Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2255
Peer-review started: March 3, 2020
First decision: April 1, 2020
Revised: April 18, 2020
Accepted: April 28, 2020
Article in press: April 28, 2020
Published online: June 6, 2020
Processing time: 96 Days and 16.6 Hours
Chronic hepatitis B virus infection remains a major global public health problem. Peginterferon-alpha-2a (PEG-IFN) has direct antiviral and immunoregulatory effects, and it has become one of the first choice drugs for the treatment of chronic hepatitis B (CHB). Cytokines play an important role in immunity, and they directly inhibit viral replication and indirectly determine the predominant pattern of the host immune response.
To determine the correlation between cytokine/chemokine expression levels and response to PEG-IFN treatment in patients with CHB.
Forty-six kinds of cytokines were analyzed before PEG-IFN therapy and at 24 wk during therapy in 26 CHB patients.
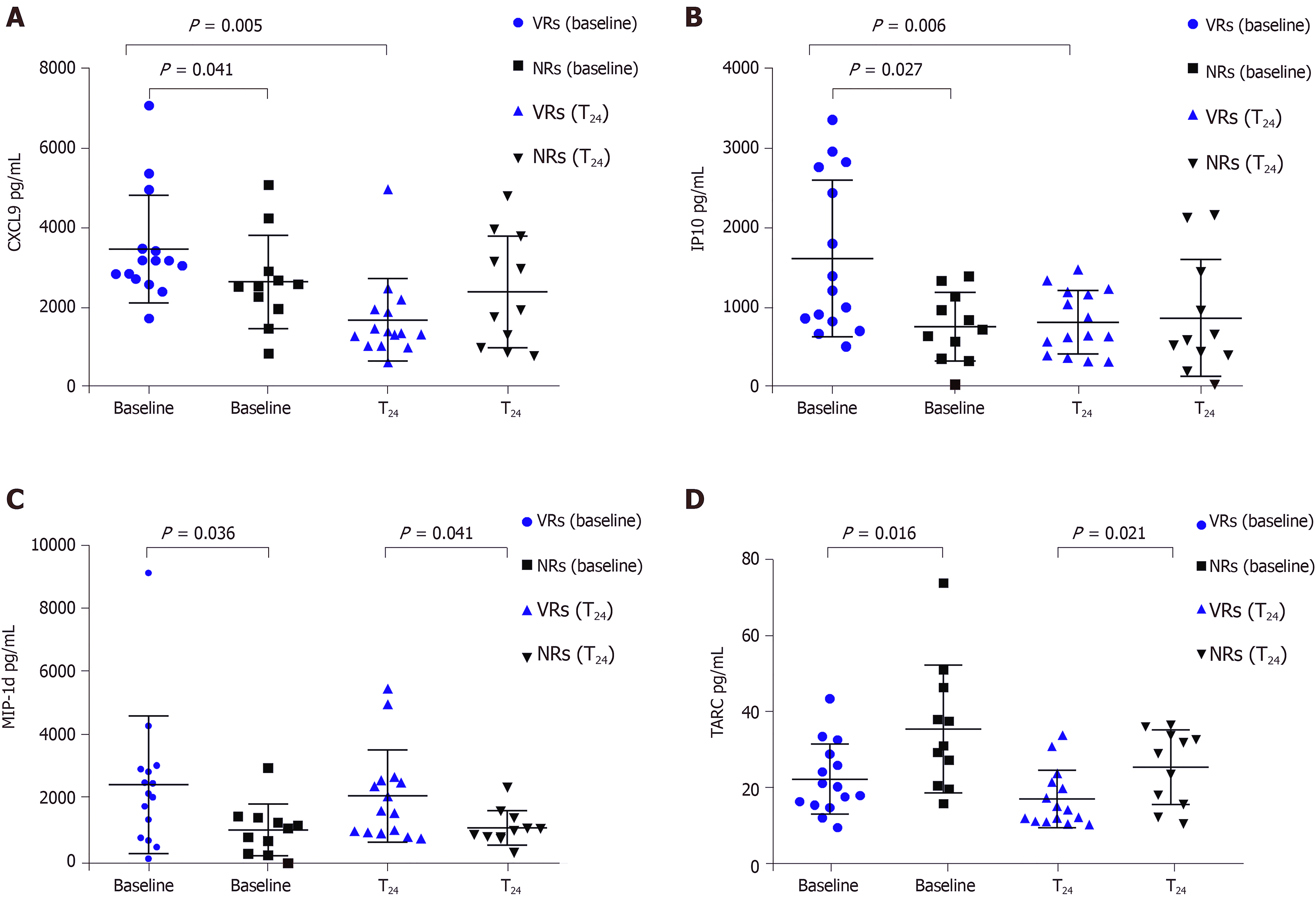
The monokine induced by INF-γ (CXCL9) and serum interferon-inducible protein 10 ( IP-10) levels at baseline were higher in virological responders than in non-virological responders (NRs) and decreased during treatment, whereas the NRs did not exhibit significant changes. The macrophage inflammatory protein 1d (MIP-1d) levels at baseline and during treatment were significantly higher in the virological responders than in the NRs, while thymus and activation-regulated chemokine (TARC) levels at baseline and during treatment were significantly lower in the virological responders than in the NRs. The CXCL9, IP-10, MIP-1d, and TARC baseline levels exhibited the expected effects for interferon treatment. The area under the receiver operating characteristic curve values of CXCL9, IP-10, MIP-1d, and TARC for predicting virological responses were 0.787, 0.799, 0.787, and 0.77 (P = 0.01, 0.013, 0.01, and 0.021), respectively.
We found that cytokine levels before and during treatment may represent potential biomarkers to select CHB patients who can respond to PEG-IFN. Therefore, cytokines can be used as an indicator of antiviral drug selection before CHB treatment.
Core tip: Analyses of the changes in cytokine expression patterns during treatment can help to understand the pathogenesis of chronic hepatitis B and predict treatment responses. We found that macrophage inflammatory protein 1d, CXCL9, CXCL6, interferon-inducible protein 10, and thymus and activation-regulated chemokine have predictive significance in interferon therapy. Therefore, further large and long-term follow-up studies are needed to determine the predictive value of cytokines in chronic hepatitis B patients receiving peginterferon-alpha-2a treatment. In addition, the differences in these factors between the response and non-response groups and their specific biological roles in hepatitis B virus infection require further investigation.
- Citation: Fu WK, Cao J, Mi NN, Huang CF, Gao L, Zhang JD, Yue P, Bai B, Lin YY, Meng WB. Cytokines predict virological response in chronic hepatitis B patients receiving peginterferon alfa-2a therapy. World J Clin Cases 2020; 8(11): 2255-2265
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2255.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2255
Despite the development of a highly effective and safe vaccine over 30 years ago, chronic hepatitis B virus (HBV) infection remains a global major public health problem, and at least 257 million people are chronically infected with HBV worldwide[1]. Approximately 887000 chronic hepatitis B (CHB) patients die annually from complications associated with the disease, including cirrhosis, hepatocellular carcinoma, and liver failure[1-10]. Effective antiviral drugs inhibit replication and reduce the risks of CHB complications[11]. Host and viral factors play key roles in the natural history of CHB, the disease activity, and the effectiveness of antiviral therapy[12]. CHB patients generally have an impaired host immune response, which may be associated with a persistent high viral load and subsequent T cell failure[11,13]. At present, the antiviral strategy for CHB mainly lies in the effective suppression of the virus and the recovery of HBV-specific immune responses[12]. Because peginterferon-alpha-2a (PEG-IFN) has direct antiviral and immunoregulatory effects, it has become one of the first choice drugs for the treatment of CHB[14]. Antiviral treatment with PEG-IFN effectively inhibits HBV replication and may lead to seroconversion of hepatitis B e antigen (HBeAg), clearance of hepatitis B surface antigen (HBsAg), normalization of alanine aminotransferase (ALT) levels, and histologic improvement[11]. If patients achieve an IFN induced virological response, they may achieve long-term therapeutic effects while reducing the risks of cirrhosis and HCC[11,14]. When patients with CHB receive PEG-IFN treatment, there is no guarantee of a sustained virology response, and studies show that only 30% of patients benefit from this treatment[15]. Since PEG-IFN has the properties of immune regulation, the host immune status may affect the efficacy of PEG-IFN in the treatment of CHB[11,12]. Several studies in recent years have shown that cytokines and chemokines may play a potential role in chronic viral hepatitis[12,16,17].
Cytokines play an important role in immunity, and they directly inhibit viral replication and indirectly determine the predominant pattern of the host immune response[18]. Chemokines are a group of small cytokines that induce the migration of circulating white blood cells to sites of inflammation or injury and enhance the phagocytosis abilities of inflammatory cells[19]. Due to the highly complex control of cytokine secretion, the interactions between cytokines, and with their receptors, are widely spread in numerous regulatory networks[19]. Screening multiple biomarkers can clarify the immune mechanism of HBV infection and predict the response to antiviral therapy. Moreover, a study has demonstrated that T cell responses and circulating cytokines are associated with HBV replication and liver function. Interleukin (IL)-10, IL -12, and IL -21 play crucial roles in the immune clearance stage; thus, they are related to the seroconversion of HBeAg[11,16,18,19]. Nevertheless, the relationship between multiple cytokines and chemokines and the responses to PEG-IFN therapy in patients with CHB has not previously been clarified.
In this study, we analyzed the serum levels of cytokines in CHB patients receiving PEG-IFN treatment and responses to the therapy. Forty-six cytokines, including macrophage inflammatory protein 1d (MIP-1d), CXCL9, CXCL6, IP10, and TARC, in serum samples of CHB patients were simultaneously tested. The predictive value of the cytokines for the responses to PEG-IFN was also assessed.
In total, 78 serum samples were prospectively collected with written informed consent from 26 CHB patients undergoing PEG-IFN therapy between August 2018 and June 2019. All patients were positive for serum HBsAg for more than 6 mo, and patients receiving PEG-IFN therapy fulfilled the treatment criteria for CHB according to the American Association for the Study of Liver Disease treatment guidelines, i.e., serum ALT levels > 80 U/L (2 × upper limit of normal) with HBV DNA > 20000 IU/mL in HBeAg positive patients[14]. The patients were treated with 180 μg of PEG-IFN per week for 48 wk. Venous blood samples were drawn from patients with consent at two to four time points as follows: Before PEG-IFN therapy and during PEG-IFN therapy (24 wk and/or 48 wk after initiation of the therapy). The patients were classified as virological responders (VRs) when the serum HBV DNA levels at the 6th mo fter the initiation of the therapy were decreased to less than 2000 IU/mL[20], and the other patients were classified as virological non-responders (NRs). This study was approved by the Institutional Review Board, the First Hospital of Lanzhou University, and complied with the standards of the Declaration of Helsinki and current ethical guidelines. All patients provided written informed consent for participation in the study.
Serum was separated within 2 h of obtaining venous blood samples. If cytokine measurements were not possible on the same day, the samples were immediately stored at -80 °C until the concentrations of cytokines were measured. A Luminex 200 analyzer and Cytokine Array I reagents (Randox, Antrim, United Kingdom) were used to quantify 46 cytokines in the serum, including BCA-1, CTACK, ENA-78, Eotoxin-2, SDF-1a, TARC, TRAIL, TSLP, CCL19, CXCL9, IP-10, EGF, Eotaxin, GRO, IL-8, and MIP-1b. All experiments are measured according to the manufacturer's instructions.
The serum HBV DNA levels were assayed using a COBAS Amplicor/COBAS TaqMan HBV test (Roche Diagnostics, Indianapolis, IN, United States) with a limit of detection of 20 IU/mL, according to the manufacturer’s instructions. HBV serologic tests for HBsAg, HBeAg, anti-HBs antibody, and HBeAb were conducted by enzyme immunoassays (AxSYM; Abbott Laboratories, Abbott Park, IL, United States). The levels of serum albumin, ALT, aspartate aminotransferase (AST), and total bilirubin were determined using a Hitachi 7600 analyzer (Hitachi Ltd., Tokyo, Japan) with dedicated reagents (Roche Diagnostics, Mannheim, Germany). The assay was carried out according to the manufacturer's instructions.
Values are expressed as the median (ranges) or the mean ± SD when appropriate. The correlations between the serum ALT and chemokine levels were tested by Spearman’s test. The Mann-Whitney U test was used to compare a series of continuous variables. The Kruskal-Wallis test and Bonferroni correction were used for multiple comparisons of continuous variables, including cytokine levels between patient subgroups and treatment stages, to compensate for alpha statistical errors. Friedman's test was also performed to compare cytokine levels between paired groups. Friedman's test was carried out to compare the cytokine levels among the paired groups. Categorical variables between the two groups were analyzed for the difference in proportion by Fisher's exact test. The areas under the receiver operating characteristic curves (AUROC) of the cytokines and HBV DNA were calculated to assess their predictive values for virological responses. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 17.0 for Windows, SPSS Inc., Chicago, IL, United States), and P < 0.05 was considered statistically significant.
The characteristics of 26 patients with CHB are summarized in Table 1. The age and male proportions were not different between the VRs (n = 15) and NRs (n = 11). Before peginterferon treatment, there was no difference in serum AST and ALT levels between VRs and NRs, but AST and ALT levels were higher in NRs during and after treatment. Serum HBV DNA levels before, during, and after the therapy were lower in the VRs than in the NRs. Among the VRs, ten (66.7%) demonstrated HBeAg seroconversion during or after the therapy.
| Variable | Virological responders (n = 15) | Virological non-responders (n = 11) | P value |
| Age (yr) | 28.8 ± 4.161 | 27.82 ± 4.446 | 0.57 |
| Gender (n of male, %) | 10 (67) | 7 (64) | 1 |
| ALT (IU/L) | |||
| Baseline | 237.5 ± 89.36 | 204.5 ± 74.36 | 0.3292 |
| T24 | 43 ± 15 | 102.8 ± 61.70 | 0.0013 |
| T48 | 29.07 ± 10.30 | 130.3 ± 117.1 | 0.0026 |
| AST (IU/L) | |||
| Baseline | 108.9 ± 42.60 | 94.64 ± 47.99 | 0.43 |
| T24 | 36.33 ± 8.541 | 76.64 ± 59.67 | 0.0157 |
| T48 | 29.4 ± 7.039 | 87 ± 89.2 | 0.0193 |
| Serum HBV DNA log10 (copies/mL) | |||
| Baseline | 6.518 ± 0.7267 | 7.477 ± 0.3533 | 0.005 |
| T24 | 1.193 ± 1.282 | 7.2 ± 0.3550 | < 0.0001 |
| T48 | 0.4867 ± 0.7605 | 7.055 ± 0.6056 | < 0.0001 |
| HBeAg seroconversion during or after Tx (n, %) | 10 (66.7) | 0 | - |
Serum levels of 46 cytokines in the phases of peginterferon therapy in the VR and NR groups are summarized in Table 2. The levels of all cytokines, with the exception of BCA-1, CTACK, ENA-78, Eotoxin-2, SDF-1a, TARC, TRAIL, TSLP, CCL19, CXCL9, IP-10, EGF, Eotaxin, GRO, IL-8, and MIP-1b, were not statistically significant between the treatment response group and the non-response group before and after treatment.
| Cytokine (pg/mL) | Virological responders (n = 15) | Virological non-responders (n = 11) | ||||||
| Baseline (n = 15) | T24 (n = 15) | P value | Baseline (n = 11) | T24 (n = 11) | P value | P1 value | P2 value | |
| 6Ckine | 144 (41.26-277) | 41.26 (19.74-239) | 0.826 | 100 (7.25-185.) | 129 (12.92-277) | 0.11 | 0.563 | 0.696 |
| BCA-1 | 33.2 (24.4-53.03) | 34.75 (26.4-50.39) | 0.394 | 33.97 (13.38-46.63) | 47.73 (33.89-94.67) | 0.01 | 0.384 | 0.264 |
| CTACK | 463 (396-541) | 575 (465-729) | 0.268 | 387 (235-479) | 430 (301-587) | 0.026 | 0.109 | 0.164 |
| ENA-78 | 219 (166-232) | 159 (81.01-217) | 0.065 | 232 (162-260) | 123 (94.48-196) | 0.023 | 0.363 | 0.474 |
| Eotoxin-2 | 221 (202-482) | 118 (60.96-226) | 0.018 | 302 (74.73-441) | 99.53 (68.48-203) | 0.091 | 0.574 | 0.721 |
| Eotoxin-3 | 177 (106-236) | 136 (77.37-256) | 0.551 | 225 (69.83-247) | 112 (74.37-255) | 0.477 | 0.929 | 0.809 |
| CCL1 | 1.82 (1.62-3.12) | 1.56 (1.01-2.22) | 0.426 | 1.42 (1.01-2.48) | 1.56 (1.01-2.22) | 0.798 | 0.11 | 0.949 |
| IL-23 | 6.45 (4.37-51.49) | 4.37 (4.37-69.89) | 0.799 | 63.29 (4.37-373) | 30.43 (4.37-535) | 0.674 | 0.18 | 0.249 |
| IL-16 | 37.38 (3.98-82.47) | 30.45 (19.15-56.61) | 0.331 | 58.74 (2.7-116) | 20.01 (2.7-50.66) | 0.26 | 0.969 | 0.296 |
| IL-20 | 23.84 (23.84-23.84) | 23.84 (23.84-23.84) | 0.197 | 23.84 (23.84-38.4) | 23.84 (23.84-23.84) | 0.655 | 0.678 | 1 |
| IL-21 | 2.63 (2.63-2.63) | 2.63 (2.63-2.63) | 0.317 | 2.63 (2.63-2.63) | 2.63 (2.63-2.63) | 0.317 | 0.423 | 0.746 |
| IL-28a | 0.99 (0.99-3.14) | 0.99 (0.99-4.48) | 0.753 | 0.99 (0.99-16.49) | 0.99 (0.99-5.95) | 0.686 | 0.47 | 0.99 |
| IL-33 | 5.78 (5.78-11.23) | 5.78 (5.78-7.26) | 0.686 | 5.78 (5.78-46.66) | 5.78 (5.78-69.88) | 0.686 | 0.3 | 0.476 |
| LIF | 3.12 (1.66-4.29) | 2.77 (1.6-3.26) | 0.691 | 1.91 (0.98-4.66) | 2.3 (1.31-3.69) | 0.441 | 0.263 | 0.711 |
| MCP-2 | 14.99 (9.58-26.36) | 14.52 (11.04-29.02) | 0.427 | 8.72 (6.74-21.44) | 18.27 (10.62-26.93) | 0.328 | 0.232 | 0.848 |
| MCP-4 | 22.38 (14.89-33.87) | 22.14 (14.07-32.12) | 0.955 | 16.78 (9.47-25.28) | 19.38 (10.36-34.95) | 0.093 | 0.124 | 0.769 |
| MIP-1d | 2230 (834-2998) | 1682 (1005-2639) | 0.496 | 1131 (331-1462) | 1051 (851-1550) | 0.248 | 0.036 | 0.041 |
| SCF | 1.86 (1.86-3.28) | 1.86 (1.86-7.51) | 0.575 | 1.86 (1.86-3.05) | 1.86 (1.86-7.32) | 0.144 | 0.771 | 0.779 |
| SDF-1a | 332 (252-765) | 767 (287-985) | 0.05 | 702 (394-1166.2) | 1261 (1016-1659) | 0.009 | 0.196 | 0.032 |
| TARC | 20.14 (15.32-28.82) | 14.03 (10.3-21.46) | 0.006 | 30.97 (20.41-46.4) | 18.97(10.97-32.62) | 0.033 | 0.016 | 0.021 |
| TPO | 188 (142-257) | 265 (179-312) | 0.173 | 190 (145-453) | 235 (131-786) | 0.374 | 0.566 | 0.655 |
| TRAIL | 2.9 (2.9-2.9) | 2.9 (2.9-5.51) | 0.028 | 2.9 (2.9-2.9) | 2.9 (2.9-31.96) | 0.046 | 0.746 | 0.457 |
| TSLP | 4.68 (4.68-4.68) | 4.68 (4.68-4.68) | 0.317 | 4.68 (4.68-6.3) | 4.68 (4.68-6.85) | 0.345 | 0.049 | 0.116 |
| CCL19 | 155 (116-183) | 191 (164-242) | 0.009 | 122 (75.74-164) | 200 (91.01-299) | 0.013 | 0.33 | 0.929 |
| CCL20 | 26.96 (9.69-75.43) | 13.99 (10.8-29.42) | 0.247 | 13.99 (6.86-27.79) | 12.95 (6.86-15.02) | 0.441 | 0.19 | 0.158 |
| CXCL9 | 3177 (2719-3474) | 1348 (1032-1951) | 0.005 | 2523 (1966-2911) | 1930 (984-3788) | 0.374 | 0.041 | 0.413 |
| CXCL6 | 160 (112-182) | 138 (101-172) | 0.426 | 110 (74.38-141) | 93.95 (70.2-123) | 0.657 | 0.059 | 0.012 |
| CXCL11 | 1847 (892-10539) | 1056 (576-2020) | 0.245 | 823 (596-2010) | 2322 (843-2714) | 0.114 | 0.13 | 0.134 |
| IL29 | 174 (153-195) | 153 (149.76-174) | 0.582 | 153 (149.76-195) | 153 (149.76-195) | 0.416 | 0.558 | 0.662 |
| M-CSF | 79.39 (79.39-79.39) | 79.39 (79.39-79.39) | 1 | 79.39 (79.39-9.39) | 79.39 (79.39-79.39) | 1 | 1 | 1 |
| XC11 | 19.72 (88.62-32.98) | 32.98 (8.62-45.45) | 0.411 | 8.62(8.62-79.25) | 8.62 (8.62-144) | 0.293 | 0.95 | 0.965 |
| EGF | 54.78 (2.6-92.2) | 9.47 (2.6-52.23) | 0.221 | 31.4 (13.8-99.06) | 10.11 (2.6-22.01) | 0.037 | 1 | 0.638 |
| FGF-2 | 16.6 (3.07-45.69) | 3.07 (3.07-40.73) | 0.241 | 3.07 (3.07-8.44) | 3.07 (3.07-29.74) | 0.715 | 0.17 | 1 |
| EOTAXIN | 389 (182-530) | 436 (278-620) | 0.394 | 262 (138-397) | 236 (118-315) | 0.374 | 0.148 | 0.015 |
| TGF-a | 2.28 (2.28-4.23) | 2.28 (2.28-2.28) | 0.128 | 2.28 (2.28-2.28) | 2.28 (2.28-2.28) | 0.317 | 0.308 | 0.614 |
| G-CSF | 2.6 (2.6-29.5) | 2.6 (2.6-7.78) | 0.345 | 2.6 (2.6-2.6) | 2.6 (2.6-2.6) | 0.18 | 0.293 | 0.113 |
| GRO | 579 (226-1378) | 469 (208-725) | 0.281 | 243 (143-541) | 85.57 (3.73-199) | 0.059 | 0.148 | 0.009 |
| IL-10 | 3.21 (3.21-3.21) | 3.21 (3.21-3.21) | 0.18 | 3.21 (3.21-3.21) | 3.21 (3.21-3.21) | 0.317 | 0.492 | 0.423 |
| MDC | 627 (184-877) | 365 (248-827) | 0.776 | 294 (192-454) | 158 (119-443) | 0.657 | 0.164 | 0.54 |
| sCD40L | 1116 (192-2402) | 1510 (737-2905) | 0.57 | 848 (242-1342) | 771 (442-1514) | 0.477 | 0.405 | 0.106 |
| IL-8 | 45.31 (2.11-153) | 2.35 (1.65-13.3) | 0.177 | 55.29 (1.65-104) | 1.65 (1.65-5.02) | 0.011 | 0.929 | 0.186 |
| IP-10 | 1154 (777-2668) | 605 (362-1282) | 0.006 | 679 (323-1086) | 549 (365-1386) | 0.859 | 0.027 | 0.574 |
| MCP-1 | 298 (116-477) | 215 (102-520) | 0.478 | 184 (76.17-267) | 118 (54.96-186) | 0.374 | 0.107 | 0.097 |
| MIP-1a | 2.51 (2.51-34.54) | 2.51 (2.51-11.46) | 0.779 | 12.02 (2.51-35.01) | 2.51 (2.51-2.51) | 0.091 | 0.705 | 0.416 |
| MIP-1b | 31.43 (1.54-174) | 1.54 (1.54-28.75) | 0.028 | 1.54 (1.54-48.57) | 1.54 (1.54-11.12) | 0.345 | 0.148 | 0.649 |
| VEGF | 42.87 (5.8-225) | 5.8 (5.8-19.43) | 0.074 | 5.8 (5.8-81.56) | 5.8 (5.8-22.63) | 0.144 | 0.307 | 0.631 |
The levels of CXCL9 and IP10 were higher in the VR group than in the NR group (P = 0.041 and 0.027, respectively). After 24 wk of treatment, CXCL9 and IP10 were significantly lower in the VR group (P = 0.005, P = 0.006), whereas the levels of these two cytokines were not significantly changed according to the phases of the therapy in the NR group (Figure 1A and B). The levels of MIP-1d in the response group at baseline and 24 wk of treatment were higher than those in the NR group (P = 0.036 and 0.041, respectively) (Figure 1C). However, the levels of TARC at baseline and 24 wk in the VRs were lower than those in the NRs (P = 0.016 and 0.021, respectively) (Figure 1D). The level of SDF-1a in the NRs was higher than that in the VRs at 24 wk of treatment (P = 0.032). The CXCL6 level was higher in the VRs than that in the NRs at 24 wk (P = 0.012).
The serum ALT and 46 chemokine levels were measured at each time point during PEG-IFN treatment for correlation analysis. ALT had positive correlations with the serum BCA-1, SCF, CCL19, CXCL9, CXCL6, and CXCL11 levels (r = 0.526, P = 0.006; r = 0.448, P = 0.022; r = 0.452, P = 0.02; r = 0.588, P = 0.002; r = 0.530, P = 0.005; and r = 0.661, P < 0.0001). ALT was inversely correlated with the serum Eotoxin-2, IL-16, IL-8, and MIP-1a (r = -0.517, P = 0.006; r = -0.458, P = 0.019; r = -0.404, P = 0.041; and r = -0.388, P = 0.05).
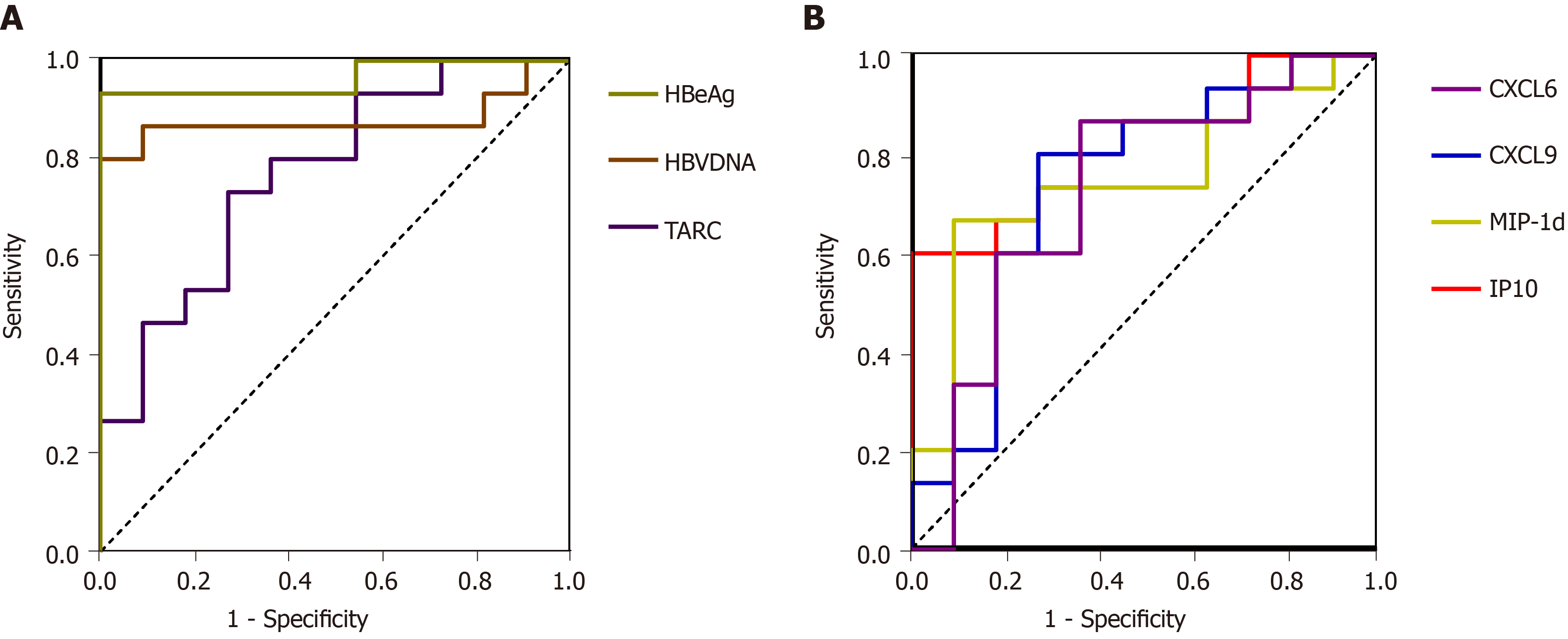
The AUROC values of the serum HBV DNA and HBeAg levels measured before peginterferon therapy were 0.879 and 0.964 (P = 0.001, P < 0.001), respectively (Figure 2A). The sensitivity and specificity of the baseline HBV DNA levels in predicting VR were 90.90% and 86.70%, respectively, when the cutoff DNA level was 7.015 IU/mL. The sensitivity and specificity of the baseline HBeAg levels in predicting VR were 100% and 93.30%, respectively, when the cutoff DNA level was 820.95 IU/mL (Figure 2A). Moreover, the AUROC value of the baseline TARC levels was 0.77 (P = 0.021) in detecting VR, and the lower TARC level was related to VR. The sensitivity and specificity of the baseline TARC level in discriminating VRs from NRs were 72.70% and 73.30%, respectively, with the cutoff value of 26.52 pg/mL. MIP-1d, CXCL9, CXCL6, and IP10 also had good predictive value. The AUROC values of the serum MIP-1d, CXCL9, CXCL6, and IP10 levels measured before peginterferon therapy were 0.787, 0.799, 0.722, and 0.787 (P = 0.01, 0.01, 0.05, and 0.013), respectively, in predicting VR. The sensitivity and specificity of the baseline MIP-1d, CXCL9, CXCL6, and IP10 levels in predicting VR were 69.20% and 84.60%, 76.90% and 76.90%, 61.50% and 84.60%, and 61.50% and 92.30%, respectively, when the cutoff values were 1662.5 pg/mL, 2840.5 pg/mL, 154.50 pg/mL, and 1332.50 pg/mL, respectively (Figure 2B). The AUROC values of all other cytokines quantified in the serum samples before the therapy were not statistically significant in predicting VR.
Liver inflammation due to HBV infection is immune mediated. Acquired immunity and innate immunity are considered related to the pathogenesis of CHB[21]. In this study, we obtained simultaneous measurements of 46 kinds of cytokines in CHB patients to assess the roles of cytokines according to the responses to PEG-IFN, which is a widely used therapeutic agent for the treatment of CHB. However, interferon therapy for CHB remains a clinical challenge. Although successful PEG-IFN therapy can induce a long-lasting response and reduce liver-related complications, the efficacy rate of conventional interferon therapy for 6 mo was only 25% to 40%[22,23]. Therefore, individualized treatment strategies according to pretreatment parameters should be established to identify patients with the highest chance of response. However, there is currently no ideal pre-treatment predictor of VR.
In our study, the serum levels of cytokines changed in the CHB patients during PEG-IFN therapy, and the baseline TARC, MIP-1d, CXCL9, CXCL6, and IP10 levels were strongly correlated with the virological response in patients with CHB. Among these cytokines, high baseline levels of MIP-1d, CXCL9, and IP10 had better prediction of the virological response to interferon therapy. CXCL6, CXCL9, and IP10 are all members of the chemokine ELR-CXC subfamily, in which CXCL9 and IP10 can be induced by IFN-γ[12,24]. These three cytokines are secreted by mono-nuclear/macrophage T lymphocytes, B lymphocytes, hepatocytes, fibroblasts, etc. CXCL9 and IP10 bind to their co-receptor CXCR3, activate T cells, NK cells, etc, and exert antiviral effects[12,25-27]. Bièche et al[28] reported that CXCL9 and IP-10 were significantly increased in the serum of patients with CHB[28]. A study found a high correlation between high baseline levels of IP10 and hepatitis C virus (HCV) clearance induced by PegIFNα treatment in HCV-infected patients[29]. The baseline levels of CXCL9 and IP10 were significantly higher in the response group than in the non-response group; however, there was a significant decrease in the response group after 24 wk of treatment, and there was no significant difference in the non-response group before and after treatment. Studies have indicated that CXCL9 and IP10 mRNAs were significantly decreased in peripheral blood mononuclear cells of CHB patients after 12-24 wk of PegIFNα treatment, which was similar to the findings in patients with chronic hepatitis C infection[30-32]. According to our study and reports in the literature, the serum levels of CXCL9 and IP10 and the mRNA levels of CXCL9 and IP10 in peripheral blood mononuclear cells decreased in patients who responded to PEG-IFN treatment, which might reflect a reduced hepatic necro-inflammatory activity after treatment. CXCL9 and IP-10 were reported to correlate with hepatic injury during hepatic flares in CHB, and a strong correlation of serum CXCL9 and IP-10 levels with ALT levels was also noted[33]. Our results also showed positive correlations of CXCL6 and CXCL9 with ALT levels (r = 0.530, P = 0.005; r = 0.588, P = 0.002). However, IP-10 was not correlated with ALT, which was different from that in the previous literature and may be related to the relatively few samples tested and individual differences of the hosts. Mac-2-binding protein glycosylation isomer (M2BPGi), also called WFA+-M2BP, is a serum biomarker for assessing liver fibrosis in patients with viral hepatitis[34], and its levels significantly correlated with serum IP-10 levels[35]. A recent study suggested that baseline serum M2BPGi level can be a useful marker in the CHB patients during PEG-IFN therapy[36]. The positive correlations between these parameters may imply that these chemokines have positive impacts on the liver inflammation and HBV control, albeit the relevance was not satisfactory due to the complex interactions between other host and viral factors that contribute to liver inflammation. These findings indicate that CXCL6, CXCL9, and IP10 are associated with hepatic necro-inflammation, and these chemokines are induced and participate in immune responses against HBV infection.
We also found that TARC in patients with an interferon-treated response was lower than that in the NRs at baseline; however, the level in the response group remained lower than that in the non-response group after 24 wk of treatment. TARC is a member of the CC chemokine group that is constitutively expressed in the thymus and is produced by dendritic cells, endothelial cells, keratinocytes, bronchial epithelial cells, and fibroblasts[37,38]. TARC is a ligand for CCR4, which is predominantly expressed on Th2 lymphocytes, basophils, and natural killer cells[38]. ARC can recruit a large number of Th2 cells, despite the fact that the Th2 cell predominance is to maintain the chronic carrying state of virus infection[39]. Th2 cell activation inhibits both acute and chronic inflammatory reactions[40]. Therefore, the higher expression of TARC in the non-response group treated with interferon is related to the poor prognosis.
MIP-1d/CCL15, a member of the CC chemokine family that is expressed only in the gut and the liver, acts mainly via the CC chemokine receptor CCR1[41,42]. MIP-1d was demonstrated to have a chemoattractant role for monocytes, lymphocytes, neutrophils, eosinophils, and dendritic cells. In addition, this molecule plays an important role in the development of inflammatory diseases[41,43]. Our study found that MIP-1d was higher in the response group than in the non-response group at baseline and after 24 wk of treatment. The higher level in the response group than in the non-response group may be associated with better recruitment of T cells in the response group.
CXCL6/GCP-2 is a member of the ELR-CXC chemokines. CXCL6 is mainly secreted by macrophages and epithelial and stromal cells[44]. The receptors of CXCL6 are CXCR1 and CXCR2, and CXCL6 has the functions of regulating immunity, tumor growth, and metastasis, as well as promoting angiogenesis. Liver tissue can express CXCL6, which can induce Kupffer cell, neutrophil, and lymphocyte infiltration in liver inflammation[44,45]. The expression of CXCL6 was elevated in liver inflammation and fibrosis, and in alcoholic hepatitis, CXCL6 levels are elevated and related to the prognosis of patients[45]. In our study, CXCL6 was significantly higher at both baseline and 24-wk in the response group than in the non-response group and showed a significant correlation with ALT.
In conclusion, our results suggest that MIP-1d, CXCL9, CXCL6, IP-10, and TARC have predictive significance in interferon therapy. Cytokines are fundamental molecules in the complex signaling network of cell-mediated immunity. Analyses of the changes in cytokine expression patterns during treatment can help to understand the pathogenesis of CHB and predict treatment responses. However, there were limited subjects in this study, and unfortunately, we could not collect all samples from each patient at all treatment phases. Therefore, the statistical power was not adequate for an assessment of the usefulness of cytokines in monitoring CHB patients. Further large and long-term follow-up studies are required to determine the predictive value of cytokines in CHB patients receiving PEG-IFN treatment. In addition, the differences in these factors between the response and non-response groups and their specific biological roles in HBV infection require further investigation.
Hepatitis B virus (HBV) infection remains a global major public health problem. Chronic hepatitis B (CHB) patients generally have an impaired host immune response, which may be associated with persistently high viral load and subsequent T cell failure. Since peginterferon-alpha-2a (PEG-IFN) has direct antiviral and immunoregulatory effects, it has become one of the first choice drugs for the treatment of CHB. PEG-IFN has the properties of immune regulation, and the host immune status may affect the efficacy of PEG-IFN in the treatment of CHB.
Recently, many studies have shown that cytokines and chemokines may play a potential role in chronic viral hepatitis. We aimed to determine the correlation between cytokine/chemokine expression levels and response to PEG-IFN treatment in patients with CHB.
Our main purpose was to analyze the serum levels of cytokines in CHB patients receiving PEG-IFN treatment and responses to the therapy, and to assess the predictive value of the cytokines for the responses to PEG-IFN.
In total, 78 serum samples were prospectively collected with written informed consent from 26 CHB patients undergoing PEG-IFN therapy. A Luminex 200 analyzer and Cytokine Array I reagents were used to quantify 46 cytokines in the serum. The serum HBV DNA levels were assayed using a COBAS Amplicor/COBAS TaqMan HBV test, and HBeAb was measured by enzyme immunoassay.
A total of 26 patients (17 males and 9 females; mean age, 28.8.3 ± 4.161 years in virological responders group, and 27.82 ± 4.446 years in virological non-responders group) were enrolled in the study. The monokine induced by INF-γ (CXCL9) and serum interferon-inducible protein 10 (IP-10) levels at baseline were higher in the virological responders than in the non-virological responders and decreased during treatment. The CXCL9, IP-10, macrophage inflammatory protein 1d (MIP-1d), and thymus and activation-regulated chemokine (TARC) baseline levels exhibited the expected effects for interferon treatment. The area under the receiver operating characteristic curve values of CXCL9, IP-10, MIP-1d, and TARC for predicting the virological response were 0.787, 0.799, 0.787, and 0.77 (P = 0.01, 0.013, 0.01, and 0.021), respectively.
In conclusion, our results suggest that MIP-1d, CXCL9, CXCL6, IP-10, and TARC have predictive significance in interferon therapy. Cytokines are fundamental molecules in the complex signaling network of cell-mediated immunity. Analyses of the changes in cytokine expression patterns during treatment can help to understand the pathogenesis of CHB and predict treatment responses.
Further large and long-term follow-up studies are required to determine the predictive value of cytokines in CHB patients receiving PEG-IFN treatment. Besides, the differences in these factors between the response and non-response groups and their specific biological roles in HBV infection require further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alsubai J, Lynch H S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Lee HM, Banini BA. Updates on Chronic HBV: Current Challenges and Future Goals. Curr Treat Options Gastroenterol. 2019;17:271-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 2. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1993] [Article Influence: 199.3] [Reference Citation Analysis (3)] |
| 3. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Ryou M. Combination therapy versus monotherapy for EUS-guided management of gastric varices: A systematic review and meta-analysis. Endosc Ultrasound. 2020;9:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (1)] |
| 4. | Samarasena J, Chang KJ. Endo-hepatology: A new paradigm. Endosc Ultrasound. 2018;7:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Huang JY. Role of EUS-guided liver biopsy in benign parenchymal disease (with video). Endosc Ultrasound. 2018;7:236-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Samarasena JB, Yu AR, Chang KJ. EUS-guided portal pressure measurement (with videos). Endosc Ultrasound. 2018;7:257-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Lisotti A, Serrani M, Caletti G, Fusaroli P. EUS liver assessment using contrast agents and elastography. Endosc Ultrasound. 2018;7:252-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Djiambou-Nganjeu H. Hepatic Encephalopathy in Patients in Lviv (Ukraine). J Transl Int Med. 2018;6:146-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Song T, Jia Z, Guo X, Zhao H, Bao W, Han D, Zhou X, Qi X. Does Hepatic Impairment Influence Renal Function Parameters in Liver Cirrhosis? J Transl Int Med. 2018;6:90-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Rössle M MD, Schultheiss M. Timing of the Treatment of Portal Vein Thrombosis in Patients with Cirrhosis: A German Hepatologist's Perspective. J Transl Int Med. 2018;6:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Tan G, Song H, Xu F, Cheng G. When Hepatitis B Virus Meets Interferons. Front Microbiol. 2018;9:1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Lee IC, Huang YH, Su CW, Wang YJ, Huo TI, Lee KC, Lin HC. CXCL9 associated with sustained virological response in chronic hepatitis B patients receiving peginterferon alfa-2a therapy: a pilot study. PLoS One. 2013;8:e76798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Wu HL, Hsiao TH, Chen PJ, Wong SH, Kao JH, Chen DS, Lu JY, Lu TP, Chen Y, Chuang EY, Tu HC, Liu CJ. Liver Gene Expression Profiles Correlate with Virus Infection and Response to Interferon Therapy in Chronic Hepatitis B Patients. Sci Rep. 2016;6:31349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2823] [Article Influence: 403.3] [Reference Citation Analysis (0)] |
| 15. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N; Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1170] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 16. | Ma SW, Huang X, Li YY, Tang LB, Sun XF, Jiang XT, Zhang YX, Sun J, Liu ZH, Abbott WG, Dong YH, Naoumov NV, Hou JL. High serum IL-21 levels after 12 weeks of antiviral therapy predict HBeAg seroconversion in chronic hepatitis B. J Hepatol. 2012;56:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Chokshi S, Cooksley H, Riva A, Phillips S, Williams R, Gaggar A, Naoumov NV. Identification of serum cytokine profiles associated with HBeAg seroconversion following antiviral treatment interruption. Viral Immunol. 2014;27:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Park Y, Park Y, Han KH, Kim HS. Serum cytokine levels in patients with chronic hepatitis B according to lamivudine therapy. J Clin Lab Anal. 2011;25:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kurihara M, Tsuge M, Murakami E, Mori N, Ohishi W, Uchida T, Fujino H, Nakahara T, Abe-Chayama H, Kawaoka T, Miki D, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Ochi H, Zhang Y, Makokha GN, Hayes CN, Chayama K. The association between serum cytokine and chemokine levels and antiviral response by entecavir treatment in chronic hepatitis B patients. Antivir Ther. 2018;23:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Park Y, Park JY, Han KH, Kim HS. Serum cytokine levels in chronic hepatitis B patients receiving peginterferon alpha-2a therapy. Hepatobiliary Pancreat Dis Int. 2012;11:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1201] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 22. | Kwon H, Lok AS. Hepatitis B therapy. Nat Rev Gastroenterol Hepatol. 2011;8:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Chien RN, Kao JH, Peng CY, Chen CH, Liu CJ, Huang YH, Hu TH, Yang HI, Lu SN, Ni YH, Chuang WL, Lee CM, Wu JC, Chen PJ, Liaw YF. Taiwan consensus statement on the management of chronic hepatitis B. J Formos Med Assoc. 2019;118:7-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Sonneveld MJ, Arends P, Boonstra A, Hansen BE, Janssen HL. Serum levels of interferon-gamma-inducible protein 10 and response to peginterferon therapy in HBeAg-positive chronic hepatitis B. J Hepatol. 2013;58:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317:620-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 741] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 26. | Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 737] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 27. | De Rose V, Cappello P, Sorbello V, Ceccarini B, Gani F, Bosticardo M, Fassio S, Novelli F. IFN-gamma inhibits the proliferation of allergen-activated T lymphocytes from atopic, asthmatic patients by inducing Fas/FasL-mediated apoptosis. J Leukoc Biol. 2004;76:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Bièche I, Asselah T, Laurendeau I, Vidaud D, Degot C, Paradis V, Bedossa P, Valla DC, Marcellin P, Vidaud M. Molecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infection. Virology. 2005;332:130-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Apolinario A, Majano PL, Lorente R, Núñez O, Clemente G, García-Monzón C. Gene expression profile of T-cell-specific chemokines in human hepatocyte-derived cells: evidence for a synergistic inducer effect of cytokines and hepatitis C virus proteins. J Viral Hepat. 2005;12:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Oo YH, Weston CJ, Lalor PF, Curbishley SM, Withers DR, Reynolds GM, Shetty S, Harki J, Shaw JC, Eksteen B, Hubscher SG, Walker LS, Adams DH. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 32. | Butera D, Marukian S, Iwamaye AE, Hembrador E, Chambers TJ, Di Bisceglie AM, Charles ED, Talal AH, Jacobson IM, Rice CM, Dustin LB. Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Tan AT, Koh S, Goh W, Zhe HY, Gehring AJ, Lim SG, Bertoletti A. A longitudinal analysis of innate and adaptive immune profile during hepatic flares in chronic hepatitis B. J Hepatol. 2010;52:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, Narimatsu H, Mizokami M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018;53:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 35. | Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Ishii A, Iijima H, Nishiguchi S. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-γ-inducible protein-10 in primary biliary cirrhosis. Hepatol Res. 2016;46:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Zhu MY, Chen PZ, Li J, Yu DM, Huang D, Zhu XJ, Han Y, Chen J, Huang W, Chen YY, Gong QM, Jiang JH, Zhang DH, Zhang Y, Zhang JM, Zhang XX. Serum M2BPGi level is a novel predictive biomarker for the responses to pegylated interferon-α treatment in HBeAg-positive chronic hepatitis B patients. J Med Virol. 2018;90:721-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Yu B, Koga T, Urabe K, Moroi Y, Maeda S, Yanagihara Y, Furue M. Differential regulation of thymus- and activation-regulated chemokine induced by IL-4, IL-13, TNF-alpha and IFN-gamma in human keratinocyte and fibroblast. J Dermatol Sci. 2002;30:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Saeki H, Tamaki K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J Dermatol Sci. 2006;43:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Monick MM, Powers LS, Hassan I, Groskreutz D, Yarovinsky TO, Barrett CW, Castilow EM, Tifrea D, Varga SM, Hunninghake GW. Respiratory syncytial virus synergizes with Th2 cytokines to induce optimal levels of TARC/CCL17. J Immunol. 2007;179:1648-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Spellberg B, Edwards JE Jr. Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 616] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 41. | Sung HJ, Kim YS, Kang H, Ko J. Human LZIP induces monocyte CC chemokine receptor 2 expression leading to enhancement of monocyte chemoattractant protein 1/CCL2-induced cell migration. Exp Mol Med. 2008;40:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Hwang J, Kim CW, Son KN, Han KY, Lee KH, Kleinman HK, Ko J, Na DS, Kwon BS, Gho YS, Kim J. Angiogenic activity of human CC chemokine CCL15 in vitro and in vivo. FEBS Lett. 2004;570:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Gao Y, Zhou Z, Lu S, Huang X, Zhang C, Jiang R, Yao A, Sun B, Wang X. Chemokine CCL15 Mediates Migration of Human Bone Marrow-Derived Mesenchymal Stem Cells Toward Hepatocellular Carcinoma. Stem Cells. 2016;34:1112-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Wuyts A, Struyf S, Gijsbers K, Schutyser E, Put W, Conings R, Lenaerts JP, Geboes K, Opdenakker G, Menten P, Proost P, Van Damme J. The CXC chemokine GCP-2/CXCL6 is predominantly induced in mesenchymal cells by interleukin-1beta and is down-regulated by interferon-gamma: comparison with interleukin-8/CXCL8. Lab Invest. 2003;83:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Dominguez M, Miquel R, Colmenero J, Moreno M, García-Pagán JC, Bosch J, Arroyo V, Ginès P, Caballería J, Bataller R. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |