5 | 5 | 10 |
The known secondary risk factors for ONFH comprise rheumatic diseases, alcohol abuse, and the use of corticosteroids. Among autoimmune diseases, SLE has a higher ONFH incidence, ranging from 5% to 30%, compared with that in the general population. Moreover, the treatment of SLE deteriorated with the onset of ONFH. Details of the pathogenesis of ONFH in SLE are unclear because patients with SLE who have not taken corticosteroids also develop ONFH. To investigate whether patients with SLE with ONFH have a genetic predisposition, we used next generation sequencing technology to analyze SNVs in reported risk genes, including CR2, NOS3, COL2A1, PTPN22, and TRPV4. Bioinformatic analyses identified 112 SNVs and 20 InDels. Most of these genomic variations were localized in coding sequence and more than the half were nonsynonymous. Almost all insertions and deletions were 1-5 bp, except for 1 deletion that was 6-10 bp. Low frequency functional mutations of NOS3, COL2A1, and CR2 were found, although the differences between the patients and controls were not significant.
NOS3 deficiency results in impaired osteoblast function and reduced bone formation. The mutations in the NOS3 identified in the present study were all previously unreported nonsynonymous SNVs. The corresponding regions of the genome were exon 6: c.814G>A: p.E272K and exon 7: c.814G>A: p.E272K. Nitric oxide, catalyzed by endothelial nitric oxide synthase (eNOS), is involved in ONFH pathogenesis by regulating angiogenesis, thrombosis, smooth muscle proliferation, and bone turnover. Excessive nitric oxide production occurs during SLE and certain other autoimmune diseases[21]. A recent study in Korean patients with SLE suggested that exonic NOS3 polymorphisms, such as rs1549758 (Asp258Asp; exon 6) and rs1799983 (Glu298Asp; exon 7) might increase the risk of ONFH[7]. SNP Glu298Asp in NOS3 exon 7 is also associated with idiopathic ONFH in Korean patients[22,23]. The c.814G>A: p.E272K mutation in exons 6 and 7 of NOS3 was also predicted to alter protein function and predicted as tolerated, possibly damaging and disease_causing damaging using several online tools. Mutations affecting the N-terminal domain of NOS3 might alter its function, leading to alterations in the enzymatic activity or expression of eNOS, thus causing ONFH in SLE.
The COL2A1 gene is 31.5 kb, comprising 54 exons that encode a protein of 1487 amino acids with a molecular mass of 134.4 kDa. Mutations in COL2A1 result in skeletal dysplasias because of failure of cartilage development and growth, which further cause epiphyseal dysplasia of the femoral head and spinal deformity.
The cause of familial idiopathic ONFH has been reported to be four types of COL2A1 mutation in six families: c.3508G>A (p.Gly1170Ser, rs121912891); c.1888G>A (p.Gly630Ser); c.2149G>A (p.Gly717Ser, rs387906558); and c.4148C>T (p.Thr1383Met, rs138498898)[24-26]. We identified two rare functional mutations in the COL2A1 gene: rs41263847: exon 29: c.1913C>T: p.T638I, exon 28: c.1706C>T: p.T569I, and rs371445823: exon 8: c.580G>A: p.A194T, exon 7: c.373G>A: p.A125T. Exons 6-48 of the COL2A1 gene encode the core region in the 330-Gly-X-Y triple-helical domain. Previous studies demonstrated that genetic mutations in the triple-helical domain can cause damage to cartilage homeostasis and long bone development. The mutations identified in COL2A1 in the present study also mapped to this domain and might impair the assembly, folding, intracellular transport, or secretion of the type II collagens in patients with SLE, ultimately resulting in ONFH.
The membrane glyocprotein CR2 binds degraded C3 fragments that are generated during complement activation. In normal immunity, CR2 has many important functions and is believed to play a role in autoimmune disease development. Previous data suggested that rs1876453 in CR2 affects gene regulation and decreases susceptibility to lupus[27]. The nonsynonymous CR2 SNP rs17615 in exon 10 (G/A, Ser639Asn) is a conserved SNV in sheep, rats, and mice, and might affect CR2 receptor function. This mutation is associated with an increased risk of ONFH in Korean patients with SLE, possibly through impairing the normal expression of CR2[6]. In the present study, we found a nonsynonymous SNV in CR2: rs45573035: exon 2: c.200C>G: p.T67S. This variation was predicted to be possibly damaging and polymorphism damaging. Thus, these mutations in exon 2 might change the function of CR2 and increase the susceptibility of patients with SLE to ONFH.
With the aid of predictive bioinformatics tools, we identified four possible pathogenic variants. Even though the size of the patient group was small, we are the first to use next generation sequencing data to identify SNVs of CR2, NOS3, COL2A1, PTPN22, and TRPV4 genes in patients with SLE and ONFH. Based on bioinformatic studies, we identified mutations of NOS3 (exon 6: c.814G>A: p.E272K and exon 7: c.814G>A: p.E272K), COL2A1 (rs41263847: exon 29: c.1913C>T: p.T638I, exon 28: c.1706C>T: p.T569I, and rs371445823: exon 8: c.580G>A: p.A194T, exon 7: c.373G>A: p.A125T) and CR2 (rs45573035: exon 2: c.200C>G: p.T67S) that are likely to be associated with the development of ONFH in SLE. These findings may have important pharmacogenetic implications. However, the detailed mechanisms of the associations need to be determined in further studies.
All the procedures that involved human participants were performed according to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All individual participants included in the study provided informed consent.
Previous publications indicated that genetic predisposition might play important roles in the onset of osteonecrosis of the femoral head (ONFH) in systemic lupus erythematosus (SLE).
Complement C3d receptor 2 (CR2), nitric oxide synthase 3 (NOS3), collagen type II alpha 1 chain (COL2A1), protein tyrosine phosphatase non-receptor type 22 (PTPN22), and transient receptor potential cation channel subfamily V member 4 (TRPV4) were reported to be involved in the onset of ONFH in SLE.
To investigate whether the risk of ONFH in SLE is associated with single nucleotide variations (SNVs) in CR2, NOS3, COL2A1, PTPN22, and TRPV4.
SNVs in the CR2, NOS3, COL2A1, PTPN22, and TRPV4 genes were examined by using FastTarget and Illumina Miseq sequencing technologies in 49 cases of SLE with ONFH. Burrows–wheeler aligner was used to align the sequencing reads to hg19, and GATK and Varscan programs were used to perform SNV calling. PolyPhen-2, SIFT, and MutationTaster were used to assess the functional effects of non-synonymous SNVs.
Six patients were confirmed to have low frequency SNVs, including one patient with SNVs in NOS3 (exon 6: c.814G>A: p.E272K and exon 7: c.814G>A: p.E272K.), four in COL2A1 (rs41263847: exon 29: c.1913C>T: p.T638I, exon 28: c.1706C>T: p.T569I, and rs371445823: exon 8: c.580G>A: p.A194T, exon 7: c.373G>A: p.A125T), and one in CR2 (rs45573035: exon 2: c.200C>G: p.T67S).
The onset of ONFH in SLE might be associated with the identified SNVs in NOS3, COL2A1, and CR2. And the low frequency functional mutations in NOS3 had never been reported previously.
These findings may have important pharmacogenetic implications. But the detailed mechanisms of the associations need to be determined in further studies.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong J, Snyder J S-Editor: Wang J L-Editor: Wang TQ E-Editor: Liu MY
| 1. | Assouline-Dayan Y, Chang C, Greenspan A, Shoenfeld Y, Gershwin ME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94-124. [PubMed] |
| 2. | Cui L, Zhuang Q, Lin J, Jin J, Zhang K, Cao L, Lin J, Yan S, Guo W, He W, Pei F, Zhou Y, Weng X. Multicentric epidemiologic study on six thousand three hundred and ninety five cases of femoral head osteonecrosis in China. Int Orthop. 2016;40:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | Leventhal GH, Dorfman HD. Aseptic necrosis of bone in systemic lupus erythematosus. Semin Arthritis Rheum. 1974;4:73-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kim TH, Baek SH, Lim JO, Lee SH, Kim SY. Genetic variation in the coagulation factor V gene and risk of femoral head osteonecrosis. Mol Med Rep. 2015;12:4434-4440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Chang JD, Hur M, Lee SS, Yoo JH, Lee KM. Genetic background of nontraumatic osteonecrosis of the femoral head in the Korean population. Clin Orthop Relat Res. 2008;466:1041-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Kim TH, Bae SC, Lee SH, Kim SY, Baek SH. Association of Complement Receptor 2 Gene Polymorphisms with Susceptibility to Osteonecrosis of the Femoral Head in Systemic Lupus Erythematosus. Biomed Res Int. 2016;2016:9208035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kim HS, Bae SC, Kim TH, Kim SY. Endothelial nitric oxide synthase gene polymorphisms and the risk of osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2013;37:2289-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Wang L, Pan H, Zhu ZA. A genetic pedigree analysis to identify gene mutations involved in femoral head necrosis. Mol Med Rep. 2014;10:1835-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sakamoto Y, Yamamoto T, Miyake N, Matsumoto N, Iida A, Nakashima Y; Research Committee on Idiopathic Osteonecrosis of the Femoral Head of the Ministry of Health, Labour and Welfare of Japan, Iwamoto Y, Ikegawa S. Screening of the COL2A1 mutation in idiopathic osteonecrosis of the femoral head. J Orthop Res. 2017;35:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Abbasi Z, Kazemi Nezhad SR, Pourmahdi-Broojeni M, Rajaei E. Association of PTPN22 rs2476601 Polymorphism with Rheumatoid Arthritis and Celiac Disease in Khuzestan Province, Southwestern Iran. Iran Biomed J. 2017;21:61-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Fan ZD, Wang FF, Huang H, Huang N, Ma HH, Guo YH, Zhang YY, Qian XQ, Yu HG. STAT4 rs7574865 G/T and PTPN22 rs2488457 G/C polymorphisms influence the risk of developing juvenile idiopathic arthritis in Han Chinese patients. PLoS One. 2015;10:e0117389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Mah W, Sonkusare SK, Wang T, Azeddine B, Pupavac M, Carrot-Zhang J, Hong K, Majewski J, Harvey EJ, Russell L, Chalk C, Rosenblatt DS, Nelson MT, Séguin C. Gain-of-function mutation in TRPV4 identified in patients with osteonecrosis of the femoral head. J Med Genet. 2016;53:705-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7731] [Cited by in RCA: 8636] [Article Influence: 308.4] [Reference Citation Analysis (0)] |
| 14. | Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7598] [Cited by in RCA: 8615] [Article Influence: 574.3] [Reference Citation Analysis (0)] |
| 15. | McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16037] [Cited by in RCA: 18846] [Article Influence: 1256.4] [Reference Citation Analysis (0)] |
| 16. | Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3230] [Cited by in RCA: 3578] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 17. | Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7976] [Cited by in RCA: 10388] [Article Influence: 692.5] [Reference Citation Analysis (0)] |
| 18. | Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812-3814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4033] [Cited by in RCA: 4475] [Article Influence: 203.4] [Reference Citation Analysis (0)] |
| 19. | Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10971] [Cited by in RCA: 10427] [Article Influence: 695.1] [Reference Citation Analysis (0)] |
| 20. | Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2146] [Cited by in RCA: 2356] [Article Influence: 157.1] [Reference Citation Analysis (0)] |
| 21. | Clancy RM, Amin AR, Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998;41:1141-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Koo KH, Lee JS, Lee YJ, Kim KJ, Yoo JJ, Kim HJ. Endothelial nitric oxide synthase gene polymorphisms in patients with nontraumatic femoral head osteonecrosis. J Orthop Res. 2006;24:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Tsukada T, Yokoyama K, Arai T, Takemoto F, Hara S, Yamada A, Kawaguchi Y, Hosoya T, Igari J. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophys Res Commun. 1998;245:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 314] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Liu YF, Chen WM, Lin YF, Yang RC, Lin MW, Li LH, Chang YH, Jou YS, Lin PY, Su JS, Huang SF, Hsiao KJ, Fann CS, Hwang HW, Chen YT, Tsai SF. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N Engl J Med. 2005;352:2294-2301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Li N, Yu J, Cao X, Wu QY, Li WW, Li TF, Zhang C, Cui YX, Li XJ, Yin ZM, Xia XY. A novel p. Gly630Ser mutation of COL2A1 in a Chinese family with presentations of Legg-Calvé-Perthes disease or avascular necrosis of the femoral head. PLoS One. 2014;9:e100505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Kannu P, O'Rielly DD, Hyland JC, Kokko LA. Avascular necrosis of the femoral head due to a novel C propeptide mutation in COL2A1. Am J Med Genet A. 2011;155A:1759-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Zhao J, Giles BM, Taylor RL, Yette GA, Lough KM, Ng HL, Abraham LJ, Wu H, Kelly JA, Glenn SB, Adler AJ, Williams AH, Comeau ME, Ziegler JT, Marion M, Alarcón-Riquelme ME; BIOLUPUS and GENLES Networks, Alarcón GS, Anaya JM, Bae SC, Kim D, Lee HS, Criswell LA, Freedman BI, Gilkeson GS, Guthridge JM, Jacob CO, James JA, Kamen DL, Merrill JT, Sivils KM, Niewold TB, Petri MA, Ramsey-Goldman R, Reveille JD, Scofield RH, Stevens AM, Vilá LM, Vyse TJ, Kaufman KM, Harley JB, Langefeld CD, Gaffney PM, Brown EE, Edberg JC, Kimberly RP, Ulgiati D, Tsao BP, Boackle SA. Preferential association of a functional variant in complement receptor 2 with antibodies to double-stranded DNA. Ann Rheum Dis. 2016;75:242-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |