Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2374
Peer-review started: January 13, 2020
First decision: April 27, 2020
Revised: April 27, 2020
Accepted: April 30, 2020
Article in press: April 30, 2020
Published online: June 6, 2020
Processing time: 146 Days and 13.3 Hours
In rare cases, odontogenic keratocysts (ODs) transform into squamous cell carcinoma. Intervals between the first attendance of a patient and the diagnosis of OD with malignant transformation vary from weeks to years. In this article, we report a case of malignancy derived from OD with a five-day delay in diagnosis.
A 54-year-old woman was referred to Tongji Hospital in Wuhan, China with complaints of moderate pain, recurrent swelling, and pus discharge around her left maxillary lateral incisor for over 10 years. Physical examination revealed a fistula at the palatine-side mucoperiosteum of the left maxillary lateral incisor and enlarged lymph node in the left neck. Cone beam computed tomography revealed a cystic lesion with massive bone destruction from the left maxillary central incisor to the left secondary maxillary premolar and local bony destruction in the left first mandibular molar. The patient was clinically diagnosed with OD. Enucleation rather than marsupialization was performed given the risk factors of long history, recent aggravated pain, and massive bony destruction. Malignant transformation of OD was confirmed by pathologists 3 d after the operation. Radical surgery was performed, and lymph node metastasis was observed. The patient was subjected to postoperative radiotherapy and synchronous chemotherapy, and no local recurrence or distant metastasis was noted at one-year follow-up.
Our case suggests that clinicians should be aware of the malignant transformation of OD, especially when patients present with a long history, massive cyst, chronic inflammation, recent persistent infections, aggravated pain, numbness around the cystic lesion, and lymph node enlargement.
Core tip: We report a rare case of odontogenic keratocyst with malignant transformation. Although distinguishing between a benign and malignant odontogenic keratocyst is clinically challenging, factors, such as long history, massive cyst, chronic inflammation, recent persistent infections, aggravated pain, numbness around the cystic lesion, and lymph node enlargement, may suggest an overlooked malignant transformation.
- Citation: Luo XJ, Cheng ML, Huang CM, Zhao XP. Reduced delay in diagnosis of odontogenic keratocysts with malignant transformation: A case report. World J Clin Cases 2020; 8(11): 2374-2379
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2374.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2374
Odontogenic keratocysts (ODs) can transform into primary intraosseous squamous cell carcinoma (PIOSCC)[1]. However, this malignant transformation rarely occurs. To the best of our knowledge, approximately 30 cases have been reported[2].
The diagnosis of PIOSCC requires caution. An important reason is clinicians’ unawareness of the malignant transformation of OD due to its rarity. In addition, similar symptoms of swelling, pain, chronic infection, and pus discharge occur between OD and PIOSCC. Imaging examinations may fail to distinguish one from the other given that the main feature of bony destruction is observed in both diseases, especially when PIOSCC is in its early stage[3,4]. Moreover, marsupialization of cystic lesions is favored by patients and clinicians. The time between patients’ attendance and the diagnosis of PIOSCC is considerably longer than that of squamous cell carcinoma in other oral and maxillofacial anatomical sites.
Information about the treatments and prognosis of PIOSCC is scarce. Although neck dissection remains in discussion, the radical excision of lesions with appropriate reconstruction and postoperative radio/chemotherapy is commonly performed[3,5-7]. The two- and five-year survival rates in adults are approximately 62% and 38%, respectively; furthermore, patients with lymph node metastasis at first clinical attendance show a poor prognosis[4]. Timely diagnosis and proper treatment may benefit these patients’ prognosis.
In this article, we report a case of maxillary PIOSCC derived from the malignant transformation of OD with reduced delay in diagnosis and lymph node metastasis. We aim to remind clinicians of the underappreciated malignant transformation of OD, especially when patients present with a long history, massive cyst, chronic inflammation, recent persistent infections, aggravated pain, and numbness around the cystic lesion.
A 54-year-old woman was referred to Tongji Hospital in Wuhan, China with complaints of moderate pain, recurrent swelling, and pus discharge around her left maxillary lateral incisor for over 10 years.
The patient received interrupted anti-inflammatory therapy. The discomfort aggravated in the recent month, and no improvement was achieved by antibiotic treatment.
The patient was generally in good health and had no history of alcohol, tobacco, or areca nut consumption.
Intraoral examination revealed a fistula at the palatine-side mucoperiosteum of the left maxillary lateral incisor, and intense pain was observed. No evident swelling was detected in the left maxilla. An abnormal looseness of maxillary tooth and enlarged lymph node were detected in the left neck.
Routine laboratory tests revealed no remarkable abnormality, except for a slightly increased D–D dimer level (0.58 µg/mL FEU).
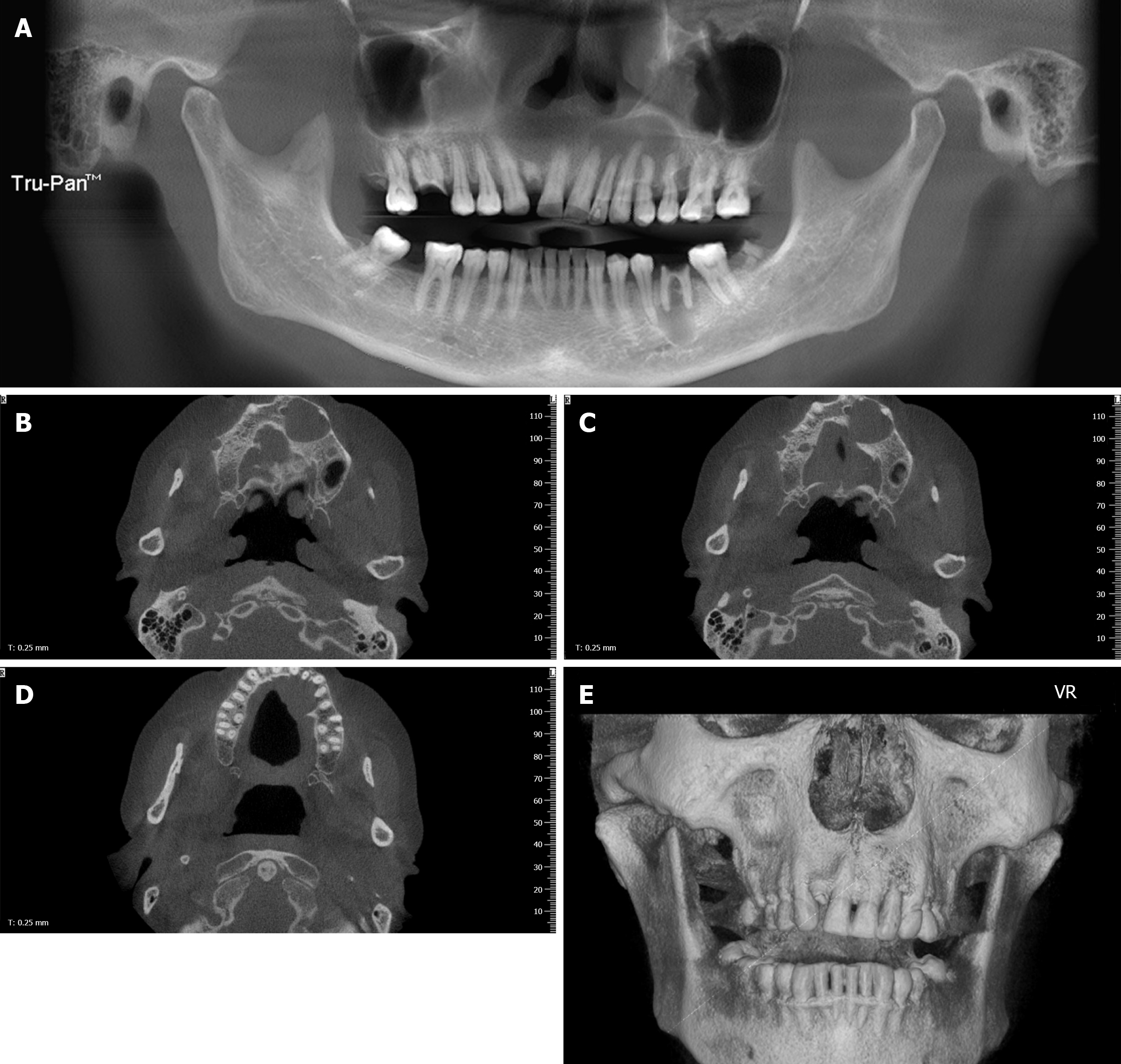
Cone beam computed tomography (CT) revealed massive bone destruction with a clear boundary from the left maxillary central incisor to the left secondary maxillary premolar and local bony destruction in the left first mandibular molar (Figure 1A-E).
Other examination results, including those of magnetic resonance imaging (MRI) for neck and cranial and chest CT, were normal.
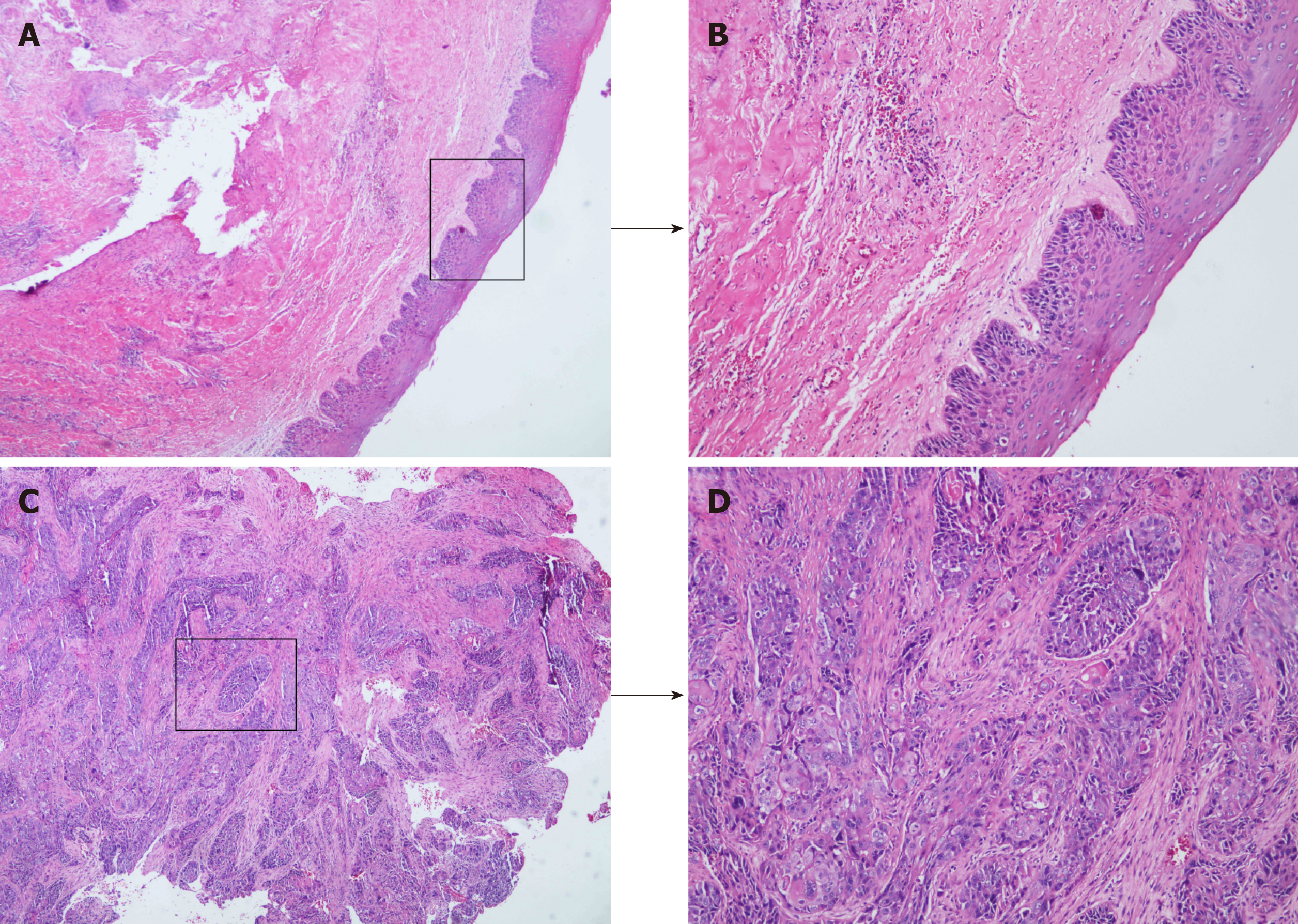
The patient was clinically diagnosed with OD. However, we were highly suspicious of squamous cell carcinoma due to the patient’s long history, recent aggravated pain, massive bony destruction, and lymph node enlargement. Such suspicion was confirmed by pathological findings 3 d after enucleation (Figure 2A-D).
One week after the diagnosis, radical surgery (left subtotal maxillectomy with reconstruction with a free anterolateral thigh flap and left supraomohyoid neck dissection) was performed. Final pathological results confirmed squamous cell carcinoma in the left maxilla, and lymph node metastasis was observed. The patient received postoperative radiotherapy (local intensity-modulated radiotherapy, 3 Gy per day for 5 d a week for 4 wk) and synchronous chemotherapy (1 g cisplatin/d per week for 4 wk).
Postoperative follow-up for one year revealed no local recurrence or distant metastasis.
PIOSCC is classified into three types: (1) A solid cancer invades marrow spaces and incudes osseous reabsorption; (2) A squamous cell carcinoma derives from an odontogenic cyst; and (3) A squamous cell carcinoma derives from other benign epithelial odontogenic tumors[8]. According to previous reports, the diagnosis of PIOSCC must meet the following common criteria: (1) The intact oral mucosa without ulcer; (2) Verified absence of invasion from nearby malignancies, such as alveolar carcinoma and metastasis from distant organs; and (3) Confirmed transformation of the normal squamous epithelium to squamous cell carcinoma within cyst linings via histopathological examination[3,9-11]. In the current case, physical examination, MRI, and CT tests revealed the intact oral mucosa and a cystic lesion of the left maxilla, excluding the invasion and metastasis of other malignancies; histopathological examination provided evidence for the malignant transformation of OD-squamous epithelium. In conjunction with the patient’s medical history, recent aggressive behavior, and intraoperative findings, we considered that the patient suffered from PIOSCC deriving from OD.
Although information about biological characteristics of malignant OD is rare, the high recurrence rate and poor survival of PIOSCC may be partly attributed to its misdiagnosis as OD; subsequent curettage is conventionally performed, followed by enucleation combined with peripheral ostectomy and radical resection for localized and large or ill-defined lesions, respectively[12]. Apart from the similarity of clinical behavior and radiologic examination results between PIOSCC and OD, the acquired malignant transformation of OD as confirmed by pathology is another huge barrier to prescribing the proper treatment for patients with PIOSCC. Although the malignant lesion was diagnosed by enucleation in this case, biopsy and enucleation have been associated with the misdiagnosis of PIOSCC in previous reports[5,6]. More information is required to assess malignant tumors before the identification of the characteristics of malignant transformation of OD.
A literature review suggests that a few cases of PIOSCC were diagnosed via surgical biopsy because of a prejudged possibility of a malignant tumor, a few cases were unsuspectedly diagnosed by enucleation, and the remaining cases were diagnosed by radical resection of lesions due to relapse and aggravation after conservative treatment[13,14]. These prudent studies and our report demonstrate cases with a long medical history, persistent inflammation, huge mass, recent aggressive behavior, including numbness and lymph node enlargement. Several recurrences and continuous uncomfortableness are commonly observed in cases with delayed diagnosis. Although these symptoms are nonspecific, clinicians should be aware of the malignant transformation of OD and synthetically judge patients’ history, clinical behavior, and CT/MRI results. For cases without these characteristics, enucleation seems to the safe way to exclude early stage PIOSCC.
This article presents a case with reduced delay in the diagnosis of PIOSCC in the maxilla. Our case suggests that for the timely diagnosis and treatment of PIOSCC, clinicians should be aware of the malignant transformation of OD, especially when patients present a cystic lesion with a long history, chronic inflammation, recent aggravated behaviors including pain and lymph node enlargement.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dereci O, Wu BL S-Editor: Tang JZ L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Nokovitch L, Bodard AG, Corradini N, Crozes C, Guyennon A, Deneuve S. Pediatric case of squamous cell carcinoma arising from a keratocystic odontogenic tumor. Int J Pediatr Otorhinolaryngol. 2018;112:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Adachi M, Inagaki T, Ehara Y, Azuma M, Kurenuma A, Motohashi M, Muramatsu Y. Primary intraosseous carcinoma arising from an odontogenic cyst: A case report. Oncol Lett. 2014;8:1265-1268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Bodner L, Manor E, Shear M, van der Waal I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst: a clinicopathologic analysis of 116 reported cases. J Oral Pathol Med. 2011;40:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Tan B, Tay SY, Shermin L, Teck KC, Yoke PC, Goh C, Balakrishnan A. Malignant transformation of keratocystic odontogenic tumor: two case reports. Am J Otolaryngol. 2013;34:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Medawela RMSHB, Jayasuriya NSS, Ratnayake DRDL, Attygalla AM, Siriwardena BSMS. Squamous cell carcinoma arising from a keratocystic odontogenic tumor: a case report. J Med Case Rep. 2017;11:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Kheur S, Mamatha GS, Bagul N, Kshirsagar K. Squamous cell carcinoma arising from keratocystic odontogenic tumor. J Oral Maxillofac Pathol. 2017;21:168-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Seethala RR. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Preface. Head Neck Pathol. 2017;11:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Sukegawa S, Matsuzaki H, Katase N, Kanno T, Mandai T, Takahashi Y, Asaumi JI, Furuki Y. Primary intraosseous squamous cell carcinoma of the maxilla possibly arising from an infected residual cyst: A case report. Oncol Lett. 2015;9:131-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Bai MR, Shen T, Chen YU, Geng N. Primary intraosseous squamous cell carcinoma in pre-existing keratocystic odontogenic tumor: A case report and literature review. Mol Clin Oncol. 2016;4:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Grisar K, Schol M, Hauben E, Schoenaers J, Politis C. Primary intraosseous squamous cell carcinoma of the mandible arising from an infected odontogenic cyst: A case report and review of the literature. Oncol Lett. 2016;12:5327-5331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Soluk-Tekkeşin M, Wright JM. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2017 (4th) Edition. Turk Patoloji Derg. 2018;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Abdelkarim AZ, Elzayat AM, Syed AZ, Lozanoff S. Delayed diagnosis of a primary intraosseous squamous cell carcinoma: A case report. Imaging Sci Dent. 2019;49:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Kikuchi K, Ide F, Takizawa S, Suzuki S, Sakashita H, Li TJ, Kusama K. Initial-Stage Primary Intraosseous Squamous Cell Carcinoma Derived from Odontogenic Keratocyst with Unusual Keratoameloblastomatous Change of the Maxilla: A Case Report and Literature Discussion. Case Rep Otolaryngol. 2018;2018:7959230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |