Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2380
Peer-review started: February 6, 2020
First decision: April 14, 2020
Revised: April 23, 2020
Accepted: May 14, 2020
Article in press: May 14, 2020
Published online: June 6, 2020
Processing time: 122 Days and 12.7 Hours
Pyloric gland adenoma (PGA) is a recently described and rare tumor. Submucosal tumor (SMT)-like PGA is more difficult to diagnose and differentiate from other submucosal lesions.
We present the case of a 69-year-old man with a 10 mm SMT-like elevated lesion with an opening in the upper part of the gastric body, referred to our hospital for further endoscopic treatment. Magnifying endoscopy with narrow-band imaging, endoscopic ultrasonography, and complete endoscopic submucosal dissection were performed on the patient. Histopathological findings revealed tightly packed tubular glands lined with cuboidal or columnar cells that had round-to-oval nuclei containing occasional prominent nucleoli and an eosinophilic cytoplasm similar to that in non-neoplastic gastric pyloric glands. Additionally, immunohistochemical analysis showed positive staining for both mucin 5AC and mucin 6. Therefore, we arrived at the final diagnosis of gastric PGA. Although there was no apparent malignant component in this tumor, PGA has been considered a precancerous disease with a high risk of transformation into adenocarcinoma.
PGA should be considered when detecting gastric SMT-like lesions. Physicians and pathologists should focus on PGA due to its malignant potential.
Core tip: Pyloric gland adenoma continues to be a rare neoplasm of the stomach. We report a case where upper gastrointestinal endoscopy revealed a 10 mm submucosal tumor-like elevated lesion located in the upper part of the gastric body. Preoperative diagnosis is difficult because of the generally deep location of the tumor. Endoscopic ultrasound and endoscopic submucosal dissection were performed in this patient. The histopathological and immunohistochemistry investigations resulted in the diagnosis of pyloric gland adenoma.
- Citation: Min CC, Wu J, Hou F, Mao T, Li XY, Ding XL, Liu H. Gastric pyloric gland adenoma resembling a submucosal tumor: A case report. World J Clin Cases 2020; 8(11): 2380-2386
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2380.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2380
Gastric adenomas are benign lesions of the stomach, and comprise less than 10% of all gastric polyps[1]. Gastric adenomas are categorized into gastric and intestinal type according to the 2019 World Health Organization classification[2]. Gastric adenomas comprise pyloric gland adenoma (PGA) and foveolar-type adenoma. PGAs are rare neoplasms with gastric differentiation, first described by Elster[3] in 1976. Subsequently, similar lesions have been gradually recognized in some case reports and small case series. This lesion most frequently occurs in the stomach, but also occurs in the duodenum, esophagus, gallbladder, bile duct, pancreas, and rectum[4-7]. In the stomach, PGAs account for 2.7% of all gastric polyps, as observed in the largest study with 90 cases[8]. PGAs endoscopically present as flat, polypoid or mass lesions[9-12]. Moreover, a PGA presenting as a submucosal tumor (SMT) is uncommon. PGAs are considered to be precancerous lesions and have a 12.2%-47% rate of transformation into adenocarcinoma[10,13,14]. However, limited information is available regarding the clinicopathological features, endoscopic performances, and pathogenesis as well as prognoses of PGAs.
Here, we report a case of gastric PGA with an SMT-like appearance that was treated with endoscopic submucosal dissection (ESD) and that could have easily been neglected and have remained under-diagnosed.
A 69-year-old Chinese man presented to our hospital for evaluation and management of an epigastric distention for over a year. He was repeatedly diagnosed with chronic gastritis, and the symptoms were alleviated by administering proton pump inhibitors. Incidentally, an SMT-like lesion with an opening on the upper part of the gastric body was discovered on gastroscopy over 3 mo prior.
He had a history of hypertension for 10 years and was ingesting oral telmisartan 80 mg every day regularly. His blood pressure was controlled and relatively stable. He had no history of surgery and allergic drugs.
He had an extensive smoking history of 40 years, with an average of 20 cigarettes per day. This patient had no familial history of genetic diseases.
Physical examination revealed that he was 170 cm in height and 73 kg in weight, with a blood pressure of 127/92 mmHg and pulse rate of 67 beats per minute. There were no other obvious abnormalities during physical examination.
After admission, the patient underwent thorough evaluations including routine investigations of the blood, urine, feces and occult blood, blood biochemistry, and common serum tumor markers such as carcinoembryonic antigen, carbohydrate antigen 19-9, alpha-fetoprotein, and carbohydrate antigen 724. No significant abnormalities were recorded in these investigations.
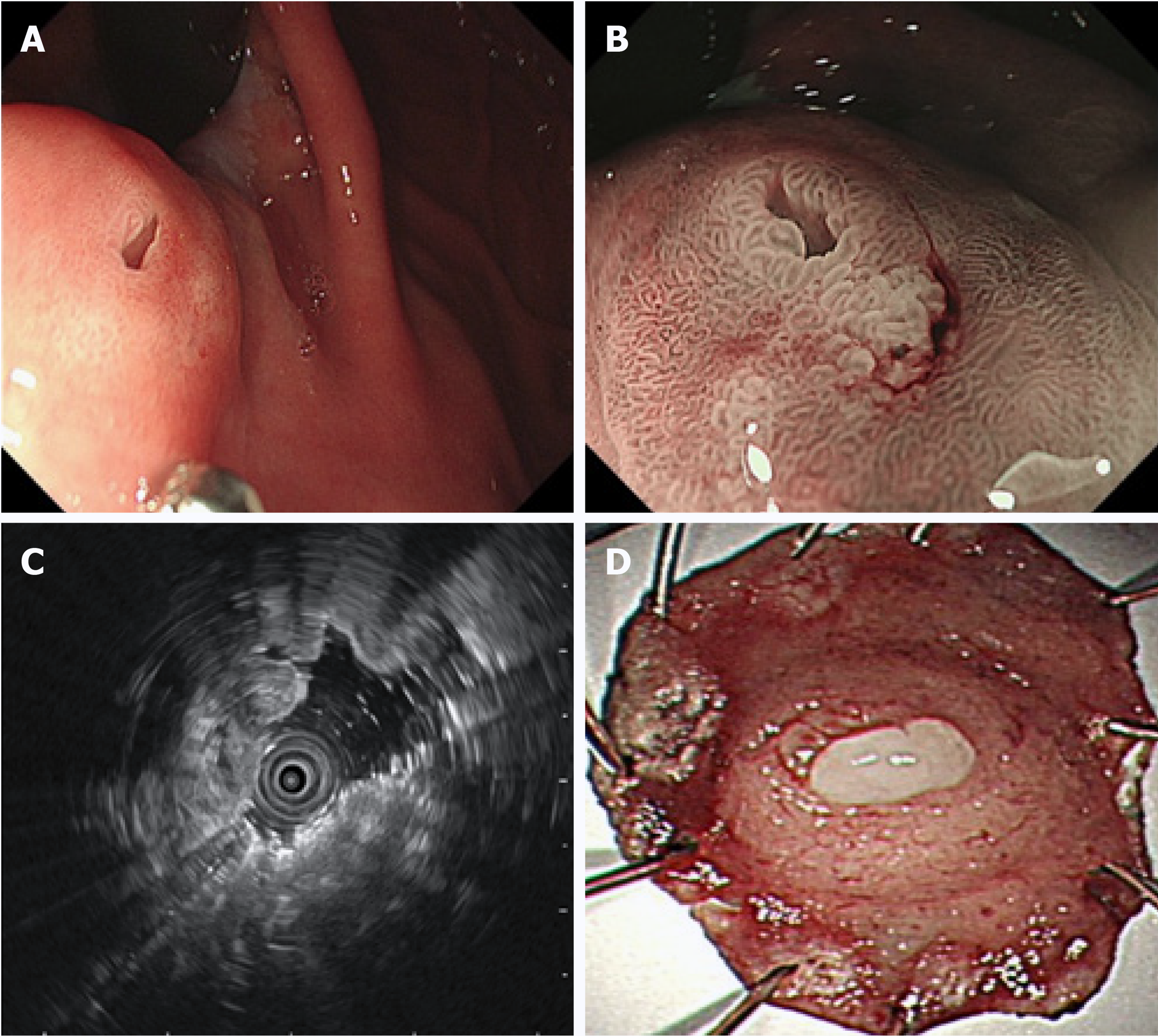
Upper gastrointestinal endoscopy revealed non-atrophic gastritis without Helicobacter pylori (H. pylori) infection in the background mucosa. An SMT-like elevated lesion with a diameter of 10 mm was located at the posterior wall of the upper part of the gastric body, with an opening on the surface of the tumor (Figure 1A). Next, magnifying endoscopy with narrow-band imaging (ME-NBI) revealed a regular surface microstructure and microvascular pattern (Figure 1B). Additionally, ME-NBI revealed that the orifice showed dilated glandular duct arising from the deeper mucosa.
Endoscopic ultrasound (EUS, 20 MHz; Olympus, Tokyo, Japan) revealed a 10.6 mm × 5.5 mm equal echoic mass with several cysts located in the submucosal layer with an intact muscularis (Figure 1C). The biopsy results indicated chronic non-atrophic, H. pylori-negative gastritis.
According to the endoscopic performance and histopathologic examination, the patient was diagnosed with PGA.
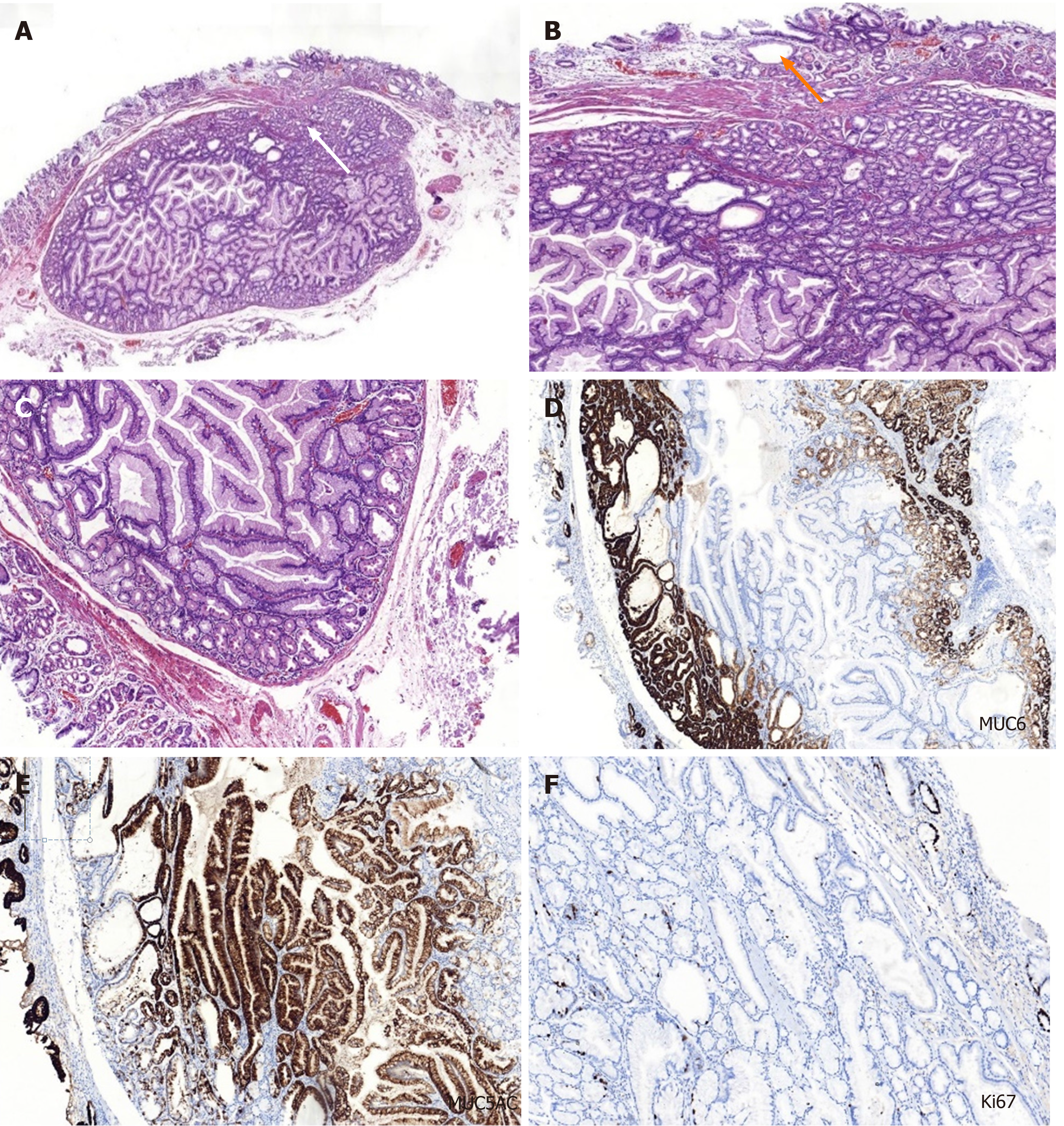
On the basis of the examination results, the diagnosis of SMT-like tumor in the upper part of the gastric body was uncertain. Diagnostic ESD was performed on the patient to confirm the diagnosis. An elevated tumor measuring 13 mm × 10 mm was identified in the ESD specimen with white mucus oozing out from the opening in the middle when squeezed (Figure 1D). In routine hematoxylin and eosin staining, the low-magnification view revealed that the lesion was located in the submucosal layer, showing a nodular appearance with clear boundaries and increasing number of glands in the lamina propria of the orifice (white arrow) (Figure 2A). The high-magnification view showed that some glandular cavity was irregular, with cystic expansion (orange arrow) and interstitial edema (Figure 2B). The tumor consisted of mucus-rich packed tubular glands lined with cuboidal or low columnar epithelial cells containing an eosinophilic cytoplasm and round nuclei, similar to that in pyloric glands (Figure 2C). There was slight epithelial dysplasia in some areas. Additionally, immunohistochemical staining demonstrated positivity for both mucin 6 (MUC6) and MUC5AC but negativity for MUC4 and p53, and the Ki-67 labeling index was about 2% (Figure 2C-F). According to the above histologic results, we finally made a diagnosis of PGA. Adenocarcinoma components were not identified in the ESD specimen. The horizontal and vertical margin were negative, resulting in complete and curative resection.
The lesion was removed completely by ESD. However, the patient will need to undergo a regular gastroscopy follow-up after surgery.
PGA is an uncommon neoplasm that has been gradually recognized in the past few decades. Since Elster[3] first described PGA in a book chapter in 1976, similar lesions were subsequently reported in some cases and small series of clinical studies. The majority of reports on PGA are published by other nations, with a lack of awareness in China.
Previous studies have shown that PGAs occur more frequently in older women[8,14]. Vieth et al[8], in one of the largest studies with 90 PGA patients, reported a 2.5:1 ratio for women to men with an average age of 73 years. The predilection site for PGA is the corpus/fundus (64%) of the stomach, followed by cardia (8%), antrum (7%) and intermediate zone (5%), and others occurred in the extra-gastric sites, similar to observations of Choi et al[10]. PGAs present with large lesions at the time of diagnosis, with an average size of 1.6-2.3 cm reported in multiple case reports and clinical studies[8-10,12,15]. In our study, the patient was an elderly man with PGA of 10 mm diameter localized in the upper part of the gastric body near the cardia. Predominantly, PGAs appear as a polypoid lesion or mass[10-12,16], but it may also present as flat lesion[9] or ulcer[10]. We reported an unusual case of PGA mimicking an SMT with an orifice on the surface of the lesion. Similarly, Yamamoto et al[9] reported a flat lesion with two openings at the greater curvature of the upper gastric body. Considering the possible deeper depth of the SMT-like lesion, we did not obtain its biopsy. But biopsy specimen from the orifice of the SMT may contribute to diagnosis. The performance of EUS is important in the diagnosis of an SMT-like lesion. To date, limited information is available on the EUS characteristics of the PGAs. Moreover, PGAs are usually located in the mucosal or submucosal layer in EUS. In the EUS by Yamamoto et al[9], multiple large cysts were found in the second and third layers and the intact fourth layer[9]. Whereas, in our case, EUS for the patient showed an isoechoic nodule with several cysts located in the submucosal layer.
The predisposing factors of PGAs have remained unclear. Eighteen cases (34%) of PGAs presented mainly with atrophic autoimmune gastritis (AIG), and they were also positive for H. pylori gastritis (30%). Moreover, only 3.8% cases have been reported with a normal gastric mucosa[8]. However, a controversy exists with regards to the background mucosa, where 22.4% of the PGAs developed with AIG background, while normal mucosa was seen in 35.8% cases[10]. The predominance of AIG in older women contributes to the frequent occurrence of PGA in these women[8]. In our study, the biopsy from the antrum indicated chronic non-atrophic gastritis without infection of H. pylori; however, its relationship with the background mucosa and underlying mechanism need to be further explored.
Furthermore, EUS revealed a few cysts within the equivalent echogenic mass located in the submucosal layer in our patient. Therefore, PGA should be distinguished from gastritis cystica profundal (GCP), which is a rare lesion of the stomach, characterized by polypoid cystic ectasia of benign gastric glands invading the submucosa. It associates with chronic inflammation and ischemia and is observed primarily in patients who have undergone gastrectomy[17]. The most frequent EUS feature of GCP was multiple anechoic cysts in the submucosal layer, as reported earlier[18,19]. However, we did not find any component of the GCP. There was an opening on the mucosal surface of the PGA that ought to be differentiated from the gastric ectopic pancreas. A dimple or umbilical opening can be observed in some heterotopic pancreases. Anechoic duct-like structure or rare cyst cavity structure can be detected within the hypoechoic mass located in the deep mucosal and submucosal layers by EUS[20]. The majority of heterotopic pancreas cases are located at the gastric antrum. No ectopic pancreatic tissues were identified in the resected specimen by ESD. Additionally, clinicians should differentiate it from other submucosal lesions such as the gastric neuroendocrine tumors[21] and SMT-like gastric aden-ocarcinoma[22,23].
Next, it was difficult to make an initial diagnosis of PGA due to the SMT-like appearance of the tumor, and an ESD was performed for final confirmation. Histopathological findings revealed mucus-rich pyloric glands lined with cuboidal or low columnar epithelial cells. Additionally, immunohistochemical staining was performed for MUC6 (specific for PGA) and MUC5AC (present in luminal foveolar type epithelium) to confirm the diagnosis of PGA. The gastric type adenoma that needed to be differentiated from PGA is foveolar-type adenoma, which is immunohistochemically positive for MUC5AC but negative for MUC6.
PGAs are considered at a high risk of malignant transformation[10,13], and have been divided into three categories: Viz, without conventional histologic dysplasia; low-grade dysplasia (LGD); and high-grade dysplasia (HGD)[14]. In a recent multicenter clinicopathologic study with 67 gastric PGA cases, lesions with HGD or adenocarcinoma were found to have larger mean size (3.5 cm) than LGD cases (1.5 cm) (P < 0.001)[10]. They concluded that the risk of developing HGD or adenocarcinoma was directly associated with the size of the lesion, presence of AIG, tubulovillous architecture, and mixed type (co-expression of both MUC6 and MUC5AC in deeper glands with MUC6 expression ranging from 20% to > 90% of the neoplastic glands)[10]. An immunohistochemical analysis of the gastric PGAs indicated higher nuclear expression of p53 in PGAs with adenocarcinoma (82.1%) than those without adenocarcinoma (59.3%)[13], suggesting that nuclear p53 may correlate with high-risk PGAs. In our study, the PGA ought to be classified as mixed type. The relatively small diameter without expression of p53 may be responsible for the lack of conventional hyperplasia histologically. Regardless of the presence of hyperplasia, all PGAs represent at least LGD, even in cases without noticeable conventional histologic dysplasia[10]. Taken together, the patient in our study would need to be followed-up regularly.
Furthermore, in our case, the SMT-like lesion was located in the upper part of the gastric body. We performed ME-NBI and EUS for the patient, but failed to obtain the biopsy of the tumor. Moreover, we could not distinguish it from other SMTs and make an accurate preoperative diagnosis. Therefore, we subsequently performed the diagnostic ESD for this patient, with confirmed diagnosis using histopathological and IHC analysis. Additionally, the resected specimen did not show signs of malignancy. Although the overall recurrence rate of PGAs was very low[10], regular follow-up with periodic gastroscopic surveillance should be suggested.
We present a case of PGA with SMT-like appearance, located in the upper part of the gastric body. It poses difficulty in distinguishing from other submucosal lesions. Deeper biopsies using larger forceps or even EUS-guided fine needle aspiration may improve the clinical diagnosis. ESD was subsequently performed to confirm the final diagnosis. It is recommended that all PGAs be completely removed if possible, particularly when they are large or show high-grade features[10]. Clinicians and pathologists should pay close attention to PGAs owing to their potential to transform to adenocarcinoma. The patient needs to be followed up with regular gastroscopy observation.
We thank all the authors helping the writing and publication of this article.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katayama Y S-Editor: Yan JP L-Editor: Filipodia E-Editor: Liu MY
| 1. | Argüello Viúdez L, Córdova H, Uchima H, Sánchez-Montes C, Ginès À, Araujo I, González-Suárez B, Sendino O, Llach J, Fernández-Esparrach G. Gastric polyps: Retrospective analysis of 41,253 upper endoscopies. Gastroenterol Hepatol. 2017;40:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2410] [Article Influence: 482.0] [Reference Citation Analysis (3)] |
| 3. | Elster K, Morson BC. Histologic classification of gastric polyps. Pathology of the gastro-intestinal tract. Berlin: Springer 1976; 78-92. |
| 4. | Miller GC, Kumarasinghe MP, Borowsky J, Choi WT, Setia N, Clauditz T, Gidwani R, Sufiyan W, Lauwers GY, Brown IS. Clinicopathological features of pyloric gland adenomas of the duodenum: a multicentre study of 57 cases. Histopathology. 2020;76:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Kushima R, Vieth M, Mukaisho K, Sakai R, Okabe H, Hattori T, Neuhaus H, Borchard F, Stolte M. Pyloric gland adenoma arising in Barrett's esophagus with mucin immunohistochemical and molecular cytogenetic evaluation. Virchows Arch. 2005;446:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Saei Hamedani F, Garcia-Buitrago M. Pyloric Gland Adenoma of Gallbladder: A Review of Diagnosis and Management. Adv Med. 2018;2018:7539694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | He C, Fukumura Y, Toriyama A, Ogura K, Sasahara N, Mitani K, Yao T. Pyloric Gland Adenoma (PGA) of the Gallbladder: A Unique and Distinct Tumor from PGAs of the Stomach, Duodenum, and Pancreas. Am J Surg Pathol. 2018;42:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Vieth M, Kushima R, Borchard F, Stolte M. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch. 2003;442:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Yamamoto M, Nishida T, Nakamatsu D, Adachi S, Inada M. Endoscopic findings of inverted pyloric gland adenoma resected by endoscopic submucosal dissection. J Gastrointestin Liver Dis. 2018;27:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Choi WT, Brown I, Ushiku T, Yozu M, Setia N, Srivastava A, Johncilla M, Pai RK, Gill RM, Fukayama M, Misdraji J, Lauwers GY. Gastric pyloric gland adenoma: a multicentre clinicopathological study of 67 cases. Histopathology. 2018;72:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Golger D, Probst A, Wagner T, Messmann H. Pyloric-gland adenoma of the stomach: case report of a rare tumor successfully treated with endoscopic submucosal dissection. Endoscopy. 2008;40 Suppl 2:E110-E111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Pei Q, Shi Y, Jing H. Early adenocarcinoma colliding with a pyloric gland adenoma in the gastric cardia. Gastrointest Endosc. 2019;90:522-523.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Vieth M, Kushima R, Mukaisho K, Sakai R, Kasami T, Hattori T. Immunohistochemical analysis of pyloric gland adenomas using a series of Mucin 2, Mucin 5AC, Mucin 6, CD10, Ki67 and p53. Virchows Arch. 2010;457:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Chen ZM, Scudiere JR, Abraham SC, Montgomery E. Pyloric gland adenoma: an entity distinct from gastric foveolar type adenoma. Am J Surg Pathol. 2009;33:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Pezhouh MK, Park JY. Gastric pyloric gland adenoma. Arch Pathol Lab Med. 2015;139:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Schaefer IM, Cameron S, Middel P, Homayounfar K, Schwörer H, Vieth M, Veits L. Pyloric gland adenoma of the cystic duct with malignant transformation: report of a case with a review of the literature. BMC Cancer. 2012;12:570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Xu G, Peng C, Li X, Zhang W, Lv Y, Ling T, Zhou Z, Zhuge Y, Wang L, Zou X, Zhang X, Huang Q. Endoscopic resection of gastritis cystica profunda: preliminary experience with 34 patients from a single center in China. Gastrointest Endosc. 2015;81:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Machicado J, Shroff J, Quesada A, Jelinek K, Spinn MP, Scott LD, Thosani N. Gastritis cystica profunda: Endoscopic ultrasound findings and review of the literature. Endosc Ultrasound. 2014;3:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Lee TH, Lee JS, Jin SY. Gastritis cystica profunda with a long stalk. Gastrointest Endosc. 2013;77:821-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (113)] |
| 20. | Attwell A, Sams S, Fukami N. Diagnosis of ectopic pancreas by endoscopic ultrasound with fine-needle aspiration. World J Gastroenterol. 2015;21:2367-2373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Corey B, Chen H. Neuroendocrine Tumors of the Stomach. Surg Clin North Am. 2017;97:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 22. | Cheng XL, Liu H. Gastric adenocarcinoma mimicking a submucosal tumor: A case report. World J Clin Cases. 2019;7:3138-3144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Yu YN, Yin XY, Sun Q, Liu H, Zhang Q, Chen YQ, Zhao QX, Tian ZB. Gastric adenocarcinoma of fundic gland type after Helicobacter pylori eradication: A case report. World J Clin Cases. 2019;7:1696-1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |