Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2219
Peer-review started: January 2, 2020
First decision: February 26, 2020
Revised: March 26, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: June 6, 2020
Processing time: 153 Days and 20.4 Hours
Persistent suspicion of prostate cancer (PCa) due to a rising prostate-specific antigen (PSA) level after repeated negative biopsies is a serious challenge in clinical practice.
To determine the role of Hiraoka’s transurethral detachment of the prostate (TUDP) combined with biopsy of the peripheral zone during the same session in patients with repeated negative biopsies in the diagnosis of PCa.
We retrospectively evaluated the records of 10 patients who were eligible for inclusion in our hospital between December 2012 and August 2017. Patient demographics, a family history of PCa, the number of biopsies, prostate volume, pathological examination, and perioperative PSA level were obtained.
Two of 10 patients were pathologically diagnosed with PCa after surgery; the Gleason scores were 4 + 4 and 4 + 3, respectively. Both patients subsequently underwent laparoscopic radical prostatectomy. The median PSA levels preoperatively, and 3 mo and 1 year postoperatively in the other eight patients who were diagnosed with benign prostate hyperplasia after surgery were 19.10 ng/mL, 1.10 ng/mL, and 1.15 ng/mL, respectively. The adjusted P values of the 3-mo and 1-year post-operative PSA level vs pre-operative PSA level were 0.003 and 0.026, respectively. None of the patients had increased PSA levels or PCa detected after a median 35 mo of follow-up.
TUDP combined with peripheral zone biopsy may improve the detection rate of PCa in patients with repeated negative biopsies. The PSA level declined rapidly in patients who had negative pathological examinations after TUDP, which remained stable 1 year after surgery.
Core tip: This retrospective study demonstrated that transurethral detachment of the prostate combined with peripheral zone biopsy may improve prostate cancer detection rate in patients with repeated negative biopsies, and abnormal prostate-specific antigen level. The prostate-specific antigen level declined rapidly 3 mo postoperatively in patients with negative pathological examinations and remained stable 1 year after surgery.
- Citation: Pan CY, Wu B, Yao ZC, Zhu XQ, Jiang YZ, Bai S. Role of Hiraoka's transurethral detachment of the prostate combined with biopsy of the peripheral zone during the same session in patients with repeated negative biopsies in the diagnosis of prostate cancer. World J Clin Cases 2020; 8(11): 2219-2226
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2219.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2219
Prostate cancer (PCa) is the most commonly diagnosed cancer in men > 65 years of age, with an estimated 1.1 million diagnosed worldwide in 2012 and accounting for 15% of all cancers diagnosed[1]. The prevalence of PCa varies widely in different geographic regions and races, and is higher in western Europe and North America, with age-standardized rates of 94.7/100000 and 97.2/100000, respectively, and lower in Asia, with an age-standardized rate of 4.5/100000[2]. PCa is the main cause of mortality for men in developed countries.
Prostate biopsy is the main strategy for diagnosing PCa; however, 25%-35% of PCas are missed at the time of the first biopsy and 10% are missed at the time of the second biopsy, even if additional transition zone and saturation biopsies are obtained[3]. The incidence of identifying cancer at the time of the third or fourth biopsies decreases to < 14%[4]. Persistent suspicion of PCa due to a rising prostate-specific antigen (PSA) level after repeated negative biopsies is common in clinical practice[5]. Although many strategies have been developed to overcome this problem, such as increasing the number of biopsy cores and biopsy of transitional or anterior zones of the gland[6-8], none of the existing strategies can resolve this problem.
Transurethral resection of the prostate (TURP) is a common treatment option for patients with lower urinary tract symptoms (LUTS) caused by bladder outlet obstruction, which allows improvement in voiding symptoms and extends the histologic examination in the transition zone, which accounts for 20%-25% of PCas[9]. Due to a lower histological positive rate compared with repeated prostate biopsies, TURP is not a reasonable approach to diagnose PCa in patients without voiding symptoms[10]. Moreover, there are several disadvantages of TURP; specifically, TURP cannot reach the prostate peripheral zone tissues and may lead to an increased surgical risk due to tissue adhesions caused by thermal injury, which necessitate postponing radical prostatectomy to avoid morbidity. Hence, either repeated biopsies or TURP cannot reliably exclude the presence of PCa.
Hiraoka’s transurethral detachment of the prostate (TUDP) was first introduced in 1989 by Hiraoka et al[11]. TUDP is a mature technique in enucleation of the prostate for treatment of benign prostate hyperplasia[12,13]. TUDP combined with biopsy of the peripheral zone during the same session is a method which may improve the PCa detection rate by obtaining a complete sample which simultaneously includes the entire transition, peripheral, and anterior fibrous zones. The purpose of this study was to determine the role of TUDP combined with biopsy of the peripheral zone during the same session in patients with repeated negative biopsies, rising PSA levels, and voiding symptoms in the diagnosis of PCa.
We retrospectively evaluated the records of 10 patients who underwent Hiraoka’s TUDP combined with biopsy of the peripheral zone during the same session and had repeated negative biopsies in the diagnosis of PCa in our hospital between December 2012 and August 2017. The inclusion criteria were as follows: had rising PSA level and negative prostate biopsies (at least twice); underwent TUDP due to bladder outlet obstruction.
Patient demographics (age and body mass index), family history of PCa, number of biopsies, prostate volume (examined by transrectal ultrasound), pathological examination, and peri-operative PSA level (preoperatively, and 3 mo and 1 year postoperatively) were obtained. The follow-up period was calculated from the date of surgery to the date of the final visit.
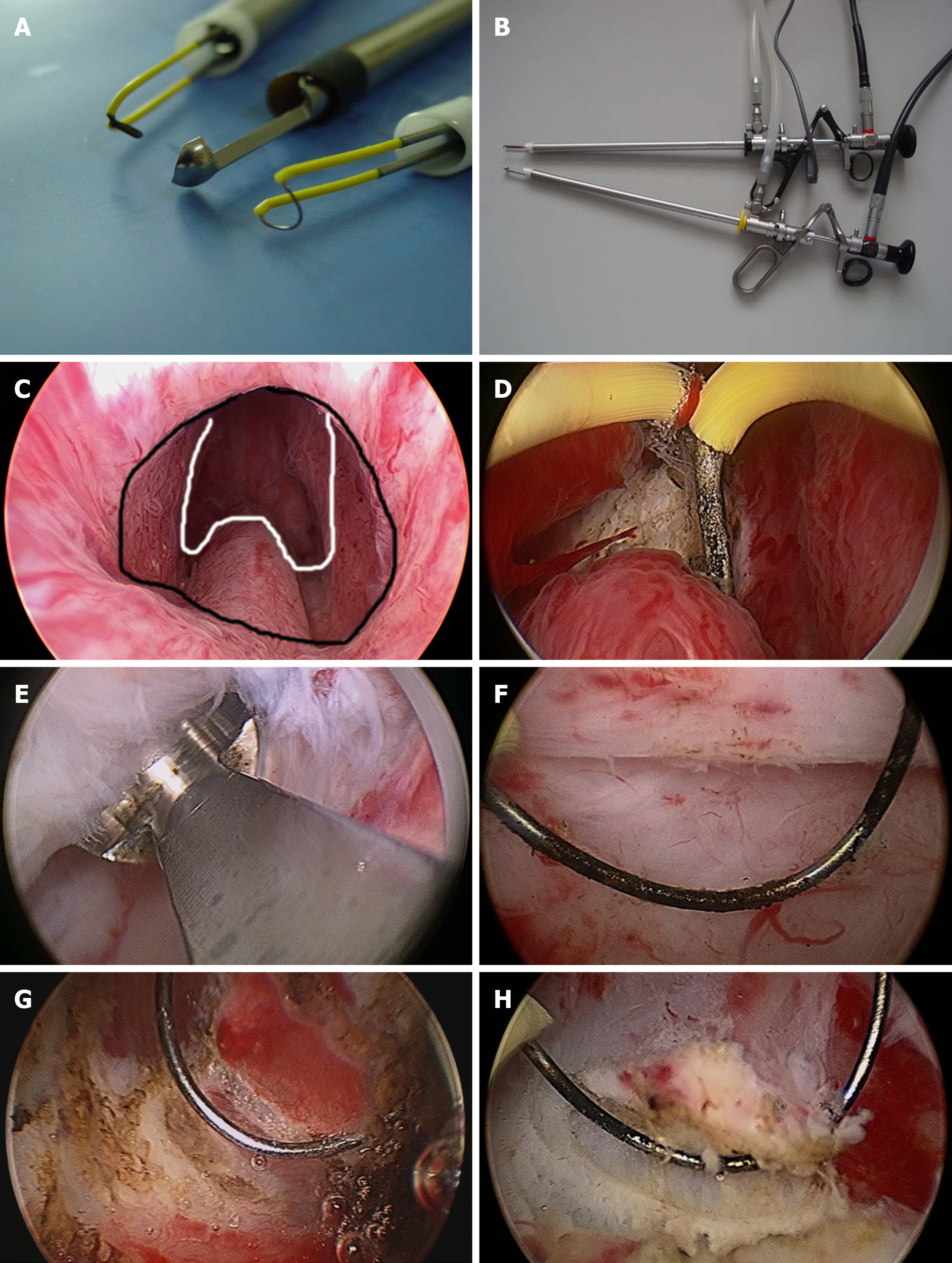
The surgical instruments include Hiraoka’s Prostate Detaching Blade (Olympus, Tokyo, Japan), Needle Electrode and Cutting Loop Electrode (Olympus, Tokyo, Japan), 26 Fr Continuous Irrigation Sheath and Resectoscope (Olympus, Tokyo, Japan; Figure 1A and B), and 26 Fr Storz Nephroscope and Lumenis Versa Tissue Morcellator (Israel).
Patients underwent surgery in the lithotomy position after anesthesia. First, we used a cutting loop electrode to mark the junction between the distal prostate gland and the proximal edge of the urethral external sphincter (Nesbit sign) using the electrocoagulation mode. The needle electrode was then used to incise the urethral mucosa in a circular incision along the marker. The depth of the circular incision was 3-4 mm and was anterior oblique (parallel to the sphincter to avoid injury). Second, Hiraoka’s Prostate Detaching Blade was used to locate and enter the surgical capsule plane at 5-7 o'clock of the circular incision, then extended forward and laterally along the surgical plane by blunt separation. A hyperplastic prostate gland is white with a dense fibrous structure, and the surgical capsule is spongy with soft and transverse vessels when viewed with a resectoscope. There is little bleeding during the detaching process, which generally does not affect the visual field. We used a cutting loop electrode to stop bleeding as needed. Third, when detaching the bladder neck, Hiraoka’s Prostate Detaching Blade was used to enucleate the anterior fibrous area in the 12 o’clock direction, then extended to both sides and stopping at 5 o’clock and 7 o’clock. The bladder neck was then cut off between 5 o’clock and 7 o’clock using a cutting loop electrode. Finally, the enucleated prostate hyperplastic gland was pushed into the bladder, which was morcellated and removed using a tissue morcellator (Figure 1C-F). A cutting loop electrode was used to biopsy the peripheral zone, which included three locations (left peripheral zone, right peripheral zone, and anterior fibrous area; Figure 1G and H).
Continuous variables with non-normal variables were reported as the median (interquartile range). Categorical data are presented as a frequency and percentage. The mean of multiple repeated continuous non-normally distributed variables was compared using the Friedman test. Statistical analyses were performed using SPSS 22.0 for Windows (SPSS, Inc., Chicago, IL, United States). A P value < 0.05 was considered statistically significant.
Ten patients underwent Hiraoka’s TUDP combined with biopsy of the peripheral zone with a long-term follow-up (median, 35 mo; range, 12-40 mo). The median age and body mass index were 66.0 years and 26.0 kg/m2, respectively. None of the patients had a family history of PCa. The median prostate volume was 55 mL (range, 40–60 mL).
Two of 10 patients were pathologically diagnosed with PCa after surgery. One patient had PCa, which was detected in the transition and peripheral zones. The other patient had PCa, which was only detected in the anterior fibrous zone. The Gleason scores were 4 + 4 and 4 + 3, respectively. Both patients subsequently underwent a laparoscopic radical prostatectomy.
The median preoperative, and 3-mo and 1-year postoperative PSA levels of the other 8 patients who were diagnosed with benign prostate hyperplasia were 19.10 ng/mL, 1.10 ng/mL, and 1.15 ng/mL, respectively. The adjusted P values for the 3-mo and 1-year post-operative PSA level vs preoperative PSA level were 0.003 and 0.026, respectively. The PSA levels of these patients declined rapidly 3 mo postoperatively and remained stable 1 year after surgery. Moreover, none of the patients had rising PSA levels and PCa detected after a median of 35 mo follow-up (Table 1).
| Characteristics | Results |
| Follow-up period (mo) | 35.0 (12.0–40.0) |
| No. of cases | 10 |
| Age (yr) | 66.0 (62.0–67.0) |
| BMI (kg/m2) | 26.0 (24.0–27.0) |
| Familial history of PCa | 0 (0) |
| Number of negative biopsy (times) | 2 (2–3) |
| Prostate volume (mL) | 55.0 (40.0–60.0) |
| Pathological examination (PCa) | 2 (0.2) |
| PSA pre-operation (ng/mL) | 19.10 (16.00 to 23.00) |
| PSA 3 mo postoperation | 1.00 (1.05–1.20) |
| PSA 1 year postoperation | 1.15 (1.10–1.20) |
Prostate biopsy is the main strategy used in diagnosing PCa. In the largest PCa screening program, 91% of PCas were detected at the time of the first or second biopsy, but < 14% of PCas were detected at the time of the third or fourth biopsy[4]. There are many methods for improving the PCa detection rate, such as increasing the number of biopsy cores and extending the transitional and anterior fibrous zones of the prostate. Nevertheless, a portion of PCas remain undetected after multiple biopsies. According to the 2018 EAU guidelines, third and fourth biopsies are not recommended because of lower sensitivities[10]. TURP is an option for patients with voiding problems, rising PSA levels, and repeated negative biopsies, which would improve voiding symptoms, normalize PSA levels, and extend the histological examination in the transition zone of the prostate[6]; however, TURP does not reach the peripheral zone, thus part of a PCa may be missed by surgery. However, this is a common issue in clinical practice for which there is currently no solution. In addition, patients with abnormal PSA levels and repeated negative biopsies can also be terrified. Katz et al[14] reported that such patients have increased cancer-related anxiety and exhibit increased sexual problems. In this study we evaluated the role of TUDP combined with peripheral zone biopsy during the same session in patients with voiding problems, rising PSA levels, and repeated negative biopsies in the diagnosis of PCa.
The most important advantage of TUDP is the nearly complete prostate tissue biopsy, which includes the entire transition zone, and parts of the peripheral and anterior fibrous zones of the prostate that can be biopsied simultaneously. It is noteworthy that the peripheral zone biopsy tissue which is resected by TURP is much more than a puncture biopsy.
Based on the literature, the PCa detection rate in TURP ranges from 1.4%-16.7%[15-19], which is less than our results (20.0%). The reason for this difference may be that more adenoma tissues are removed in TUDP than TURP, and when simultaneously combined with a peripheral zone resection biopsy, a nearly complete sampling would be obtained and the cancer detection rate would be increased.
Puppo et al[5] reported a similar technique to TURP combined with a peripheral zone biopsy in the diagnosis of PCa for patients with repeated negative biopsies, a persistently abnormal PSA level, and no voiding problems. Eight of 14 patients had PCa, which was higher than repeated biopsies. Therefore, Puppo et al[5] concluded that this technique had high diagnostic power for such patients. In contrast, many studies suggest that TURP should be performed only in patients with voiding symptoms due to lower sensitivity than repeated biopsies[10,20]. In this study all of the patients had moderate-to-severe LUTS and an impaired quality of life.
Puppo et al[5] stated that radical prostatectomy is more difficult in patients after TURP than routine cases, with good continence and poor erection function. In this study there were two patients with positive biopsies, both of whom had aggressive PCa. The patients underwent laparoscopic radical prostatectomies within 1 mo after TUDP, did not have severe morbidities perioperatively, and had good continence and erection function.
Postoperative follow-up is also important. Zigeuner et al[10] reported that every fourth cancer is missed by previous biopsies and surgery, which were detected during follow-up. Kitamura et al[20] reported that four of 139 patients who underwent TURP with repeated negative biopsies were shown to have PCa during the follow-up period. In a retrospective study, Radhakrishnan et al[21] reported that three of 14 patients had PCa during the follow-up period. Philip et al[7] also described five patients who had PCa and underwent radical prostatectomy and emphasized that PCa was mainly located in the anterior fibrous zone. In our study none of the patients had rising PSA levels and PCa detected after a median of 35 mo follow-up, possibly because the anterior and peripheral zones were both examined during surgery.
van Renterghem et al[6] reported that TURP could improve voiding symptoms, quality of life, and normalize the PSA level. The average PSA level decreased from 8.2 ng/mL to 0.9 ng/mL 1 year after surgery and 97.2% of patients (35/36) had a permanently normal PSA. Cho et al[22] stated that 95.5% of patients (106/112) had normalized PSA 3 mo after TURP that persisted in 97.2% of patients 3 years after surgery. In agreement with this finding, we demonstrated that 100% of patients (8/8) with negative biopsies had normalized PSA levels 3 mo after TUDP that persisted for at least 1 year. The reason for this finding could be that the prostate volume and voiding symptoms are closely related to the PSA level.
TUDP resects the entire hyperplasic prostate adenoma. In contrast, TURP only removes one-half of the adenoma on average[23]. Helfand et al[16] reported a 93.0% decrease in the PSA level from baseline in patients undergoing open prostatectomy and a 60% decrease in the PSA level in patients undergoing TURP, which presumably reflects the completeness of the resection. We noted a 94.0% decrease in the PSA level in our study.
We are of the opinion that the other types of transurethral enucleation of the prostate, such as Holmium laser enucleation of the prostate, could also be combined with peripheral zone biopsies in diagnosing PCa; however, we would not recommend vaporization in these patients as vaporization does not allow pathological examination[24].
There were several limitations in this study that should be noted. First, this was a retrospective study with inherent flaws. Second, relatively few patients were included in one center. A large multi-center study is required to support the advantages of this technique. In addition, other surgeons are needed to confirm the repeatability before widespread acceptance. Nevertheless, this type of technique is demanding compared with other kinds of techniques.
In conclusion, TUDP combined with peripheral zone biopsy may improve the detection rate of PCa in patients with repeated negative biopsies. The PSA level declined rapidly in patients who had negative pathological examinations after TUDP, which remained stable 1 year after surgery.
After repeated negative biopsies, persistent suspicion of prostate cancer (PCa) due to a rising prostate-specific antigen (PSA) level is a serious challenge in clinical practice.
Transurethral detachment of the prostate (TUDP) combined with biopsy of the peripheral zone during the same session is a method which may improve the PCa detection rate by obtaining a complete sample which simultaneously includes the entire transition, peripheral, and anterior fibrous zones.
Our aim was to determine the role of Hiraoka's TUDP combined with biopsy of the peripheral zone during the same session in patients with repeated negative biopsies in the diagnosis of PCa.
We retrospectively evaluated the records of 10 patients who were eligible for inclusion in our hospital between December 2012 and August 2017. Patient demographics, a family history of PCa, the number of biopsies, prostate volume, pathological examination, and perioperative PSA level were obtained.
Two of 10 patients were pathologically diagnosed with PCa after surgery; the Gleason scores were 4 + 4 and 4 + 3, respectively. Both of the patients subsequently underwent laparoscopic radical prostatectomy. The median PSA levels preoperatively, and 3 mo and 1 year postoperatively in the other eight patients who were diagnosed with benign prostate hyperplasia after surgery were 19.10 ng/mL, 1.10 ng/mL, and 1.15 ng/mL, respectively. The adjusted P values of the 3-mo and 1-year post-operative PSA level vs pre-operative PSA level were 0.003 and 0.026, respectively. None of the patients had rising PSA levels or PCa detected after a median 35 mo of follow-up.
TUDP combined with peripheral zone biopsy may improve the PCa detection rate in patients with repeated negative biopsies.
The PSA level declined rapidly in patients who had negative pathological examinations after TUDP, which remained stable 1 year after surgery.
We give special thanks to all the teachers at the Department of Urology of Shengjing Hospital for their help and support.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bugaj AM, Markic D, Sugimura H S-Editor: Dou Y L-Editor: Webster JR E-Editor: Qi LL
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 2. | Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15:3866-3871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Abedi AR, Fallah-Karkan M, Allameh F, Ranjbar A, Shadmehr A. Incidental prostate cancer: a 10-year review of a tertiary center, Tehran, Iran. Res Rep Urol. 2018;10:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Puppo P, Introini C, Calvi P, Naselli A. Role of transurethral resection of the prostate and biopsy of the peripheral zone in the same session after repeated negative biopsies in the diagnosis of prostate cancer. Eur Urol. 2006;49:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | van Renterghem K, Van Koeveringe G, Achten R, van Kerrebroeck P. A new algorithm in patients with elevated and/or rising prostate-specific antigen level, minor lower urinary tract symptoms, and negative multisite prostate biopsies. Int Urol Nephrol. 2010;42:29-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Philip J, Dutta Roy S, Scally J, Foster CS, Javlé P. Importance of TURP in diagnosing prostate cancer in men with multiple negative biopsies. Prostate. 2005;64:200-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Chon CH, Lai FC, McNeal JE, Presti JC. Use of extended systematic sampling in patients with a prior negative prostate needle biopsy. J Urol. 2002;167:2457-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Onder AU, Yalcin V, Arar O, Yaycioglu O, Citci A, Solok V. Impact of transition zone biopsies in detection and evaluation of prostate cancer. Eur Urol. 1998;33:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Zigeuner R, Schips L, Lipsky K, Auprich M, Salfellner M, Rehak P, Pummer K, Hubmer G. Detection of prostate cancer by TURP or open surgery in patients with previously negative transrectal prostate biopsies. Urology. 2003;62:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Hiraoka Y, Akimoto M. Transurethral enucleation of benign prostatic hyperplasia. J Urol. 1989;142:1247-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Iwamoto K, Hiraoka Y, Shimizu Y. Transurethral detachment prostatectomy using a tissue morcellator for large benign prostatic hyperplasia. J Nippon Med Sch. 2008;75:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Hiraoka Y, Shimizu Y, Iwamoto K, Takahashi H, Abe H. Trial of complete detachment of the whole prostate lobes in benign prostate hyperplasia by transurethral enucleation of the prostate. Urol Int. 2007;79:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Katz DA, Jarrard DF, McHorney CA, Hillis SL, Wiebe DA, Fryback DG. Health perceptions in patients who undergo screening and workup for prostate cancer. Urology. 2007;69:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Voigt S, Hüttig F, Koch R, Propping S, Propping C, Grimm MO, Wirth M. Risk factors for incidental prostate cancer-who should not undergo vaporization of the prostate for benign prostate hyperplasia? Prostate. 2011;71:1325-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Helfand BT, Anderson CB, Fought A, Kim DY, Vyas A, McVary KT. Postoperative PSA and PSA velocity identify presence of prostate cancer after various surgical interventions for benign prostatic hyperplasia. Urology. 2009;74:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Yoo C, Oh CY, Kim SJ, Kim SI, Kim YS, Park JY, Seong do H, Song YS, Yang WJ, Chung HC, Cho IR, Cho SY, Cheon SH, Hong S, Cho JS. Preoperative clinical factors for diagnosis of incidental prostate cancer in the era of tissue-ablative surgery for benign prostatic hyperplasia: a korean multi-center review. Korean J Urol. 2012;53:391-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Trpkov K, Thompson J, Kulaga A, Yilmaz A. How much tissue sampling is required when unsuspected minimal prostate carcinoma is identified on transurethral resection? Arch Pathol Lab Med. 2008;132:1313-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Otto B, Barbieri C, Lee R, Te AE, Kaplan SA, Robinson B, Chughtai B. Incidental prostate cancer in transurethral resection of the prostate specimens in the modern era. Adv Urol. 2014;2014:627290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Kitamura H, Masumori N, Tanuma Y, Yanase M, Itoh N, Takahashi A, Tsukamoto T, Adachi H, Hotta H. Does transurethral resection of the prostate facilitate detection of clinically significant prostate cancer that is missed with systematic sextant and transition zone biopsies? Int J Urol. 2002;9:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Radhakrishnan S, Dorkin TJ, Sheikh N, Greene DR. Role of transition zone sampling by TURP in patients with raised PSA and multiple negative transrectal ultrasound-guided prostatic biopsies. Prostate Cancer Prostatic Dis. 2004;7:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Cho HJ, Shin SC, Cho JM, Kang JY, Yoo TK. The role of transurethral resection of the prostate for patients with an elevated prostate-specific antigen. Prostate Int. 2014;2:196-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Shimizu Y, Hiraoka Y, Iwamoto K, Takahashi H, Abe H. Measurement of residual adenoma after transurethral resection of the prostate by transurethral enucleation technique. Urol Int. 2005;74:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | van Renterghem K, Van Koeveringe G, Achten R, van Kerrebroeck P. Prospective study of the role of transurethral resection of the prostate in patients with an elevated prostate-specific antigen level, minor lower urinary tract symptoms, and proven bladder outlet obstruction. Eur Urol. 2008;54:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |