Published online Jun 26, 2020. doi: 10.12998/wjcc.v8.i12.2473
Peer-review started: January 30, 2020
First decision: April 21, 2020
Revised: May 9, 2020
Accepted: May 13, 2020
Article in press: May 13, 2020
Published online: June 26, 2020
Processing time: 145 Days and 21.2 Hours
Multiple system atrophy (MSA) is a serious progressive neurodegenerative disease. Early diagnosis of MSA is very difficult, and diagnostic biomarkers are limited. Growth differentiation factor 15 (GDF15) is involved in the differentiation and progression of the central nervous system, and is widely distributed in peripheral blood, which may be a novel biomarker for MSA.
To determine serum GDF15 levels, related factors and their potential diagnostic value in MSA patients, compared with Parkinson’s disease (PD) patients and healthy controls.
A case-control study was conducted, including 49 MSA patients, 50 PD patients and 50 healthy controls. Serum GDF15 levels were measured by human enzyme-linked immunosorbent assay, and the differences between the MSA, PD and control groups were analyzed. Further investigations were performed in different MSA subgroups according to age of onset, sex, clinical subtypes, diagnostic criteria, and disease duration. Receiver-operating characteristic curve analysis was used to evaluate the diagnostic value of GDF15, especially for the differential diagnosis between MSA and PD.
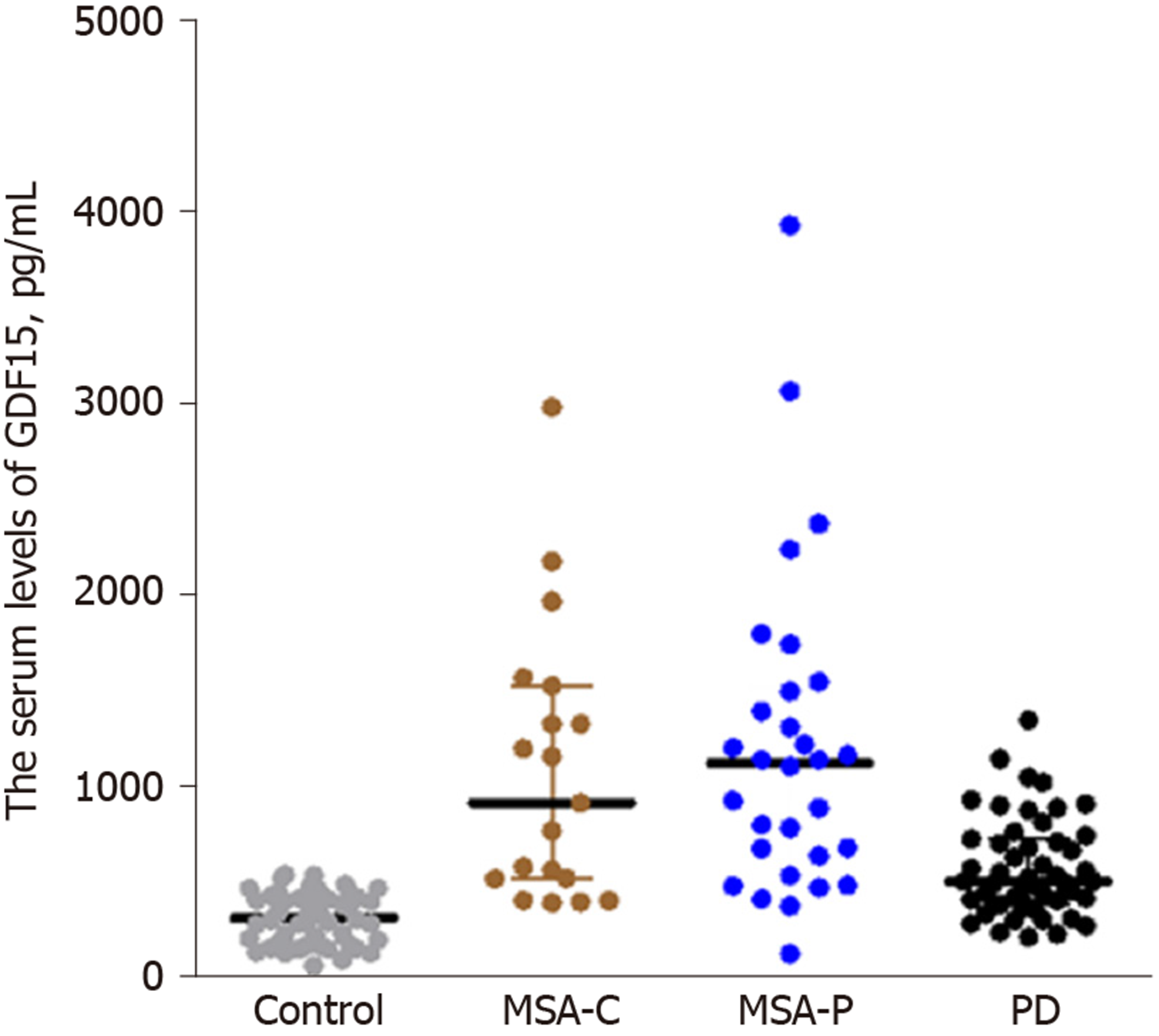
Serum GDF15 levels were significantly higher in MSA patients than in PD patients and healthy controls (P = 0.000). Males and those with a disease duration of more than three years showed higher serum GDF15 levels (P = 0.043 and 0.000; respectively). Serum GDF15 levels may be a potential diagnostic biomarker for MSA patients compared with healthy controls and PD patients (cutoff: 470.42 pg/mL, sensitivity: 85.7%, specificity: 88.0%; cutoff: 1075.91 pg/mL, sensitivity: 51.0%, specificity: 96.0%; respectively).
Serum GDF15 levels are significantly higher in MSA patients and provide suggestions on the etiology of MSA.
Core tip: In this case-control study, we determined serum growth differentiation factor 15 (GDF15) levels in multiple system atrophy (MSA) patients, Parkinson’s disease (PD) patients and healthy controls. We found that serum GDF15 levels in MSA patients were significantly higher compared with PD patients and healthy controls. These findings suggest the potential value of serum GDF15 levels as a diagnostic biomarker of MSA, and serum GDF15 contributes to the differential diagnosis between MSA and PD.
- Citation: Yue T, Lu H, Yao XM, Du X, Wang LL, Guo DD, Liu YM. Elevated serum growth differentiation factor 15 in multiple system atrophy patients: A case control study. World J Clin Cases 2020; 8(12): 2473-2483
- URL: https://www.wjgnet.com/2307-8960/full/v8/i12/2473.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i12.2473
Multiple system atrophy (MSA) is a rare progressive neurodegenerative disease characterized by parkinsonism, cerebellar impairment, and autonomic dysfunction[1]. The degeneration of striatonigral and olivopontocerebellar neurons has commonly been observed in MSA patients, accompanied by deposition of fibrillized α-synuclein proteins[2-4]. The mechanisms underlying these pathological changes are not fully understood. According to the predominance of motor symptoms, patients can be further divided into the parkinsonian type (MSA-P) and the cerebellar type (MSA-C)[2]. It is difficult to provide an accurate diagnosis of MSA due to the heterogeneity in clinical manifestations and symptoms that are similar to other neurodegenerative diseases at the early stage, especially the differential diagnosis between MSA-P and Parkinson’s disease (PD)[5,6]. Thus, there is an urgent need for biomarkers for early diagnosis, prognostic prediction of disease progression, and monitoring the therapeutic response[7].
Growth differentiation factor 15 (GDF15), a member of the transforming growth factor-β superfamily, is widely expressed in both the central and peripheral nervous systems[8,9]. GDF15 is involved in a vast array of physiological processes, including cell proliferation, differentiation and repair[10], and might participate in the progression of numerous pathological conditions[11]. Apart from cancer, metabolic diseases, cardiovascular events, cerebrovascular diseases and mitochondrial disorders[11-16], abnormal concentrations of GDF15 are also considered to have a close association with neurodegenerative diseases and may be a potential biomarker for early diagnosis, as well as prognostic utility[17-19].
Additionally, it has been demonstrated that elevated levels of GDF15 were found in patients with Alzheimer’s disease, cognitive impairment, Lewy body dementia and PD[17,19,20]. Animal studies have shown that GDF15 might play a protective role in response to stress and contribute to neurogenesis[19,21-23]. Machado et al[24] found that GDF15 deficiency could exacerbate dopaminergic neuron survival and decrease the inflammatory response in the 6-hydroxydopamine mouse model of PD. Furthermore, in vitro studies have demonstrated that GDF15 was highly expressed in ventral tegmental area astrocytes and might selectively protect ventral tegmental area dopaminergic neurons[25]. MSA has shown a similar pathogenesis to PD[26], however, whether GDF15 also participates in the progression of MSA is unknown. In this regard, we hypothesized that GDF15 may be higher in MSA patients than in normal controls and perhaps higher than in PD patients.
Therefore, in this case-control study, we aimed to determine serum levels of GDF15 in MSA patients and its potential diagnostic utility. Forty-nine MSA patients, 50 PD patients and 50 healthy controls were enrolled. The serum levels of GDF15 in the different groups and in different subgroups of MSA patients were analyzed. Receiver-operating characteristic (ROC) analysis was performed to assess the diagnostic utility.
This study was approved by the ethics committee of Qilu Hospital of Shandong University, and all participants signed a written informed consent. MSA and PD patients were recruited from the outpatient and inpatient clinics of the Department of Neurology, Qilu Hospital of Shandong University, from August 2015 to September 2016.
Both MSA and PD patients were diagnosed, by an experienced specialist majoring in PD and movement disorders, according to the consensus statement by the American Autonomic Society and the American Academy of Neurology[2] and the Movement Disorders Society[27].
Eleven MSA patients were excluded for the following reasons: Coronary heart disease; diabetes; hypertension; lung disorders; chronic kidney disease; acute or chronic infection; cancer; dementia; or psychological disorders. Finally, 49 MSA patients were enrolled and 50 PD patients and 50 healthy controls that were matched for age (± 2 years) were selected. All the PD patients enrolled also fulfilled the exclusion criteria for MSA patients. All healthy volunteers in the control group were from the Physical Examination Center of Qilu Hospital.
Basic clinical information, including age, sex, age of onset of the disease, and duration of the disease was collected for all MSA patients and PD patients. In addition, the Unified MSA Rating Scale (UMSARS)-II scores and the Unified PD Rating Scale (UPDRS)-III scores were used to assess the motor severity in MSA and PD patients, respectively. For the MSA patients, the clinical subtypes (MSA-P/MSA-C) and diagnostic criteria (probable/possible) were also recorded according to the relevant consensus criteria[2].
Blood samples were collected from all participants. After centrifugation, serum samples were stored at -80°C. A high-sensitivity human enzyme-linked immunosorbent assay kit (Abcam, Cambridge, MA, United States) was used to detect serum levels of GDF15, according to the manufacturer’s instructions.
The levels of serum GDF15 were compared among the MSA patients, PD patients and healthy controls using the Kruskal-Wallis H test. The levels of GDF15 in the MSA patients with different clinical features, including sex (female/male), clinical subtypes (MSA-P/MSA-C), and diagnostic certainty (probable/possible MSA), were compared using the Mann-Whitney test. Data are expressed as the median (interquartile range) or means ± SD. Categorical variables are expressed as numbers (%). The χ2 test was used for categorical variables. SPSS 22.0 software (Chicago, IL, United States) was used for the statistical analyses. P < 0.05 was regarded as statistically significant.
A ROC curve was used to analyze the sensitivity and specificity of GDF15 for differentiating MSA patients from PD patients and healthy controls, and to evaluate the diagnostic value of GDF15. The area under the curve (AUC) gives an indication of predictive values, with AUC = 0.5 for a random association and AUC = 1 for perfect discrimination. The optimum cutoff value was determined by the Youden index. Statistical significance was accepted at two-tailed P < 0.05.
The clinical characteristics of the participants in this study are presented in Table 1. The ages and the sex composition of the participants in the MSA group, PD group and healthy control group were similar. In addition, the onset age, and clinical severity (scores on the clinical rating scales) of the MSA group and PD group are displayed and showed no differences. The patients in the MSA group were classified as MSA-P or MSA-C types and probable or possible types. Some common nonmotor symptoms, motor symptoms and imaging features of the MSA-P and MSA-C patients are presented and showed no differences between the subgroups (Supplementary Table 1).
| MSA | PD | Control | P value | |
| All | ||||
| Subjects, n | 49 | 50 | 50 | - |
| Age, yr | 61.7 ± 7.7 | 61.4 ± 7.5 | 58.5 ± 7.5 | 0.077 |
| Female/male, n | 26/23 | 26/24 | 30/20 | 0.683 |
| Age of onset, yr | 58.7 ± 7.4 | 56.6 ± 7.1 | - | |
| MSA-P/MSA-C, n | 30/20 | - | - | - |
| Probable/Possible, n | 31/18 | - | - | - |
| Clinical rate scales (UMSARSII/UPDRSIII) | 19.5 ± 6.4 (UMSARSII) | 26.1 ± 13.2 (UPDRSIII) | - - | - |
| GDF15, pg/mL | 1105.69 (984.24) | 506.70 (346.80) | 313.85 (247.76) | 0.000 |
The serum GDF15 levels in the MSA patients [1105.69 (984.24)] were significantly higher than those in PD patients [506.70 (346.80); P = 0.000] and healthy controls [313.85 (247.76); P = 0.000] (Table 1). Further analysis showed that serum GDF15 levels in MSA-P patients [1121.82 (897.09)] and MSA-C patients [916.14 (1009.59)] were both significantly higher compared with PD patients [506.70 (346.80); P = 0.000, 0.000, respectively] (Figure 1).
Serum GDF15 levels were significantly higher in male patients with MSA than in female patients [1311.84 (1214.16) vs 795.31 (698.54), respectively; P = 0.043]. Patients with disease duration of more than 3 years also showed higher serum GDF15 levels than those with a shorter disease duration [1393.79 (1021.36) vs 612.31 (527.96), respectively; P = 0.000]. However, the serum GDF15 levels in patients with different ages of onset [> 50 years vs ≤ 50 years; 1156.99 (1028.41) vs 795.32 (605.42), respectively; P = 0.185], clinical subtypes [MSA-P vs MSA-C; 1121.82 (897.09) vs 916.14 (1009.59), respectively; P = 0.622], or diagnostic criteria [probable vs possible; 887.55 (1004.50) vs 1182.16 (753.32), respectively; P = 0.561] showed no differences (Table 2).
| Subgroups | GDF15, pg/mL | P value |
| Age of onset | ||
| > 50 yr | 1156.99 (1028.41) | 0.185 |
| ≤ 50 yr | 795.32 (605.42) | |
| Gender | ||
| Female | 795.31 (698.54) | 0.043 |
| Male | 1311.84 (1214.16) | |
| Clinical subtype | ||
| MSA-P | 1121.82 (897.09) | 0.622 |
| MSA-C | 916.14 (1009.59) | |
| Diagnosis criteria | ||
| Probable | 887.55 (1004.50) | 0.561 |
| Possible | 1182.16 (753.32) | |
| Duration | ||
| > 3 yr | 1393.79 (1021.36) | 0.000 |
| ≤ 3 yr | 612.31 (527.96) |
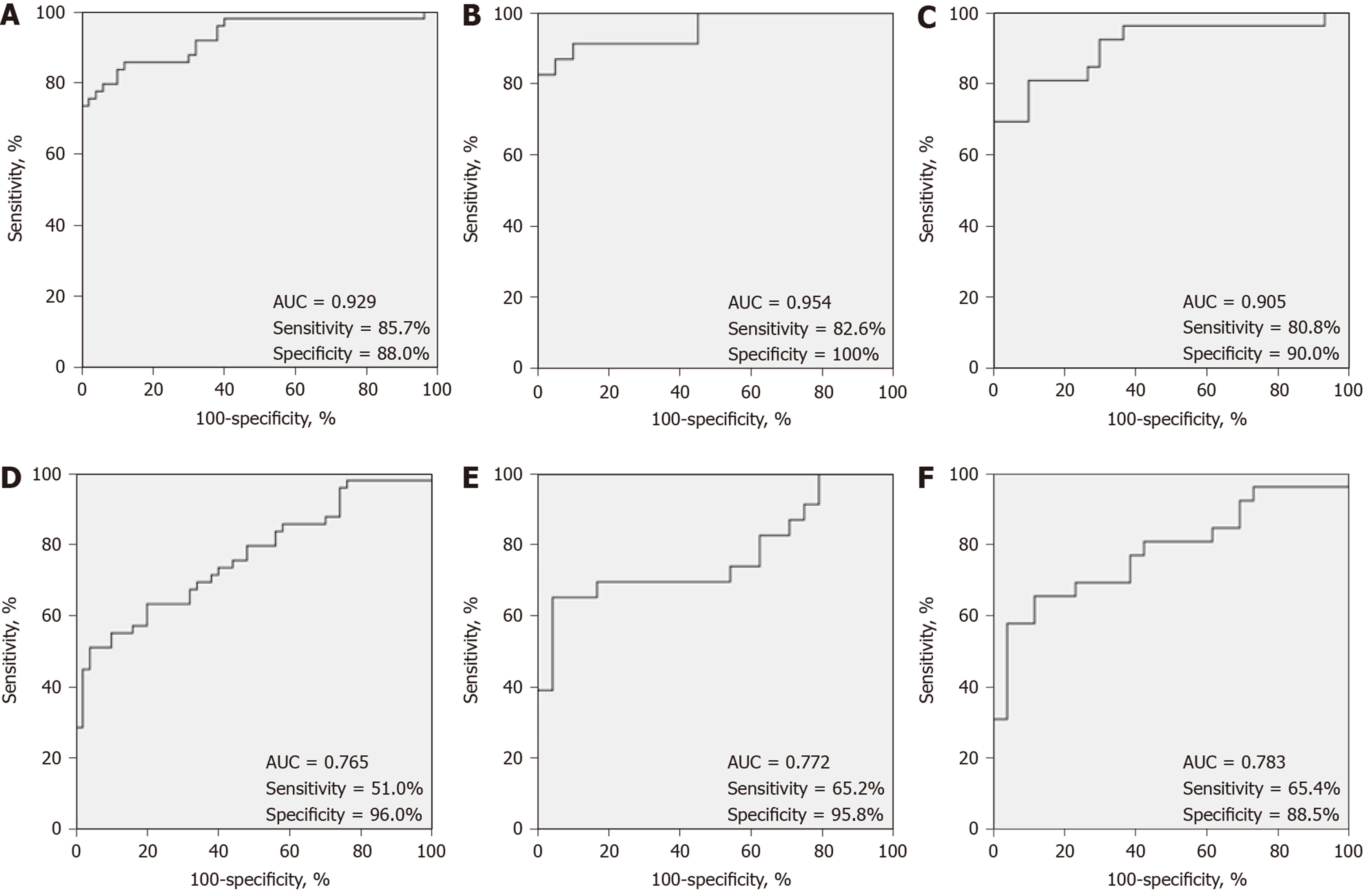
ROC curve analysis was performed to assess the potential diagnostic value of serum GDF15 levels in MSA patients. As shown in Figure 2, the best cutoff concentration was 470.42 pg/mL for all MSA patients, and the AUC was 0.929 (SE: 0.027, 95%CI: 0.876-0.981, P = 0.000), while the sensitivity and specificity were 85.7% and 88.0%, respectively. For males, the AUC was 0.954 (SE: 0.030, 95%CI: 0.896-1.000, P = 0.000), with a sensitivity of 82.6% and specificity of 100.0%, where the optimal cutoff value for serum GDF15 levels was 552.10 pg/mL. In females, the AUC was 0.905 (SE: 0.044, 95%CI: 0.820-0.990, P = 0.000), with a sensitivity of 80.8% and specificity of 90.0%, where the optimal cutoff value for serum GDF15 levels was 470.42 pg/mL (Table 3, Figure 2).
| Group | Subtype | AUC | SE | 95%CI | P value | Cutoff, pg/mL | Sensitivity, % | Specificity, % |
| MSA vs Control | Overall | 0.929 | 0.027 | 0.876-0.981 | 0.000 | 470.42 | 85.7 | 88.0 |
| Male | 0.954 | 0.030 | 0.896-1.000 | 0.000 | 552.10 | 82.6 | 100.0 | |
| Female | 0.905 | 0.044 | 0.820-0.990 | 0.000 | 470.42 | 80.8 | 90.0 | |
| MSA vs PD | Overall | 0.765 | 0.048 | 0.671-0.859 | 0.000 | 1075.91 | 51.0 | 96.0 |
| Male | 0.772 | 0.072 | 0.630-0.914 | 0.001 | 1075.91 | 65.2 | 95.8 | |
| Female | 0.783 | 0.066 | 0.654-0.911 | 0.000 | 616.39 | 65.4 | 88.5 |
The potential value of serum GDF15 levels in the differential diagnosis between MSA and PD was also assessed. The best cutoff concentration was 1075.91 pg/mL for all MSA patients, and the AUC was 0.765 (SE: 0.0458, 95%CI: 0.671-0.859, P = 0.000), while the sensitivity and specificity were 51.0% and 95.0%, respectively. For males, the AUC was 0.772 (SE: 0.072, 95%CI: 0.630-0.914, P = 0.001), with a sensitivity of 65.2% and specificity of 95.8%, where the optimal cutoff value for serum GDF15 levels was 1075.91 pg/mL. In females, the AUC was 0.783 (SE: 0.066, 95%CI: 0.654-0.911, P = 0.000), with a sensitivity of 65.4% and specificity of 88.5%, where the optimal cutoff value for serum GDF15 levels was 616.39 pg/mL (Table 3, Figure 2).
In this case-control study, we determined the serum GDF15 levels in MSA patients, PD patients and healthy controls and compared the differences between different MSA subgroups, which were classified by age of onset, sex, clinical subtype, diagnostic criteria or disease duration. In addition, the potential value of serum GDF15 levels as a diagnostic biomarker of MSA was analyzed, especially for the differential diagnosis between MSA and PD. We found that serum GDF15 levels in MSA patients were significantly higher compared with PD patients and healthy controls. Male patients with MSA showed higher serum GDF15 levels than female patients, and serum GDF15 levels were also higher in those with a disease duration of more than 3 years.
Studies on blood and cerebrospinal fluid biomarkers for MSA have been carried out, which have focused on total α-syn, markers of axonal degeneration, or catecholamines[7]. Due to the complexity of the diagnostic process and the lack of effective treatment for the disorder, the priority is identification of potential and non-invasive biomarkers, which would facilitate the development of clinical trials[28]. The ideal biomarker would be detectable in easily accessible samples, i.e., blood, urine, saliva, or exhaled air, as subideal biomarkers such as cerebrospinal fluid, muscle, liver, or heart require more invasive procedures. A recent study revealed that mean serum GDF15 levels in PD patients were significantly higher than those in the healthy control group[17]. Therefore, we investigated serum GDF15 in MSA patients as blood collection is non-invasive and easily accessed.
GDF15 is a cytokine of the transforming growth factor-β superfamily and is expressed mainly in the placenta, kidney, liver, lung, pancreas, and prostate. It has an essential role in regulating the cellular response to stress signals and inflammation and is involved in the suppression of inflammation in early pregnancy, cancer, and cardiovascular diseases. Moreover, GDF15 is expressed in the choroid plexus and acts as a potent neurotrophic factor for motor and sensory neurons[29].
Serum GDF15 levels were found to be higher in patients with PD, dementia and Alzheimer’s disease[17,19,20]. Therefore, whether these differences are also present in other neurodegenerative diseases seemed worthy of study. It has been reported that peripheral levels of GDF15 were associated with cognitive impairment in patients with dementia and Alzheimer’s disease patients with white matter hyperintensities[20]. Another study suggested that GDF15 could be considered a marker of age-related cognitive decline and brain structural changes[30]. Similarly, elevated plasma GDF15 was associated with slower gait speed and lower physical performance in very healthy community-dwelling adults[31], while another study confirmed that baseline GDF15 values predicted declining physical function in older patients with hypertension[32]. However, it has not been previously reported whether serum GDF15 levels are also higher in patients with MSA. In the present study, we found that the mean GDF15 level in the MSA group was approximately 3-fold higher than those in the control group.
The clinical history and neurological exam are the basis of the diagnosis of MSA, which can be assisted by ancillary tests (olfactory testing, autonomic testing, neuroimaging, urological evaluation), and the diagnosis can only be confirmed pathologically after the patient’s death. The diagnostic accuracy of MSA varies greatly between different centers, from as little as 29% to 86% (approximately 62% according to autopsies), despite well-established diagnostic criteria[2,33-36]. There were no significant imaging abnormalities in the early disease stages and it has been challenging to study patients with a postmortem diagnosis of MSA. The most common diseases misdiagnosed as MSA have included PD, progressive supranuclear paralysis and Lewy body dementia. The large difference in diagnostic accuracy may be due to the duration and stage of MSA, and to the presence of various pathological changes in patients with MSA-P[37-39]. However, such differentiation is important for prognosis and treatment.
Due to the heterogeneity of clinical manifestations, it is extremely difficult to make an exact distinction between MSA and PD patients, especially in the early stage[34,35]. Although significant differences between MSA, PD, and other parkinsonian disorders were found for some markers, none provided sufficient sensitivity and specificity for a differential diagnosis[7]. This study showed that serum GDF15 levels might contribute to this effort to some extent. The patients with MSA showed significantly higher serum GDF15 levels than the PD patients and healthy controls. In addition, the ROC curve analysis revealed that serum GDF15 levels also had high specificity, while the sensitivity seemed unsatisfactory in the differential diagnosis between MSA and PD. According to a recent study, autopsy studies show that approximately 5% of PD patients diagnosed by community neurologists turn out to have MSA at autopsy[40], which might be correlated with the sensitivity of serum GDF15 levels which was not satisfactory for the differential diagnosis between MSA and PD.
Further analysis revealed that sex and disease duration might be correlated with higher serum levels of GDF15 in MSA patients. Similar to PD patients, male patients with MSA had a greater elevation in serum GDF15 levels. A previous study found no influence of sex on overall survival, but males had a shorter duration from diagnosis to death[41]. In addition, MSA patients with a longer disease duration also had higher serum GDF15 levels. The mean disease duration for the 46 patients with MSA was 3.0 ± 1.9 years[42]. In addition, in a comprehensive large‐scale study of 143 Chinese MSA patients, the mean disease duration was 3.54 ± 1.96 years. Therefore, in this study, we compared the serum GDF15 levels between patients with disease duration longer than 3 years and those with disease duration shorter than 3 years. The MSA patients with a longer disease duration showed higher serum GDF15 levels.
It has been suggested that MSA patients with older age of onset[43-47], sex (male or female)[43,46,48,49], with a particular subtype of MSA-P[46,51,52], and with early or initial autonomic symptoms[46,47,51-53] were associated with worse survival, while the late development of autonomic failure has been reported as a favorable prognostic factor[54,55]. Surprisingly, patients with different ages of onset (> 50 years/≤ 50 years), clinical subtypes (MSA-P/MSA-C), or diagnostic criteria (probable/possible) showed no differences in serum levels of GDF15.
After adjusting for disease duration, the MSA‐P patients showed more severe motor impairment and disability compared with the MSA‐C patients, and severe depression, anxiety, hyposmia, and cognitive deficits were more common among the MSA‐P patients than the MSA‐C patients. On the other hand, there was no difference in overall survival between the MSA-P and MSA-C patients. Consistent with the clinical manifestations of MSA-P and MSA-C patients, no differences were found in GDF15 levels.
MSA is a rare and fatal neurodegenerative disorder that is characterized by a variable combination of parkinsonism, cerebellar impairment, and autonomic dysfunction[2], and the exact mechanisms underlying the pathogenesis are not well understood. Mitochondrial dysfunction (MD) has been connected to the neurodegenerative process in MSA[56,57].
Serum GDF15 (sGDF15) has been identified in recent years as a potential diagnostic biomarker for MDs[58]. The role of sGDF15 as a biomarker for MDs has been tested in large patient cohorts with different mitochondrial defects, and showed high sensitivity and specificity[59]. It was shown that sGDF15 does not correlate with disease severity in a large cohort of adult A3243G mutation carriers, which suggested that GDF15 seems to be more indicative of MD regardless of clinical phenotype[58]. To further explore whether serum GDF15 levels are consistent with clinical manifestations requires studies with larger sample sizes in the future.
Taken together, this study revealed that serum GDF15 may be a potential diagnostic biomarker in MSA. However, some limitations also exist and need to be improved upon in subsequent studies. Firstly, the sample size was limited, and it was difficult to obtain better insight into the relevant risk factors for the elevated serum GDF15 levels in MSA patients. More importantly, patients covering the broad range of disease stages are necessary, especially those in the prodromal stage, and this might contribute greatly to the early diagnosis of MSA.
In summary, serum GDF15 levels are significantly higher in MSA patients and might be a potential diagnostic biomarker and provide some suggestions regarding the etiology of MSA.
Multiple system atrophy (MSA) is a serious progressive neurodegenerative disease. The early diagnosis of MSA is very difficult, and diagnostic biomarkers are limited. There is an urgent need for biomarkers for early diagnosis, prognostic prediction of disease progression, and monitoring the therapeutic response.
Growth differentiation factor 15 (GDF15) is a member of the transforming growth factor-β superfamily. Abnormal concentrations of GDF15 are considered to have a close association with neurodegenerative diseases and might be a potential biomarker for early diagnosis. It has not been previously reported whether serum GDF15 levels are also higher in patients with MSA.
This study aimed to determine serum GDF15 levels, related factors and their potential diagnostic value in MSA patients.
A case-control study was conducted, which included 49 MSA patients, 50 Parkinson’s disease (PD) patients and 50 healthy controls. Serum GDF15 levels were measured by human enzyme-linked immunosorbent assay and the differences between the MSA, PD and control groups were analyzed.
Serum GDF15 levels were significantly higher in MSA patients than in PD patients and healthy controls (P = 0.000). Males and those with a disease duration of more than three years showed higher serum GDF15 levels (P = 0.043 and 0.000; respectively). Serum GDF15 levels might be a potential diagnostic biomarker for MSA patients compared with healthy controls and PD patients (cutoff: 470.42 pg/mL, sensitivity: 85.7%, specificity: 88.0%; cutoff: 1075.91 pg/mL, sensitivity: 51.0%, specificity: 96.0%; respectively).
We found that serum GDF15 levels in MSA patients were significantly higher compared with PD patients and healthy controls. Male patients with MSA had higher serum GDF15 levels than female patients, and serum GDF15 levels were also higher in those with disease duration of more than 3 years. The receiver-operating characteristic curve analysis revealed that serum GDF15 levels had high specificity, while the sensitivity seemed unsatisfactory in the differential diagnosis between MSA and PD. Serum GDF15 levels might be a potential diagnostic biomarker for MSA patients.
This study revealed that serum GDF15 might be a potential diagnostic biomarker in MSA. To further explore whether serum GDF15 levels are consistent with clinical manifestations requires studies with larger sample sizes in the future. The difference between serum GDF15 and cerebrospinal fluid GDF15 should be considered.
We thank all medical staff and technicians of dialysis centers who agreed to participate in this study.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Member of Parkinson's Disease and Motor Disorders Group, Neurology Branch, Chinese Medical Association.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishizawa K S-Editor: Dou Y L-Editor: Webster JR E-Editor: Liu JH
| 1. | Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. 2015;372:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 548] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 2. | Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2050] [Cited by in RCA: 2353] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 3. | Wong YC, Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 638] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 4. | Woerman AL, Watts JC, Aoyagi A, Giles K, Middleton LT, Prusiner SB. α-Synuclein: Multiple System Atrophy Prions. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Kim HJ, Jeon BS, Jellinger KA. Diagnosis and differential diagnosis of MSA: boundary issues. J Neurol. 2015;262:1801-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tsuda M, Asano S, Kato Y, Murai K, Miyazaki M. Differential diagnosis of multiple system atrophy with predominant parkinsonism and Parkinson's disease using neural networks. J Neurol Sci. 2019;401:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Laurens B, Constantinescu R, Freeman R, Gerhard A, Jellinger K, Jeromin A, Krismer F, Mollenhauer B, Schlossmacher MG, Shaw LM, Verbeek MM, Wenning GK, Winge K, Zhang J, Meissner WG. Fluid biomarkers in multiple system atrophy: A review of the MSA Biomarker Initiative. Neurobiol Dis. 2015;80:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018;28:353-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 9. | Wang X, Krebbers J, Charalambous P, Machado V, Schober A, Bosse F, Müller HW, Unsicker K. Growth/differentiation factor-15 and its role in peripheral nervous system lesion and regeneration. Cell Tissue Res. 2015;362:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Strelau J, Sullivan A, Böttner M, Lingor P, Falkenstein E, Suter-Crazzolara C, Galter D, Jaszai J, Krieglstein K, Unsicker K. Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. J Neurosci. 2000;20:8597-8603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Corre J, Hébraud B, Bourin P. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med. 2013;2:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Villanueva MT. Obesity: GDF15 tells the brain to lose weight. Nat Rev Drug Discov. 2017;16:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Wollert KC, Kempf T, Wallentin L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin Chem. 2017;63:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 394] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 14. | Meloux A, Rigal E, Rochette L, Cottin Y, Bejot Y, Vergely C. Ischemic Stroke Increases Heart Vulnerability to Ischemia-Reperfusion and Alters Myocardial Cardioprotective Pathways. Stroke. 2018;49:2752-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Gröschel K, Schnaudigel S, Edelmann F, Niehaus CF, Weber-Krüger M, Haase B, Lahno R, Seegers J, Wasser K, Wohlfahrt J, Vollmann D, Stahrenberg R, Wachter R. Growth-differentiation factor-15 and functional outcome after acute ischemic stroke. J Neurol. 2012;259:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yatsuga S, Fujita Y, Ishii A, Fukumoto Y, Arahata H, Kakuma T, Kojima T, Ito M, Tanaka M, Saiki R, Koga Y. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. 2015;78:814-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 17. | Yao X, Wang D, Zhang L, Wang L, Zhao Z, Chen S, Wang X, Yue T, Liu Y. Serum Growth Differentiation Factor 15 in Parkinson Disease. Neurodegener Dis. 2017;17:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Maetzler W, Deleersnijder W, Hanssens V, Bernard A, Brockmann K, Marquetand J, Wurster I, Rattay TW, Roncoroni L, Schaeffer E, Lerche S, Apel A, Deuschle C, Berg D. GDF15/MIC1 and MMP9 Cerebrospinal Fluid Levels in Parkinson's Disease and Lewy Body Dementia. PLoS One. 2016;11:e0149349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Kim DH, Lee D, Chang EH, Kim JH, Hwang JW, Kim JY, Kyung JW, Kim SH, Oh JS, Shim SM, Na DL, Oh W, Chang JW. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer's disease model. Stem Cells Dev. 2015;24:2378-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 20. | Chai YL, Hilal S, Chong JP, Ng YX, Liew OW, Xu X, Ikram MK, Venketasubramanian N, Richards AM, Lai MK, Chen CP. Growth differentiation factor-15 and white matter hyperintensities in cognitive impairment and dementia. Medicine (Baltimore). 2016;95:e4566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 375] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Kim KH, Kim SH, Han DH, Jo YS, Lee YH, Lee MS. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci Rep. 2018;8:6789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 23. | Carrillo-García C, Prochnow S, Simeonova IK, Strelau J, Hölzl-Wenig G, Mandl C, Unsicker K, von Bohlen Und Halbach O, Ciccolini F. Growth/differentiation factor 15 promotes EGFR signalling, and regulates proliferation and migration in the hippocampus of neonatal and young adult mice. Development. 2014;141:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Machado V, Haas SJ, von Bohlen Und Halbach O, Wree A, Krieglstein K, Unsicker K, Spittau B. Growth/differentiation factor-15 deficiency compromises dopaminergic neuron survival and microglial response in the 6-hydroxydopamine mouse model of Parkinson's disease. Neurobiol Dis. 2016;88:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Kostuk EW, Cai J, Iacovitti L. Subregional differences in astrocytes underlie selective neurodegeneration or protection in Parkinson's disease models in culture. Glia. 2019;67:1542-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Ubhi K, Low P, Masliah E. Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci. 2011;34:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30:1600-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (0)] |
| 28. | Steele HE, Horvath R, Lyon JJ, Chinnery PF. Monitoring clinical progression with mitochondrial disease biomarkers. Brain. 2017;140:2530-2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Montero R, Yubero D, Villarroya J, Henares D, Jou C, Rodríguez MA, Ramos F, Nascimento A, Ortez CI, Campistol J, Perez-Dueñas B, O'Callaghan M, Pineda M, Garcia-Cazorla A, Oferil JC, Montoya J, Ruiz-Pesini E, Emperador S, Meznaric M, Campderros L, Kalko SG, Villarroya F, Artuch R, Jimenez-Mallebrera C. GDF-15 Is Elevated in Children with Mitochondrial Diseases and Is Induced by Mitochondrial Dysfunction. PLoS One. 2016;11:e0148709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Jiang J, Wen W, Sachdev PS. Macrophage inhibitory cytokine-1/growth differentiation factor 15 as a marker of cognitive ageing and dementia. Curr Opin Psychiatry. 2016;29:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Semba RD, Gonzalez-Freire M, Tanaka T, Biancotto A, Zhang P, Shardell M, Moaddel R; CHI Consortium, Ferrucci L. Elevated Plasma Growth and Differentiation Factor 15 Is Associated With Slower Gait Speed and Lower Physical Performance in Healthy Community-Dwelling Adults. J Gerontol A Biol Sci Med Sci. 2020;75:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Barma M, Khan F, Price RJG, Donnan PT, Messow CM, Ford I, McConnachie A, Struthers AD, McMurdo MET, Witham MD. Association between GDF-15 levels and changes in vascular and physical function in older patients with hypertension. Aging Clin Exp Res. 2017;29:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Chelban V, Bocchetta M, Hassanein S, Haridy NA, Houlden H, Rohrer JD. An update on advances in magnetic resonance imaging of multiple system atrophy. J Neurol. 2019;266:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 34. | Stamelou M, Bhatia KP. Atypical parkinsonism: diagnosis and treatment. Neurol Clin. 2015;33:39-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology. 2016;86:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 510] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 36. | Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85:404-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 37. | Ozawa T, Paviour D, Quinn NP, Josephs KA, Sangha H, Kilford L, Healy DG, Wood NW, Lees AJ, Holton JL, Revesz T. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127:2657-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 379] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 38. | Brenneis C, Egger K, Scherfler C, Seppi K, Schocke M, Poewe W, Wenning GK. Progression of brain atrophy in multiple system atrophy. A longitudinal VBM study. J Neurol. 2007;254:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Shigemoto Y, Matsuda H, Kamiya K, Maikusa N, Nakata Y, Ito K, Ota M, Matsunaga N, Sato N. In vivo evaluation of gray and white matter volume loss in the parkinsonian variant of multiple system atrophy using SPM8 plus DARTEL for VBM. Neuroimage Clin. 2013;2:491-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Joutsa J, Gardberg M, Röyttä M, Kaasinen V. Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord. 2014;20:840-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 41. | Coon EA, Sletten DM, Suarez MD, Mandrekar JN, Ahlskog JE, Bower JH, Matsumoto JY, Silber MH, Benarroch EE, Fealey RD, Sandroni P, Low PA, Singer W. Clinical features and autonomic testing predict survival in multiple system atrophy. Brain. 2015;138:3623-3631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Yamamoto T, Yamanaka Y, Sugiyama A, Hirano S, Uchiyama T, Asahina M, Sakakibara R, Kuwabara S. The severity of motor dysfunctions and urinary dysfunction is not correlated in multiple system atrophy. J Neurol Sci. 2019;400:25-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Wenning GK, Ben Shlomo Y, Magalhães M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 499] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 44. | Ben-Shlomo Y, Wenning GK, Tison F, Quinn NP. Survival of patients with pathologically proven multiple system atrophy: a meta-analysis. Neurology. 1997;48:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Klockgether T, Lüdtke R, Kramer B, Abele M, Bürk K, Schöls L, Riess O, Laccone F, Boesch S, Lopes-Cendes I, Brice A, Inzelberg R, Zilber N, Dichgans J. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121:589-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 254] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | O'Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131:1362-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 47. | Figueroa JJ, Singer W, Parsaik A, Benarroch EE, Ahlskog JE, Fealey RD, Parisi JE, Sandroni P, Mandrekar J, Iodice V, Low PA, Bower JH. Multiple system atrophy: prognostic indicators of survival. Mov Disord. 2014;29:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Schrag A, Selai C, Mathias C, Low P, Hobart J, Brady N, Quinn NP. Measuring health-related quality of life in MSA: the MSA-QoL. Mov Disord. 2007;22:2332-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Kim HJ, Jeon BS, Lee JY, Yun JY. Survival of Korean patients with multiple system atrophy. Mov Disord. 2011;26:909-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Saito Y, Matsuoka Y, Takahashi A, Ohno Y. Survival of patients with multiple system atrophy. Intern Med. 1994;33:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain. 2002;125:1070-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 428] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 52. | Tada M, Onodera O, Tada M, Ozawa T, Piao YS, Kakita A, Takahashi H, Nishizawa M. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol. 2007;64:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Low PA, Reich SG, Jankovic J, Shults CW, Stern MB, Novak P, Tanner CM, Gilman S, Marshall FJ, Wooten F, Racette B, Chelimsky T, Singer W, Sletten DM, Sandroni P, Mandrekar J. Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 2015;14:710-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 54. | Petrovic IN, Ling H, Asi Y, Ahmed Z, Kukkle PL, Hazrati LN, Lang AE, Revesz T, Holton JL, Lees AJ. Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord. 2012;27:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Calandra-Buonaura G, Guaraldi P, Sambati L, Lopane G, Cecere A, Barletta G, Provini F, Contin M, Martinelli P, Cortelli P. Multiple system atrophy with prolonged survival: is late onset of dysautonomia the clue? Neurol Sci. 2013;34:1875-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Fernagut PO, Dehay B, Maillard A, Bezard E, Perez P, Pavy-Le Traon A, Rascol O, Foubert-Samier A, Tison F, Meissner WG. Multiple system atrophy: a prototypical synucleinopathy for disease-modifying therapeutic strategies. Neurobiol Dis. 2014;67:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Jellinger KA. Interaction between pathogenic proteins in neurodegenerative disorders. J Cell Mol Med. 2012;16:1166-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 58. | Kalko SG, Paco S, Jou C, Rodríguez MA, Meznaric M, Rogac M, Jekovec-Vrhovsek M, Sciacco M, Moggio M, Fagiolari G, De Paepe B, De Meirleir L, Ferrer I, Roig-Quilis M, Munell F, Montoya J, López-Gallardo E, Ruiz-Pesini E, Artuch R, Montero R, Torner F, Nascimento A, Ortez C, Colomer J, Jimenez-Mallebrera C. Transcriptomic profiling of TK2 deficient human skeletal muscle suggests a role for the p53 signalling pathway and identifies growth and differentiation factor-15 as a potential novel biomarker for mitochondrial myopathies. BMC Genomics. 2014;15:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Koene S, de Laat P, van Tienoven DH, Weijers G, Vriens D, Sweep FC, Timmermans J, Kapusta L, Janssen MC, Smeitink JA. Serum GDF15 Levels Correlate to Mitochondrial Disease Severity and Myocardial Strain, but Not to Disease Progression in Adult m.3243A>G Carriers. JIMD Rep. 2015;24:69-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |