Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2350
Peer-review started: January 21, 2020
First decision: April 14, 2020
Revised: April 17, 2020
Accepted: April 29, 2020
Article in press: April 29, 2020
Published online: June 6, 2020
Processing time: 138 Days and 19.7 Hours
A myxofibrosarcoma (MFS) is a malignant fibroblastic tumor that tends to occur in the lower and upper extremities. The reported incidence of head and neck MFSs is extremely rare. We report a 46-year-old male with “a neoplasm in the scalp” who was hospitalized and diagnosed with an MFS (highly malignant with massive necrotic lesions) based on histologic and immunohistochemistry evaluations. The magnetic resonance imaging manifestations did not demonstrate the “tail sign” mentioned in several studies, which resulted in a great challenge to establish an imaging diagnosis. The treatment plan is closely associated with the anatomic location and histologic grade, and more importantly, aggressive surgery and adjuvant radiotherapy may be helpful. Hence, we report the case and share some valuable information about the disease.
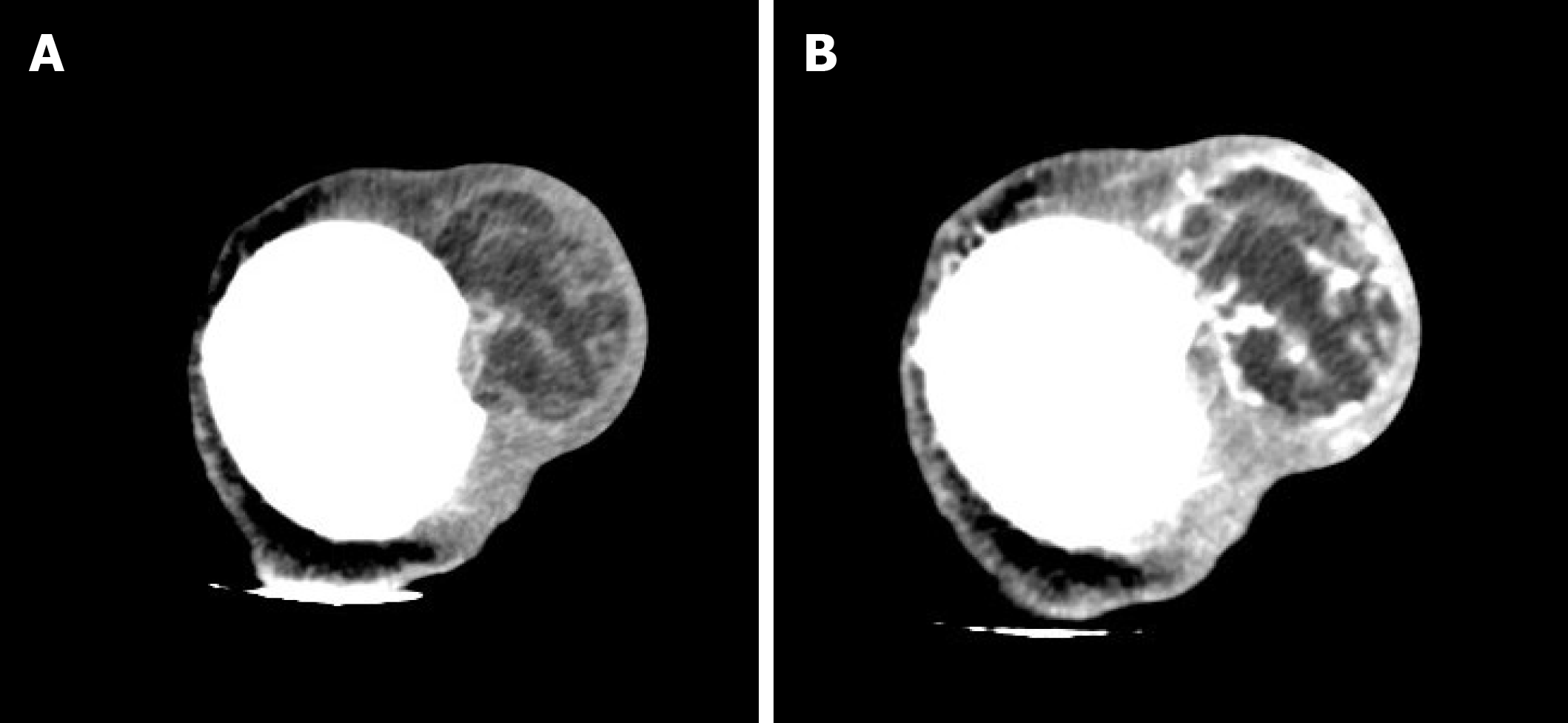
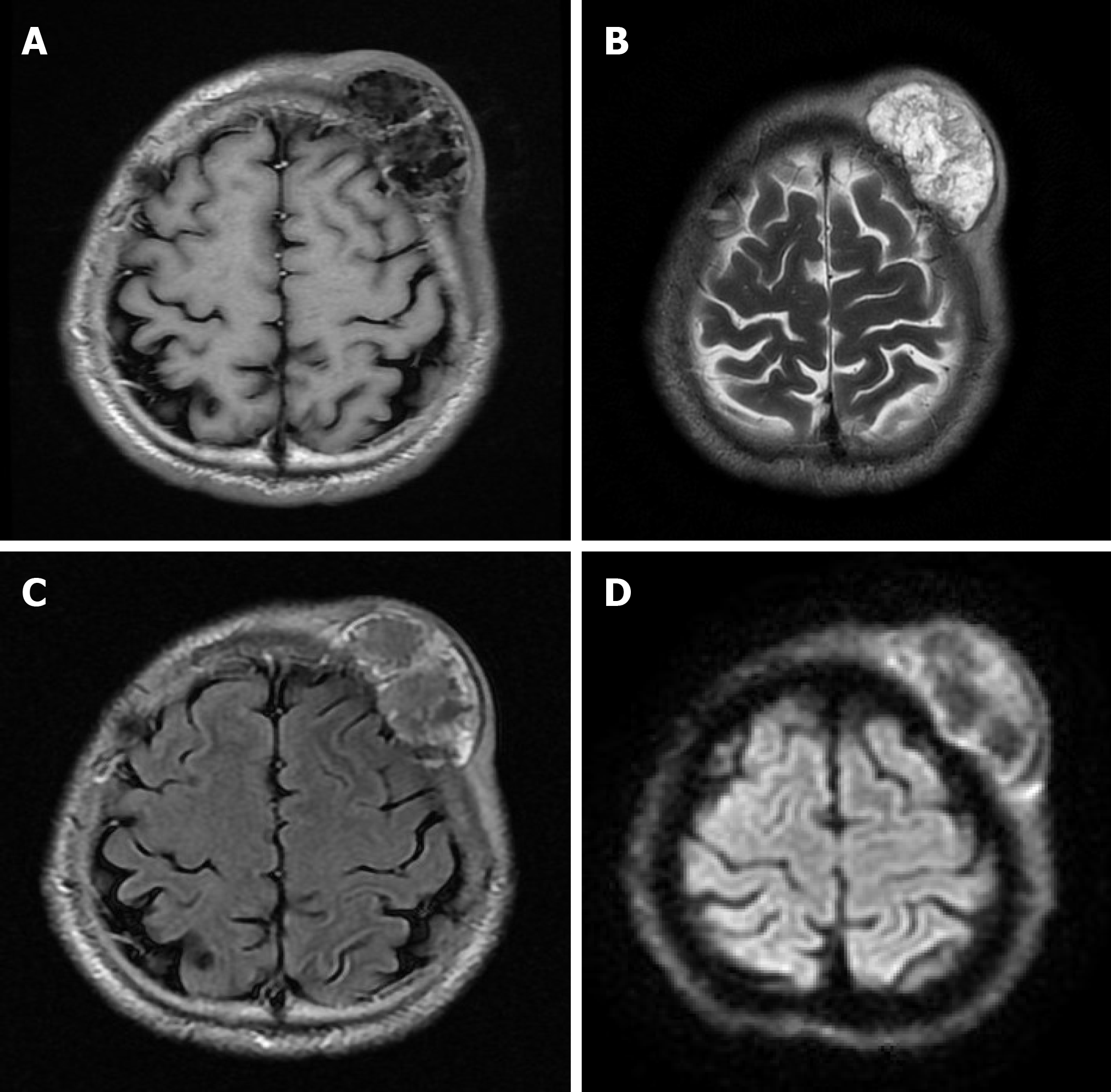
A 46-year-old male with “a neoplasm in the scalp for 6 mo” was hospitalized. Initially, the tumor was about the size of a soybean, without algesia or ulceration. The patient ignored the growth, did not seek treatment, and thus, did not receive treatment. Recently, the tumor increased to the size of an egg; there was no bleeding or algesia. His family history was unremarkable. No abnormalities were found upon laboratory testing, including routine hematologic, biochemistry, and tumor markers. Computed tomography showed an ovoid mass (6.25 cm × 3.29 cm × 3.09 cm in size) in the left frontal scalp with low density intermingled with equidense strips in adjacent areas of the scalp. Magnetic resonance imaging revealed a lesion with an irregular surface and an approximate size of 3.55 cm × 6.34 cm in the left frontal region, with clear boundaries and visible separation. Adjacent areas of the skull were damaged and the dura mater was involved. Contrast enhancement showed an uneven enhancement pattern. Surgery was performed and postoperative adjuvant radiotherapy was administered to avoid recurrence or metastasis. The post-operative pathologic diagnosis confirmed an MFS. A repeat computed tomography scan showed no local recurrence or distant metastasis 19 mo post-operatively.
The case reported herein of MFS was demonstrated in an extremely rare location on the scalp and had atypical magnetic resonance imaging findings, which serves as a reminder to radiologists of the possibility of this diagnosis to assist in clinical treatment. Given the special anatomic location and the high malignant potential of this rare tumor, combined surgical and adjuvant radiotherapy should be considered to avoid local recurrence and distant metastasis. The significance of regular follow-up is strongly recommended to improve the long-term survival rate.
Core tip: Myxofibrosarcoma (MFS) is a malignant fibroblastic tumor that has a predilection for lower and upper extremities. Rare occurrences have been reported in the scalp. We describe a 46-year-old male diagnosed with a MFS of the scalp (highly malignant with massive necrotic lesions) by histologic examination and immunohistochemistry testing. The magnetic resonance imaging findings did not conform to the reported typical “tail sign”, which may be confused with other tumors and lead to the correct diagnosis being missed. The definitive diagnosis of MFS is based on immunohistologic features. Considering the location and non-specific imaging manifestations of this case, the treatment is also worthy of discussion. Surgical excision combined with postoperative adjuvant radiotherapy was effective in our case.
- Citation: Ke XT, Yu XF, Liu JY, Huang F, Chen MG, Lai QQ. Myxofibrosarcoma of the scalp with difficult preoperative diagnosis: A case report and review of the literature. World J Clin Cases 2020; 8(11): 2350-2358
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2350.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2350
Myxofibrosarcoma (MFS) is a fibroblast malignant tumor with a matrix of myxoid, visible arc-like vessels, and tumor cells showing varying degrees of atypia. A MFS is the most common soft tissue sarcoma that appears in late adult life, is mainly a low-grade malignancy, and occurs primarily in the lower extremities (77%), followed by the trunk (12%), and retroperitoneum or mediastinum (8%)[1]. Rare occurrences have been reported in the cranial cavity[2], orbit[3], maxilla[4], parotid gland[5], hypopharynx[6], sinus piriformis[7], vocal folds[8], thyroid gland[9], esophagus[10], breast[2], heart[11], aorta[2], scapular region[12], buttock[13], scrotum[14], pterygopalatine fossa[1,2], liver[2], and scalp. MSF of the scalp is extremely rare. We report a case of MFS of the scalp. A 46-year-old male with “a neoplasm in the scalp” was hospitalized and diagnosed with an MFS (highly malignant with massive necrotic lesions) by histologic evaluation and immunohistochemistry testing. A computed tomography (CT) scan and magnetic resonance imaging (MRI) indicated a mass in the scalp, but no typical "tail sign" was observed. Due to the lack of characteristic imaging features and the extremely unusual location, the diagnosis was missed. Lefkowitz et al[15] reported that the “tail sign” cannot be considered of diagnostic value for MFS as the sensitivity and specificity were approximately 80%. This case had unusual imaging findings. Moreover, the treatment for MFS is a matter of international discussion. In our case, aggressive surgery and adjuvant radiotherapy was effective. Therefore, we describe a 46-year-old male with a rare case of MFS originating from the scalp and report the unusual imaging findings to offer some reference for researchers. We discuss the MRI findings, treatment, and histologic evaluation and immunohistochemical testing in this rare case.
A 46-year-old male with “a neoplasm in the scalp for 6 mo” was hospitalized.
His medical history was unremarkable.
His family history was unremarkable.
On physical examination, a mass on the left forehead was palpated and measured approximately 6 cm × 3 cm. The tumor was hard without algesia or ulcerations.
No abnormalities were found on laboratory examinations, including routine hematologic, biochemistry, and tumor markers.
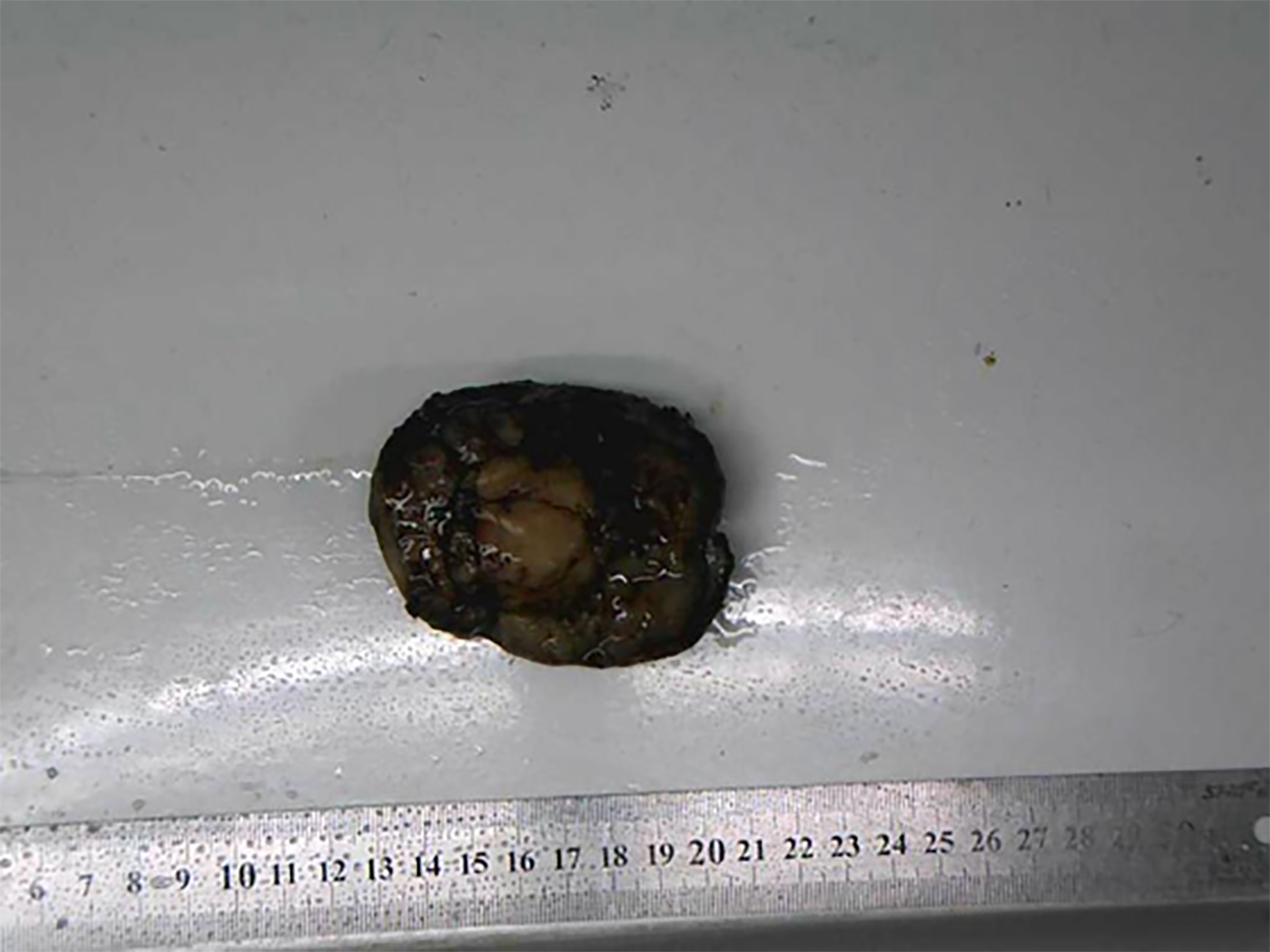
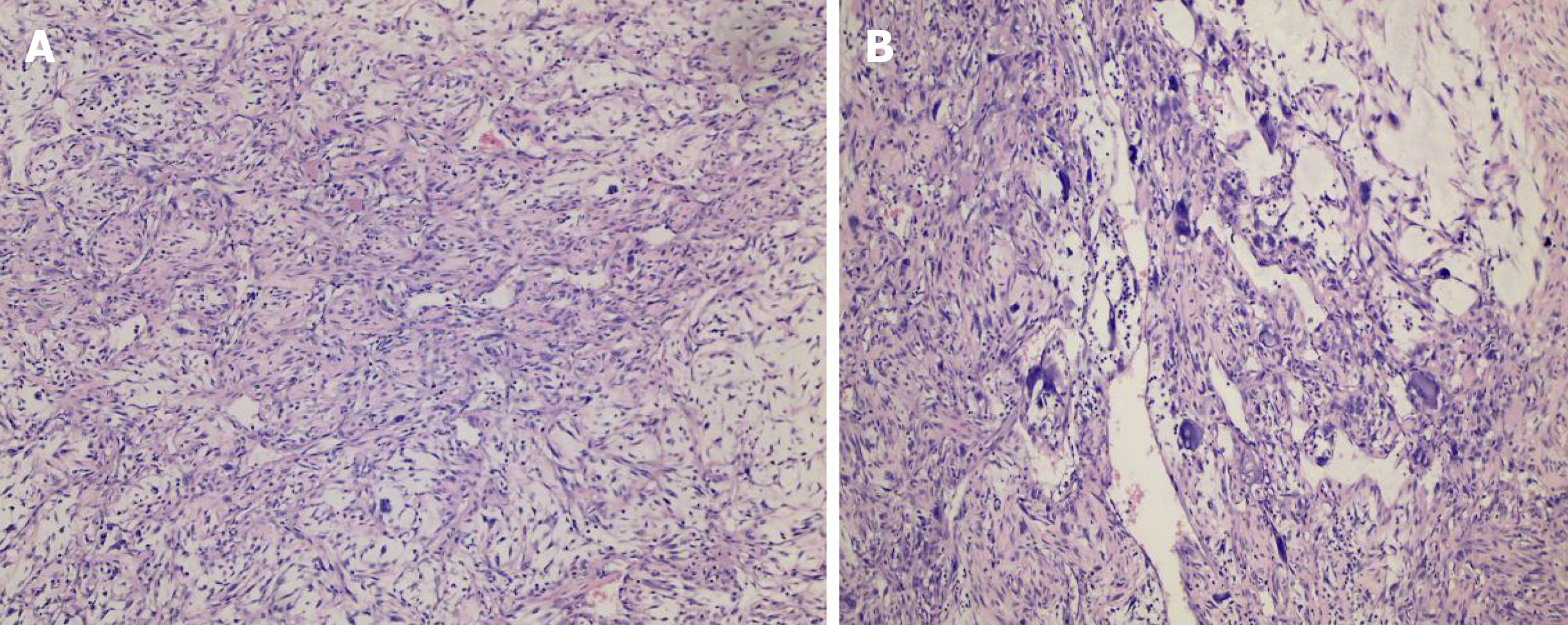
A CT scan (Figure 1) showed an ovoid mass approximately 6.25 cm × 3.29 cm × 3.09 cm in the left frontal scalp with low density intermingled with equidense strips involving adjacent areas of the scalp. Contrast enhancement showed an uneven enhancement pattern. MRI (Figure 2) revealed a lesion in the left frontal region with an irregular surface, approximately 3.55 cm × 6.34 cm in size, and clear boundaries and visible separation. The adjacent skull was damaged and the dura mater was involved. The images of different sequences are as follows: T1, complex signal with dramatic low signal; T2, complex signal with dramatic high signal; T2 FLAIR, high marginal and low central signals; and DWI, high marginal and low central signals. The specimen (Figure 3) was visible to the naked eye as a mass in the scalp involving the skull and approximately 7.5 cm × 6.0 cm × 3.0 cm in size. Histologically (Figure 4), there were abundant heteromorphic spindle cells and partial nodular mucus arranged in a woven pattern with rare nuclear fission and abundant blood vessels in the interstitium. Immunohistochemical stains demonstrated the following: Vim (+); SMA (+); S-100 (-); GFAP (-); CD34 (+); and Ki-67 (+).
Myxofibrosarcoma of the scalp.
A tumor resection (superficial) and cranioplasty were performed under general anesthesia. A horseshoe incision was made, approximately 24 cm in length, in the left frontotemporal parietal. The scalp was incised to the periosteum and the subcutaneous tumor was separated along the tumor margin. The tumor boundaries were clear and approximately 6.5 cm × 4.0 cm × 4.5 cm in size. Following surgery, the patient was returned to the ward in a stable condition. Subsequently, the patient underwent appropriate radiotherapy.
After surgery and subsequent radiotherapy, the patient recovered uneventfully without local recurrence or distant metastasis during a 19-mo follow-up period.
MFS is a type of malignant tumor with an unknown etiology that occurs in late adult life and mainly affects the lower and upper extremities, followed by the trunk, and retroperitoneum or mediastinum. The occurrence of head and neck MFS is rare, with a reported incidence of 2%-4%[16]. Only 21 cases of MFS in the head and neck have been reported in the literature, the clinical features of which (including our case) are summarized in Table 1.
| Patient | Ref. | Sex/age | Location | Treatment | Results |
| 1 | Blitzer et al[17], 1981 | Male/66 | Sphenoid sinus | Radiotherapy | Died after 3 mo |
| 2 | Pomerantz et al[18], 1982 | Male/58 | Maxillary sinus | Surgery | Unknown |
| 3 | Barnes and Kanbour[19], 1988 | Female/67 | Sphenoid sinus-cavernous sinus | Surgery, adjuvant radiotherapy | Alive after 8 mo |
| 4 | Imai et al[20], 2000 | Female/52 | Orbit | Surgery | NA |
| 5 | Iguchi et al[21], 2002 | Male/NA | Maxillary | NA | NA |
| 6 | Song and Miller[10], 2002 | Male/40 | Esophagus | Surgery | NA |
| 7 | Nishimura et al[6], 2006 | Male/69 | Hypopharynx | Surgery | Alive after 16 mo |
| 8 | Udaka et al[22], 2006 | Male/55 | Neck | Surgery | Alive after 27 mo |
| 9 | Enoz and Suoglu[23], 2007 | Female/36 | Maxillary sinus | Surgery | Alive after 2 yr |
| 10 | Gugatschka et al[8], 2010 | Male/79 | Vocal folds | Surgery | NA |
| 11 | Li et al[5], 2010 | Female/37 | Parotid | Surgery, radiotherapy | Alive after 8 mo |
| 12 | Zhang et al[24], 2010 | Female/27 | Orbit | Surgery, radiotherapy | Alive after 6 mo |
| 13 | Zouloumis et al[25], 2010 | Male/23 | Mandible | Surgery, radiotherapy | Alive 39 mo |
| 14 | Norval et al[26], 2011 | Male/69 | Maxillary sinus | Radiotherapy, chemotherapy | Died after 1 yr |
| 15 | Srinivasan et al[27], 2011 | Female/78 | Parotid | Surgery, radiotherapy | Died after 24 mo |
| 16 | Krishnamurthy et al[28], 2011 | Female/42 | Infratemporal space | Surgery, radiotherapy | Alive after 26 mo |
| 17 | Nakahara et al[4], 2012 | Male/52 | Maxilla | Surgery, radiotherapy | Alive after 20 mo |
| 18 | Qiubei et al[7], 2012 | Male/42 | Hypopharynx | Surgery | NA |
| 19 | Dell’Aversana Orabona et al[30], 2014 | Male/35 | Pterygopalatine fossa | Surgery, radiotherapy | Alive after 27 mo |
| 20 | Wong et al[29], 2017 | Female/61 | Maxillary sinus | Surgery, radiotherapy | NA |
| 21 | Clair et al[30], 2018 | Female/87 | Orbit | Surgery, radiotherapy | Alive after 48 mo |
| 22 | Present case | Male/46 | Scalp | Surgery, radiotherapy | Alive after 19 mo |
It as shown in Table 1[17-30], there was no significant difference in the incidence of MFS in the head and neck between men and women. The age ranged from 23-87 years, and the median age was 52 years. The prognosis varied greatly depending on the time of discovery and the degree of malignancy. MFS of the scalp has not been reported; thus, the diagnosis of MFS by radiologists is difficult.
To our knowledge, a large volume, extracompartmental extension, broad interface with the underlying fascia, inhomogeneous MR signal intensity, high signal intensity on T2-weighted MR images, invasion of bone or neurovascular structures, intratumoral necrosis, and marked, primarily peripheral enhancement have been reported in the literature as malignant imaging features of soft tissue tumors[31]. The mucous component of MFS, including more water molecules, shows a higher signal at T2. The degree of high signals in T2 varies with the proportion of the mucinous component in the tumor. MRI findings of MSF contribute to establishing a diagnosis. In T2-weighted MRI, the infiltrative spread of the tumor along the fascial plane is manifested by a curvilinear shape, commonly defined as a “tail,” which extends from the primary mass of the MFS[32]; however, in several studies, MFS with a “tail-like” pattern is significantly related to a superficial (subcutaneous) origin[15]. In our case, MRI revealed a lesion with an irregular surface and a size of approximately 3.55 cm × 6.34 cm in the left frontal region, with clear boundaries and visible separation. The adjacent skull was damaged and the dura mater was involved. The MFS of the scalp of this case was a tumor of superficial (subcutaneous) origin, but the relevant MRI findings did not conform to the so-called “tail-like” pattern. Such atypical imaging findings, combined with the uncommon location, may lead radiologists to miss the correct diagnosis. Therefore, it is crucial to differentiate a MFS from other tumors with similar MRI findings, such as a low-grade fibromyxoid sarcoma (LGFS). MFS shares similar characteristics with LGFST on T1 low signals, T2 mixed signals, and an enhancing pattern; thus, the histopathologic features are required to identify a MFS[33]. In addition, compared with the apparent diffusion coefficient (ADC) value of non-myxoid tumors, that of mucinous tumors is obviously high, and DWI MR imaging has been testified as a helpful way to assess the composition of tumor cells in soft tissue sarcomas[34]. Surov et al[35] indicated that sarcomas require further study using a standardized MR program to compare the ADC values of various types of sarcomas. This idea may provide a new way for researchers to study MR of MFS in the future.
MFS can be diagnosed accurately based on immunohistologic and ultrastructural studies[16]. Histologically, myxoid cells are mixed with spindle cells. The spindle cell area is characterized by large atypical cells and more mitotic features. Mononuclear or multinucleate giant cells, curved blood vessels, spoke-like structures, and inflammatory cells are observed[32]. MFS is classified into low-grade tumors with low metastatic potential and high-grade tumors[16]. The specimen in our case was visible to the naked eye as a mass on the scalp involving the skull and approximately 7.5 cm × 6.0 cm × 3.0 cm in size. Histologically, there were abundant heteromorphic spindle cells and partial nodular mucus arranged in a woven pattern with rare nuclear fission and abundant blood vessels in the interstitium. Immunohistochemical staining demonstrated the following: Vim (+); SMA (+); S-100 (-); GFAP (-); CD34 (+); and Ki-67 (+). The histologic features combined with immunohistochemical findings in our case were consistent with mucinous fibrosarcoma (highly malignant with massive necrotic lesions). Based on the FNCLCC system, this MFS in the scalp was grade 3. In addition, the histologic findings and evaluation provided several options for the differential diagnosis, such as neurilemoma, dedifferentiated liposarcoma, and fibromatosis[16]. A MFS of grade 2-3 can be differentiated from dedifferentiated liposarcoma by immunohistochemical staining; the latter has distinctive immunohistochemical stains that are strongly positive for CKD4 and MDM2[36]. The characteristic histopathologic features of neurilemoma are the presence of abundant Wagner-Meissner corpuscle-like structures and a lack of neoplastic spindle cell nests, as seen in conventional neurofibroma and diffusely positive for S-100 by immunohistochemical analysis[37].
Based on a literature review, there has been no internationally uniform conclusion on the treatment of MFS. As far as malignant tumors are concerned, intact mass excision is advised. Additionally, the value of pre- and post-operative chemotherapy and radiotherapy is still being discussed[38]. Over the past several decades, progress in understanding sarcoma management has promoted the application of combined modality therapies to improve survival. FNCLCC grade plays a significant role in the treatment and prognosis. Therefore, the grade should be an important reference basis for clinical treatment. Some researchers have suggested that local radiotherapy of the mass for patients with FNCLCC grade 1-2 and grade 3 may be supplemented by appropriate chemotherapy and other treatments. Several studies have proposed significant reference factors for local recurrence and metastases, including tumor size, depth, extent of histologic myxoid areas, mitotic rate, and grade[15,32]. It is reported that the high rates of local recurrence of MFS are 50%-60% and distal metastases are significantly more common with high-grade MFS at a rate of 33%[16]. Given the highly malignant MFS in our case, combined with the anatomic location, size, and other factors, surgery was performed and adjuvant radiotherapy was delivered to avoid local and distant recurrences. Importantly, follow-up should be encouraged. The patient recovered without complications, without local recurrence and distant metastases after a follow-up period of 19 mo. Although there is no gold standard of treatment, a complete tumor resection with sufficient resection margins, assisted by adjuvant radiotherapy, may be effective. Dell'Aversana Orabona et al[1] has proposed that a possible re-excision of recurrent lesions is a way to enhance survival. Additionally, the recognition of the “tail” on MRI may be valuable in pre-operative planning to ameliorate the quality of the excision, thus, reducing the risk of local recurrence[15,32]. The treatment for MSF of the scalp (highly malignant) without a “tail sign” reported in this case may provide a reference for subsequent cases that are equally atypical.
The case reported herein of MFS occurred in an extremely rare location on the scalp and had atypical MRI findings, which serves as a reminder to radiologists of the possibility of this diagnosis to assist in clinical treatment. Although there is no gold standard of treatment, a complete tumor resection with clear resection margins, assisted by adjuvant radiotherapy, may be effective.
Manuscript source: Unsolicited manuscript.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: El-Razek AA, Sawazaki H S-Editor: Zhang L L-Editor: Webster JR E-Editor: Xing YX
| 1. | Dell'Aversana Orabona G, Iaconetta G, Abbate V, Piombino P, Romano A, Maglitto F, Salzano G, Califano L. Head and neck myxofibrosarcoma: a case report and review of the literature. J Med Case Rep. 2014;8:468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Shao Z, Jiao B, Yu J, Liu H. Primary low grade myxofibrosarcoma of the liver with benign presentation but malignant outcome: a case report. BMC Cancer. 2019;19:1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Pujari A, Ali MJ, Honavar SG, Mittal R, Naik M. Orbital myxofibrosarcoma: a clinicopathologic correlation of an extremely rare tumor. Ophthalmic Plast Reconstr Surg. 2014;30:e111-e113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Nakahara S, Uemura H, Kurita T, Suzuki M, Fujii T, Tomita Y, Yoshino K. A case of myxofibrosarcoma of the maxilla with difficulty in preoperative diagnosis. Int J Clin Oncol. 2012;17:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Li X, Chen X, Shi ZH, Chen Y, Ye J, Qiao L, Qiu JH. Primary myxofibrosarcoma of the parotid: case report. BMC Cancer. 2010;10:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Nishimura G, Sano D, Hanashi M, Yamanaka S, Tanigaki Y, Taguchi T, Horiuchi C, Matsuda H, Mikami Y, Tsukuda M. Myxofibrosarcoma of the hypopharynx. Auris Nasus Larynx. 2006;33:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Qiubei Z, Cheng L, Yaping X, Shunzhang L, Jingping F. Myxofibrosarcoma of the sinus piriformis: case report and literature review. World J Surg Oncol. 2012;10:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Gugatschka M, Beham A, Stammberger H, Schmid C, Friedrich G. First case of a myxofibrosarcoma of the vocal folds: case report and review of the literature. J Voice. 2010;24:374-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Darouassi Y, Attifi H, Zalagh M, Rharrassi I, Benariba F. Myxofibrosarcoma of the thyroid gland. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:385-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Song HK, Miller JI. Primary myxofibrosarcoma of the esophagus. J Thorac Cardiovasc Surg. 2002;124:196-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Sanchez-Uribe M, Retamero JA, Gomez Leon J, Montoya Perez J, Quiñonez E. Primary intermediate-grade cardiac myxofibrosarcoma with osseous metaplasia: an extremely rare occurrence with a previously unreported feature. Cardiovasc Pathol. 2014;23:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Sakamoto A, Shiba E, Hisaoka M. Short-term spontaneous regression of myxofibrosarcoma in the scapular region. Skeletal Radiol. 2014;43:1487-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Picardo NE, Mann B, Whittingham-Jones P, Shaerf D, Skinner JA, Saifuddin A. Bilateral symmetrical metachronous myxofibrosarcoma: a case report and review of the literature. Skeletal Radiol. 2011;40:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Ozkan B, Ozgüroğlu M, Ozkara H, Durak H, Talat Z. Adult paratesticular myxofibrosarcoma: report of a rare entity and review of the literature. Int Urol Nephrol. 2006;38:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Lefkowitz RA, Landa J, Hwang S, Zabor EC, Moskowitz CS, Agaram NP, Panicek DM. Myxofibrosarcoma: prevalence and diagnostic value of the "tail sign" on magnetic resonance imaging. Skeletal Radiol. 2013;42:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Quimby A, Estelle A, Gopinath A, Fernandes R. Myxofibrosarcoma in Head and Neck: Case Report of Unusually Aggressive Presentation. J Oral Maxillofac Surg. 2017;75:2709.e1-2709.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Blitzer A, Lawson W, Zak FG, Biller HF, Som ML. Clinical-pathological determinants in prognosis of fibrous histiocytomas of head and neck. Laryngoscope. 1981;91:2053-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Pomerantz JM, Sanfacon DG, Dougherty TP, Hanson S. Myxofibrosarcoma of the maxillary sinus. Del Med J. 1982;54:147-152. [PubMed] |
| 19. | Barnes L, Kanbour A. Malignant fibrous histiocytoma of the head and neck. A report of 12 cases. Arch Otolaryngol Head Neck Surg. 1988;114:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Imai Y, Sugawara Y, Okazaki M, Harii K. Low grade myxofibrosarcoma in the orbit: a case report. Japanese J Plastic Reconstructive Surg. 2000;43:401-409. |
| 21. | Iguchi Y, Takahashi H, Yao K, Nakayama M, Nagai H, Okamoto M. Malignant fibrous histiocytoma of the nasal cavity and paranasal sinuses: review of the last 30 years. Acta Otolaryngol Suppl. 2002;75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Udaka T, Yamamoto H, Shiomori T, Fujimura T, Suzuki H. Myxofibrosarcoma of the neck. J Laryngol Otol. 2006;120:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Enoz M, Suoglu Y. Myxofibrosarcoma of the maxillary sinus. Int J Head Neck Surg. 2007;1:1-4. |
| 24. | Zhang Q, Wojno TH, Yaffe BM, Grossniklaus HE. Myxofibrosarcoma of the orbit: a clinicopathologic case report. Ophthalmic Plast Reconstr Surg. 2010;26:129-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Zouloumis L, Ntomouchtsis A, Lazaridis N. Giant myxofibrosarcoma of the mandible. Balkan J Stomatol. 2010;14:41-44. |
| 26. | Norval EJ, Raubenheimer EJ. Myxofibrosarcoma arising in the maxillary sinus: a case report with a review of the ultrastructural findings and differential diagnoses. J Maxillofac Oral Surg. 2011;10:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Srinivasan B, Ethunandan M, Hussain K, Ilankovan V. Epitheloid myxofibrosarcoma of the parotid gland. Case Rep Pathol. 2011;2011:641621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Krishnamurthy A, Vaidhyanathan A, Majhi U. Myxofibrosarcoma of the infratemporal space. J Cancer Res Ther. 2011;7:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Wong A, Chan Woo Park R, Mirani NM, Eloy JA. Myxofibrosarcoma of the maxillary sinus. Allergy Rhinol (Providence). 2017;8:95-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Clair BC, Salloum G, Carruth BP, Bersani TA, Hill RH. Orbital Myxofibrosarcoma: Case Report and Review of Literature. Ophthalmic Plast Reconstr Surg. 2018;34:e180-e182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Razek AA, Huang BY. Soft tissue tumors of the head and neck: imaging-based review of the WHO classification. Radiographics. 2011;31:1923-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Sambri A, Spinnato P, Bazzocchi A, Tuzzato GM, Donati D, Bianchi G. Does pre-operative MRI predict the risk of local recurrence in primary myxofibrosarcoma of the extremities? Asia Pac J Clin Oncol. 2019;15:e181-e186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Yue Y, Liu Y, Song L, Chen X, Wang Y, Wang Z. MRI findings of low-grade fibromyxoid sarcoma: a case report and literature review. BMC Musculoskelet Disord. 2018;19:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Razek A, Nada N, Ghaniem M, Elkhamary S. Assessment of soft tissue tumours of the extremities with diffusion echoplanar MR imaging. Radiol Med. 2012;117:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Surov A, Nagata S, Razek AA, Tirumani SH, Wienke A, Kahn T. Comparison of ADC values in different malignancies of the skeletal musculature: a multicentric analysis. Skeletal Radiol. 2015;44:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Shen J, Fang Z, Zhang Y, Hou J. In-situ recurrence of the primary cardiac dedifferentiated liposarcoma: To resect or not? J Card Surg. 2020;35:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 37. | Miyasaka C, Ishida M, Kouchi Y, Morimoto N, Kusumoto K, Okabe H, Tsuta K. Wagner-Meissner neurilemmoma of the lip occurring in a patient with neurofibromatosis type 1: A case report. Mol Clin Oncol. 2020;12:41-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Look Hong NJ, Hornicek FJ, Raskin KA, Yoon SS, Szymonifka J, Yeap B, Chen YL, DeLaney TF, Nielsen GP, Mullen JT. Prognostic factors and outcomes of patients with myxofibrosarcoma. Ann Surg Oncol. 2013;20:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |